Multiple-Use Zoning Model for Private Forest Owners in Agricultural Landscapes: A Case Study
Abstract
:1. Introduction
2. Materials and Methods
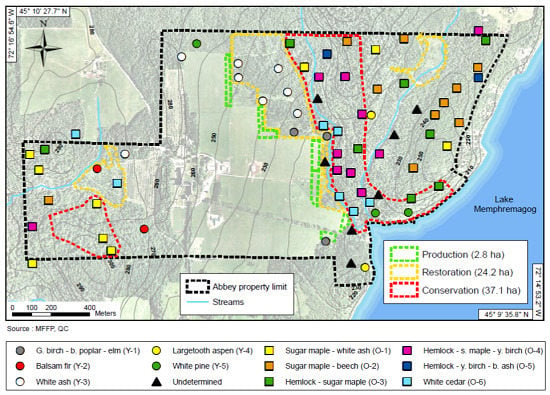
2.1. Study Site Description

2.2. Natural Disturbance Regime, Historical Forest Composition, Settlement Impacts, and Contemporary Stressors Affecting the Northern Hardwoods Forest
2.3. Forest Vegetation Analysis
2.3.1. Data Collection for Forest Vegetation Analysis
2.3.2. Forest Community Determination Using Ordination Analyses


2.3.3. Young Forests Community Types Description
| Tree Species and Community Structure | Grey Birch—Balsam Poplar—Elm Y-1 (3 plots) | Balsam Fir Y-2 (2 plots) | White Ash Y-3 (8 plots) | Largetooth Aspen Y-4 (2 plots) | White Pine Y-5 (3 plots) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sap. | Trees | Sap. | Trees | Sap. | Trees | Sap. | Trees | Sap. | Trees | |
| Abies balsamea | 48 (2) | 13 (6) | 2 (2) | 8 (1) | 1 (1) | |||||
| Acer pensylvanicum | 6 (2) | |||||||||
| Acer rubrum | 1 (1) | 54 (1) | + (1) | 10 (2) | 10 (3) | 1 (1) | ||||
| Acer saccharum | 1 (1) | 8 (4) | 8 (3) | 16 (2) | 7 (2) | 14 (1) | 1 (1) | |||
| Betula alleghaniensis | 3 (2) | 17 (1) | 3 (1) | |||||||
| Betula papyrifera | 3 (1) | 20 (2) | 10 (1) | + (1) | 4 (2) | 3 (2) | 5 (1) | 1 (1) | ||
| Betula populifolia | 78 (3) | 7 (5) | 1 (1) | 9 (1) | 4 (1) | |||||
| Fagus grandifolia | + (1) | 26 (2) | ||||||||
| Fraxinus americana | 13 (1) | 43 (8) | 45 (8) | 8 (2) | 1 (1) | 22 (2) | ||||
| Fraxinus nigra | 4 (1) | 7 (2) | 4 (1) | |||||||
| Fraxinus pennsylvanica | 2 (1) | 21 (1) | + (1) | |||||||
| Juglans cinerea | 4 (1) | |||||||||
| Ostrya virginiana | 8 (5) | 1 (1) | 2 (1) | |||||||
| Picea abies | 21 (1) | |||||||||
| Picea glauca | 10 (1) | |||||||||
| Pinus strobus | 11 (3) | 89 (3) | ||||||||
| Populus balsamifera | 10 (2) | 67 (2) | ||||||||
| Populus deltoides | 1 (1) | |||||||||
| Populus grandidentata | 1 (1) | 7 (1) | 11 (1) | 69 (2) | ||||||
| Populus tremuloides | 15 (1) | 3 (1) | 10 (2) | |||||||
| Prunus serotina | + (1) | 1 (1) | 4 (1) | |||||||
| Thuja occidentalis | + (1) | |||||||||
| Tilia americana | 1 (1) | + (1) | 1 (1) | 1 (1) | 4 (2) | |||||
| Tsuga canadensis | 1 (1) | |||||||||
| Ulmus americana | 4 (1) | 33 (1) | 2 (1) | 7 (8) | 18 (6) | 2 (1) | ||||
| Mean sapling (5–10 cm) density (n/ha) | 292 | 1,838 | 878 | 900 | 583 | |||||
| Mean tree (>10 cm) density (n/ha) | 567 | 775 | 994 | 1,000 | 717 | |||||
| Mean tree (>20 cm) basal area (m2/ha) | 10 | 5 | 12 | 25 | 32 | |||||
| Mean total basal area (saplings + trees) (m2/ha) | 18 | 46 | 30 | 40 | 43 | |||||
| Tree Species and Community Structure | Sugar Maple—White Ash O-1 (9 plots) | Sugar Maple—Beech O-2 (10 plots) | Hemlock—Sugar Maple O-3 (8 Plots) | Hemlock—Sugar Maple—Yellow Birch O-4 (10 Plots) | Hemlock—Yellow Birch—Black Ash O-5 (2 Plots) | White Cedar O-6 (7 Plots) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sap. | Trees | Sap. | Trees | Sap. | Trees | Sap. | Trees | Sap. | Trees | Sap. | Trees | |
| Abies balsamea | 1 (1) | 8 (4) | 6 (1) | 6 (6) | 4 (2) | 48 (7) | 7 (4) | |||||
| Acer pensylvanicum | 5 (3) | 24 (8) | 6 (4) | 21 (7) | 1 (1) | |||||||
| Acer rubrum | 6 (1) | 6 (2) | 1 (2) | 5 (2) | 1 (1) | 5 (2) | 3 (2) | 5 (6) | 1 (1) | 8 (1) | 1 (1) | 2 (1) |
| Acer saccharum | 46 (9) | 53 (9) | 2 (6) | 70 (10) | 9 (4) | 17 (8) | 3 (2) | 15 (8) | 11 (1) | + (1) | ||
| Betula alleghaniensis | + (1) | 2 (2) | 1 (2) | 6 (6) | 2 (2) | 5 (4) | 11 (5) | 9 (5) | 4 (2) | 23 (2) | 8 (4) | 7 (4) |
| Betula papyrifera | 5 (2) | 2 (1) | + (1) | + (1) | + (1) | 1 (1) | ||||||
| Betula populifolia | + (1) | 2 (1) | 1 (1) | |||||||||
| Fagus grandifolia | 15 (7) | 9 (4) | 61 (10) | 14 (7) | 39 (8) | 8 (3) | 22 (8) | 9 (5) | 4 (1) | |||
| Fraxinus americana | 8 (3) | 14 (5) | 3 (6) | 3 (4) | 10 (4) | 7 (3) | 1 (2) | 5 (4) | 6 (2) | 13 (2) | 5 (3) | 3 (2) |
| Fraxinus nigra | 4 (2) | 3 (2) | + (1) | 66 (2) | 8 (1) | 23 (5) | 6 (3) | |||||
| Juglans cinerea | 3 (1) | 4 (1) | ||||||||||
| Ostrya virginiana | 11 (4) | 3 (4) | 4 (4) | 2 (2) | 3 (1) | 2 (3) | 1 (1) | |||||
| Picea glauca | 4 (1) | + (1) | 1 (1) | |||||||||
| Pinus strobus | 1 (1) | 5 (1) | ||||||||||
| Populus grandidentata | + (1) | 3 (1) | 2 (1) | + (1) | 1 (1) | |||||||
| Prunus pensylvanica | + (1) | 2 (1) | 4 (3) | 1 (1) | ||||||||
| Prunus serotina | 7 (3) | + (1) | 1 (3) | + (1) | 1 (1) | + (1) | ||||||
| Thuja occidentalis | 1 (1) | 2 (3) | 1 (1) | 4 (4) | 55 (7) | |||||||
| Tilia americana | + (1) | 2 (2) | 1 (1) | 3 (2) | 1 (1) | + (1) | 4 (2) | 1 (1) | 2 (1) | |||
| Tsuga canadensis | 2 (2) | 1 (3) | 1 (1) | 4 (2) | 43 (8) | 25 (9) | 53 (10) | 3 (2) | 37 (2) | 2 (3) | 6 (5) | |
| Ulmus americana | + (1) | 1 (1) | 3 (2) | |||||||||
| Mean sapling (5–10 cm) density (n/ha) | 544 | 938 | 497 | 400 | 1,613 | 754 | ||||||
| Mean tree (>10 cm) density (n/ha) | 611 | 640 | 622 | 848 | 1,100 | 1,214 | ||||||
| Mean tree (>20 cm) basal area (m2/ha) | 23 | 18 | 24 | 32 | 9 | 25 | ||||||
| Mean total basal area (saplings + trees) (m2/ha) | 33 | 30 | 32 | 42 | 32 | 43 | ||||||
| Ecological Factors, Stand Age and Deadwood Abundance | Forest Community Types | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y-1 | Y-2 | Y-3 | Y-4 | Y-5 | O-1 | O-2 | O-3 | O-4 | O-5 | O-6 | Fields | |
| Site Factors | ||||||||||||
| Elevation (m) | 227 | 273 | 258 | 238 | 239 | 268 | 248 | 242 | 238 | 239 | 238 | - |
| Slope (%) | 0 | 1 | 7 | 13 | 16 | 13 | 11 | 14 | 12 | 6 | 3 | - |
| Topographical situation 1 | 9 | 8.5 | 7.1 | 5.5 | 4.7 | 6.1 | 4.3 | 6 | 5.4 | 3.5 | 8.3 | - |
| Rocks on soil surface (%) | 12 | 2 | 18 | 15 | 10 | 9 | 15 | 16 | 14 | 27 | 7 | - |
| Soil drainage class2 | 3.3 | 3 | 2.5 | 2 | 1.7 | 2 | 2.3 | 2 | 2.7 | 3.5 | 5.4 | - |
| Soil Factors | ||||||||||||
| pH (water) | 5.7 | 5.6 | 5.3 | 5.2 | 5.1 | 5.0 | 4.8 | 4.9 | 4.9 | 5.7 | 5.4 | 6.3 |
| CEC (meq/100g) | 27 | 25 | 24 | 18 | 19 | 18 | 23 | 27 | 27 | 29 | 24 | 20 |
| P (kg/ha) | 22 | 29 | 18 | 14 | 16 | 58 | 19 | 22 | 19 | 17 | 24 | 98 |
| Ca (kg/ha) | 6053 | 4305 | 3495 | 1745 | 2083 | 1746 | 1877 | 3474 | 3408 | 6190 | 4511 | 4903 |
| K (kg/ha) | 191 | 134 | 155 | 134 | 117 | 110 | 115 | 121 | 127 | 127 | 114 | 226 |
| Mg (kg/ha) | 927 | 891 | 509 | 341 | 278 | 263 | 333 | 493 | 546 | 1198 | 550 | 502 |
| N (mg N/g) | 5.1 | 16.7 | 4.9 | 4.1 | 3.1 | 3.6 | 4.6 | 6.3 | 5.8 | 7.1 | 6.7 | 4.2 |
| C:N | 10.9 | 14.7 | 11.4 | 12.4 | 12.6 | 10.8 | 14.3 | 15.5 | 16.6 | 15.4 | 15.3 | 9.6 |
| Organic matter (%) | 11 | 15 | 14 | 9 | 7 | 7 | 12 | 17 | 17 | 20 | 14 | 8 |
| Stand Age | ||||||||||||
| Mean age of largest trees | 40 | 43 | 47 | 47 | 65 | 81 | 99 | 115 | 128 | 86 | 97 | - |
| Deadwood | ||||||||||||
| Fallen deadwood 3 (m/ha) | 7.5 | 162.5 | 302.5 | 370 | 337.5 | 615 | 327.5 | 447.5 | 580 | 457.5 | 730 | |
| Large snag density 4 (snags/ha) | 0 | 100 | 31 | 12 | 25 | 22 | 35 | 12 | 32 | 25 | 43 | - |
| Total snag density (snags/ha) | 58 | 137 | 144 | 87 | 25 | 50 | 50 | 19 | 72 | 37 | 136 | - |
2.3.4. Old Forests Community Types Description
2.4. Delineating the Conservation Zone


2.5. Delineating Ecosystem Management Zones
2.5.1. Oak Restoration Zones: Determining Restoration Environments Based on Results from a Long-Term Experiment

2.5.2. White Pine, Walnut and White Cedar Restoration
| Species | Species Environmental Requirements | Potential Restoration Environments within Study Site |
|---|---|---|
| Red oak | Low to moderate soil fertility Rapidly to moderately-well drained soils Intermediate to high canopy openness | Aspen or ash stands regenerated on old-fields Forest/old-field edges |
| Bur oak | High soil fertility Well to imperfectly drained soils Very high canopy openness | Alder or willow thickets regenerated on imperfectly drained old-fields Forest/old-field edges |
| White pine | Low to moderate soil fertility Rapidly to imperfectly drained soils Intermediate to high canopy openness | Young grey birch, balsam poplar and aspen stands regenerated on old-fields |
| Black walnut | Very high soil fertility Well to moderately-well drained soils Very high canopy openness | Aspen or ash stands regenerated on old-fields Forest/old-field edges |
2.5.3. Silviculture in the Ecosystem Management Zones
2.6. Delineating Intensive Production Zones

2.6.1. Why Use Hybrid Poplars in Buffer Strips along Field Margins?
2.6.2. Yield and Rotation Length Projections
2.6.3. Area of Intensive Poplar Production Required to Compensate for the Creation of Conservation Zones
3. Results and Discussion
3.1. Zoning Scenarios for the St-Benoît-du-Lac Abbey Property
| Scenarios | Zones | Land Type | Land Area (%) | Land Area (ha) | Yield 1 (t/ha/year) | Timber Flow (t/year) |
|---|---|---|---|---|---|---|
| 0. Current status | Forest | 100 | 150 | 1 | 150 | |
| Fields | 0 | 0 | 0 | 0 | ||
| 1. Base case | Conservation | Forest | 25 | 37 | 0 | 0 |
| Ecosystem | Forest | 75 | 113 | 1 | 113 | |
| Intensive | Fields | 5.6 | 2.8 | 13 | 37 | |
| Total | 150 | |||||
| 2. Low productivity | Conservation | Forest | 25 | 37 | 0 | 0 |
| Ecosystem | Forest | 75 | 113 | 1 | 113 | |
| Intensive | Fields | 9.2 | 4.6 | 8 | 37 | |
| Total | 150 | |||||
| 3. High productivity | Conservation | Forest | 25 | 37 | 0 | 0 |
| Ecosystem | Forest | 75 | 113 | 1 | 113 | |
| Intensive | Fields | 4.1 | 2.1 | 18 | 37 | |
| total | 150 | |||||
| 4. Energy | Conservation | Forest | 25 | 37 | 0 | 0 |
| Ecosystem | Forest | 75 | 113 | 1 | 113 | |
| Intensive | Fields | 51 | 26 | 13 | 337 | |
| Total | 450 | |||||
| 5. Conservation | Conservation | Forest | 50 | 75 | 0 | 0 |
| Ecosystem | Forest | 50 | 75 | 1 | 75 | |
| Intensive | Fields | 11 | 5.8 | 13 | 75 | |
| Total | 150 |
3.2. Management Considerations and Limitations of Applying Forest Zoning at the Single Property Scale: Towards a Multiple-Landowner Approach at the Landscape Level
4. Conclusions
Supplementary Materials
Survival, Volume and Biomass Yield of Red and Bur Oak Plantations after 18 Years at St-Benoît-du-Lac
- Old-field—herbicide: located at the forest edge (6 l/ha of glyphosate active ingredient applied in early June in the first 6 growing seasons) (H-field)
- Old-field—control: located at the forest edge (no herbicide treatment) (Field)
- Alder (Alnus rugosa) thicket regenerated in an old-field (Alder)
- Aspen (Populus tremuloides) stand regenerated in an old-field (Poplar)
- Red pine (Pinus resinosa) plantation (Pine)
- Sugar maple (Acer saccharum) forest located on a hilltop (Maple)
- Degraded sugar maple stand located on a cliff (Cliff)
- For large trees (DBH > 15 cm), stem volume was calculated for five sections of the stem: (1) tree base diameter to DBH; (2) DBH to 15 cm diameter; (3) 15 to 10 cm diameter; (4) 10 to 5 cm diameter; and (5) 5 to 2 cm diameter.
- For medium size trees (DBH = 10–15 cm), stem volume was calculated for four sections of the stem: (1) tree base diameter to DBH; (2) DBH to 10 cm diameter; (3) 10 to 5 cm diameter; and (4) 5 cm diameter to 2 cm diameter.
- For small size trees (DBH = 5–10 cm), stem volume was calculated for three sections of the stem: (1) tree base diameter to DBH; (2) DBH to 5 cm diameter; and (3) 5 cm diameter to 2 cm diameter.
- For very small trees (DBH > 5 cm) volume was calculated for two sections of the stem: (1) tree base diameter to DBH; and (2) DBH to 2 cm diameter.



Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pan, D.; Domon, G.; de Blois, S.; Bouchard, A. Temporal (1958–1993) and spatial patterns of land use changes in Haut-Saint-Laurent (Quebec, Canada) and their relation to landscape physical attributes. Landsc. Ecol. 1999, 14, 35–52. [Google Scholar] [CrossRef]
- Bürgi, M.; Turner, M.G. Factors and Processes Shaping Land Cover and Land Cover Changes Along the Wisconsin River. Ecosystem 2002, 5, 184–201. [Google Scholar]
- Rey Benayas, J.M.; Martins, A.; Nicolau, J.M.; Schulz, J.J. Abandonment of agricultural land: An overview of drivers and consequences. Perspect. Agric. Vet. Sci. Nat. Res. 2007, 2, 14. [Google Scholar] [CrossRef]
- Jobin, B.; Latendresse, C.; Baril, A.; Maisonneuve, C.; Boutin, C.; Côté, D. A half-century analysis of landscape dynamics in southern Québec, Canada. Environ. Monit. Assess. 2014, 186, 2215–2229. [Google Scholar] [CrossRef] [PubMed]
- York, A.M.; Munroe, D.K. Urban encroachment, forest regrowth and land-use institutions: Does zoning matter? Land Use Policy 2010, 27, 471–479. [Google Scholar] [CrossRef]
- Wiersum, K.F.; Elands, B.M.; Hoogstra, M. Small-scale forest ownership across Europe: Characteristics and future potential. Small-Scale For. 2005, 4, 1–19. [Google Scholar]
- Guimond, L.; Simard, M. Gentrification and neo-rural populations in the Québec countryside: Representations of various actors. J. Rural Stud. 2010, 26, 449–464. [Google Scholar] [CrossRef]
- Butler, B.J.; Leatherberry, E.C. America’s family forest owners. J. For. 2004, 102, 4–14. [Google Scholar]
- Abrams, J.; Gosnell, H.; Gill, N.; Klepeis, P. Re-creating the Rural, Reconstructing Nature: An International Literature Review of the Environmental Implications of Amenity Migration. Conserv. Soc. 2012, 10, 270–284. [Google Scholar] [CrossRef]
- Mander, Ü.; Helming, K.; Wiggering, H. Multifunctional land use: Meeting future demands for landscape goods and services. In Multifunctional Land Use; Mander, Ü., Wiggering, H., Helming, K., Eds.; Springer Berlin: Heidelberg, Germany, 2007; pp. 1–13. [Google Scholar]
- Paquette, S.; Domon, G. Trends in rural landscape development and sociodemographic recomposition in southern Quebec (Canada). Landsc. Urban Plan. 2001, 55, 215–238. [Google Scholar] [CrossRef]
- Paquette, S.; Domon, G. Changing ruralities, changing landscapes: Exploring social recomposition using a multi-scale approach. J. Rural Stud. 2003, 19, 425–444. [Google Scholar] [CrossRef]
- Dupras, J.; Alam, M. Urban Sprawl and Ecosystem Services: A Half Century Perspective in the Montreal Area (Quebec, Canada). J. Environ. Policy Plan. 2014, 1–21. [Google Scholar] [CrossRef]
- CAAAQ. Agriculture et Agroalimentaire: Assurer et Bâtir L’avenir, Propositions pour une Agriculture Durable et en Santé. Rapport de la Commission sur L’avenir de L’agriculture et de L’agroalimentaire Québécois; Québec, QC, Canada, 2008.
- Commission d’étude sur la gestion de la forêt publique québécoise. Chapitre 2—Les Forêts Québécoises: Territoire, Ressources et Usages; Gouvernement du Québec: Québec, QC, Canada, 2004; pp. 7–34.
- Côté, M.-A.; Gilbert, D.; Nadeau, S. Caractérisation des Profils, des Motivations et des Comportements des Propriétaires Forestiers Québécois par Territoire D’agence régionale de Mise en Valeur des Forêts Privées. Rapport Produit pour le Compte des Agences Régionales de Mise en Valeur des Forêts Privées et du Ministère des Ressources Naturelles du Québec; La Fédération des Producteurs Forestiers du Québec; Le Groupe AGÉCO et Ressources Naturelles Canada: Québec, QC, Canada, 2012. [Google Scholar]
- Brassard, F.; Bouchard, A.R.; Boisjoly, D.; Poisson, F.; Bazoge, A.; Bouchard, M.-A.; Lavoie, G.; Tardif, B.; Bergeron, M.; Perron, J.; et al. Portrait du Réseau D’aires Protégées au Québec: Période 2002–2009; Ministère du Développement durable de l’Environnement et des parcs du Québec: Québec, QC, Canada, 2010; p. 41.
- Götmark, F.; Thorell, M. Size of nature reserves: Densities of large trees and dead wood indicate high value of small conservation forests in southern Sweden. Biodivers. Conserv. 2003, 12, 1271–1285. [Google Scholar] [CrossRef]
- Alexandrov, G. Carbon stock growth in a forest stand: The power of age. Carbon Balance Manag. 2007, 2, 1–5. [Google Scholar] [CrossRef]
- Hooker, T.D.; Compton, J.E. Forest ecosystem carbon and nitrogen accumulation during the first century after agricultural abandonnement. Ecol. Appl. 2003, 13, 299–313. [Google Scholar] [CrossRef]
- Lewis, D.B.; Castellano, M.J.; Kaye, J.P. Forest succession, soil carbon accumulation, and rapid nitrogen storage in poorly remineralized soil organic matter. Ecology 2014, 95, 2687–2693. [Google Scholar] [CrossRef]
- Rousseau, A.N.; Mailhot, A.; Slivitzky, M.; Villeneuve, J.-P.; Rodriguez, M.J.; Bourque, A. Usages et approvisionnement en eau dans le sud du Québec Niveau des connaissances et axes de recherche à privilégier dans une perspective de changements climatiques. Can. Water Res. J. 2004, 29, 121–134. [Google Scholar] [CrossRef]
- Hudon, C.; Carignan, R. Cumulative impacts of hydrology and human activities on water quality in the St. Lawrence River (Lake Saint-Pierre, Quebec, Canada). Can. J. Fish. Aquat. Sci. 2008, 65, 1165–1180. [Google Scholar] [CrossRef]
- De La Chenelière, V.; Brodeur, P.; Mingelbier, M. Restauration des habitats du lac Saint-Pierre: Un prérequis au rétablissement de la perchaude. Nat. Can. 2014, 138, 50–61. [Google Scholar] [CrossRef]
- Galvez-Cloutier, R.; Sanchez, M. Trophic Status Evaluation for 154 Lakes in Quebec, Canada: Monitoring and Recommendations. Water Qual. Ressour. J. Can. 2007, 42, 252–268. [Google Scholar]
- Chu, C.; Minns, C.K.; Lester, N.P.; Mandrak, N.E. An updated assessment of human activities, the environment, and freshwater fish biodiversity in Canada. Can. J. Fish. Aquat. Sci. 2015, 72, 135–148. [Google Scholar] [CrossRef]
- Simard, H.; Bouchard, A. The precolonial 19th century forest of the Upper St. Lawrence Region of Quebec: A record of its exploitation and transformation through notary deeds of wood sales. Can. J. For. Res. 1996, 26, 1670–1676. [Google Scholar] [CrossRef]
- AFCE. Les Grands Pins au Québec: Un Choix D’avenir; Association Forestière des Cantons-de-l’Est: Québec, QC, Canada, 2008; p. 34. [Google Scholar]
- Giguère, M. L’industrie de la Transformation du Bois au Québec; Direction du Développement de L’industrie des Produits Forestiers; Ministère des Ressources Naturelles: Québec, QC, Canada, 2012; p. 126.
- Truax, B.; Gagnon, D. Effects of straw and black plastic mulching on the initial growth and nutrition of butternut, white ash and bur oak. For. Ecol. Manag. 1993, 57, 17–27. [Google Scholar] [CrossRef]
- Truax, B.; Lambert, F.; Gagnon, D. Herbicide-free plantations of oaks and ashes along a gradient of open to forested mesic environments. For. Ecol. Manag. 2000, 137, 155–169. [Google Scholar] [CrossRef]
- Lambert, F.; Truax, B.; Gagnon, D.; Chevrier, N. Growth and N nutrition, monitored by enzyme assays, in a hardwood plantation: Effects of mulching materials and glyphosate application. For. Ecol. Manag. 1994, 70, 231–244. [Google Scholar] [CrossRef]
- Cogliastro, A.; Gagnon, D.; Bouchard, A. Experimental determination of soil characteristics optimal for the growth of ten hardwoods planted on abandoned farmland. For. Ecol. Manag. 1997, 96, 49–63. [Google Scholar] [CrossRef]
- Gardiner, E.S.; Stanturf, J.A.; Schweitzer, C.J. An afforestation system for restoring bottomland hardwood forests: Biomass accumulation of nuttall oak seedlings interplanted beneath eastern cottonwood. Restor. Ecol. 2004, 12, 525–532. [Google Scholar] [CrossRef]
- Neumann, P.D.; Krahn, H.J.; Krogman, N.T.; Thomas, B.R. “My grandfather would roll over in his grave”: Family farming and tree plantation on farmland. Rural Sociol. 2007, 72, 111–135. [Google Scholar] [CrossRef]
- McKenney, D.W.; Yemshanov, D.; Fraleigh, S.; Allen, D.; Preto, F. An economic assessment of the use of short-rotation coppice woody biomass to heat greenhouses in southern Canada. Biomass Bioenergy 2011, 35, 374–384. [Google Scholar] [CrossRef]
- Lantz, V.A.; Chang, W.-Y.; Pharo, C. Benefit-cost analysis of hybrid willow crop production on agricultural land in eastern Canada: Assessing opportunities for on-farm and off-farm bioenergy use. Biomass Bioenergy 2014, 63, 257–267. [Google Scholar] [CrossRef]
- Rockwood, D.L.; Naidu, C.V.; Carter, D.R.; Rahmani, M.; Spriggs, T.A.; Lin, C.; Alker, G.R.; Isebrands, J.G.; Segrest, S.A. Short-rotation woody crops and phytoremediation: Opportunities for agroforestry? Agrofor. Syst. 2004, 61–62, 51–63. [Google Scholar]
- Ferrarini, A.; Serra, P.; Almagro, M.; Trevisan, M.; Amaducci, S. Linking Bioenergy and ecological services along field margins: The hedge-biomass project. In Proceedings of the 22nd European Biomass Conference and Exibition, Hamburg, Germany, 23–26 June 2014; pp. 257–273.
- Licht, L.A.; Isebrands, J.G. Linking phytoremediated pollutant removal to biomass economic opportunities. Biomass Bioenergy 2005, 28, 203–218. [Google Scholar] [CrossRef]
- Fortier, J.; Gagnon, D.; Truax, B.; Lambert, F. Understory plant diversity and biomass in hybrid poplar riparian buffer strips in pastures. New For. 2011, 42, 241–265. [Google Scholar] [CrossRef]
- Fortier, J.; Truax, B.; Gagnon, D.; Lambert, F. Mature hybrid poplar riparian buffers along farm streams produce high yields in response to soil fertility assessed using three methods. Sustainability 2013, 5, 1893–1916. [Google Scholar] [CrossRef]
- Fortier, J.; Truax, B.; Gagnon, D.; Lambert, F. Biomass carbon, nitrogen and phosphorus stocks in hybrid poplar buffers, herbaceous buffers and natural woodlots in the riparian zone on agricultural land. J. Environ. Manag. 2015, 154, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Pageault, D. Effet de Bandes Riveraines Plantées de Peupliers Hybrides sur la Présence et L’abondance de Micromammifères et de Picidés en Zone Agricole du sud du Québec. Master’s Thesis, Université du Québec à Montréal, Montréal, QC, Canada, 2013. [Google Scholar]
- Truax, B.; Gagnon, D.; Fortier, J.; Lambert, F. Biomass and volume yield in mature hybrid poplar plantations on temperate abandoned farmland. Forests 2014, 5, 3107–3130. [Google Scholar] [CrossRef]
- Gouvernement du Québec. Loi sur L’Aménagement Durable du Territoire Forestier; Éditeur officiel du Québec, 2015.
- Jetté, J.-P.; Leblanc, M.; Bouchard, M.; Villeneuve, N. Intégration des Enjeux Écologiques dans les Plans D’aménagement Forestier Intégré, Partie I—Analyse des Enjeux; Gouvernement du Québec; Ministère des Ressources Naturelles; Direction de L’aménagement et de L’environnement Forestiers: Québec, QC, Canada, 2013; p. 150.
- Grenon, F.; Jetté, J.-P.; Leblanc, M. Manuel de Référence pour L’aménagement Écosystémique des Forêts au Québec—Module 1—Fondements et Démarche de la Mise en Oeuvre; Centre D’enseignement et de Recherche en Foresterie de Sainte-Foy Inc. et Ministère des Ressources Naturelles et de la Faune; Direction de L’environnement et de la Protection des Forêts: Québec, QC, Canada, 2010. [Google Scholar]
- Binkley, C. Ecosystem management and plantation forestry: New directions in British Columbia. New For. 1999, 18, 75–88. [Google Scholar] [CrossRef]
- Hunter, M.L. Wildlife, Forests, and Forestry: Principles for Managing Forests for Biological Diversity; Prentice Hall: Englewood Cliffs, NJ, USA, 1990; p. 370. [Google Scholar]
- Sahajananthan, S.; Haley, D.; Nelson, J. Planning for Sustainable Forests in British Columbia through Land Use Zoning. Can. Public Policy 1998, 24, S73–S81. [Google Scholar] [CrossRef]
- Messier, C.; Tittler, R.; Kneeshaw, D.D.; Gélinas, N.; Paquette, A.; Berninger, K.; Rheault, H.; Meek, P.; Beaulieu, N. TRIAD zoning in Quebec: Experiences and results after 5 years. For. Chron. 2009, 85, 885–896. [Google Scholar] [CrossRef]
- Montigny, M.K.; MacLean, D.A. Triad forest management: Scenario analysis of forest zoning effects on timber and non-timber values in New Brunswick, Canada. For. Chron. 2006, 82, 496–511. [Google Scholar] [CrossRef]
- Simoncic, T.; Boncina, A.; Rosset, C.; Binder, F.; Meo, I.D.; Cavlovic, J.; Gal, J.; Matijasic, D.; Schneider, J.; Singer, F.; et al. Importance of priority areas for multi-objective forest planning: A central european perspective. Int. For. Rev. 2013, 15, 509–523. [Google Scholar] [CrossRef]
- Grumbine, R.E. What is ecosystem management? Conserv. Biol. 1994, 8, 27–38. [Google Scholar] [CrossRef]
- Association Maritime du Québec. Lac Memphrémagog. Available online: http://www.navigationquebec.com/fiche_lac.php?l_id=46 (accessed on 14 June 2015).
- Robitaille, A.; Saucier, J.-P. Paysages Régionaux du Québec Méridional; Les Publications du Québec: Ste-Foy, QC, Canada, 1998; p. 213. [Google Scholar]
- Westveld, M. Natural forest vegetation zones of New England. J. For. 1956, 54, 332–338. [Google Scholar]
- Cogbill, C.V.; Burk, J.; Motzkin, G. The forests of presettlement New England, USA: Spatial and compositional patterns based on town proprietor surveys. J. Biogeogr. 2002, 29, 1279–1304. [Google Scholar] [CrossRef]
- Cann, D.B.; Lajoie, P. Études des Sols des Comtés de Stanstead, Richmond, Sherbrooke et Compton dans la Province de Québec; Ministère de l’Agriculture: Ottawa, ON, Canada, 1943; p. 58.
- Comité d’experts sur la prospection pédologique d’Agriculture Canada. Le Système Canadien de Classification des Sols. Publication No. 1646, 2nd ed.; Agriculture Canada: Ottawa, ON, Canada, 1987; p. 170. [Google Scholar]
- Lorimer, C.G. The presettlement forest and natural disturbance cycle of northeastern Maine. Ecology 1977, 58, 139–148. [Google Scholar] [CrossRef]
- Seymour, R.S.; White, A.S.; deMaynadier, P.G. Natural disturbance regimes in northeastern North America—evaluating silvicultural systems using natural scales and frequencies. For. Ecol. Manag. 2002, 155, 357–367. [Google Scholar] [CrossRef]
- Nichols, G.E. The hemlock-white pine-northern hardwood region of eastern North America. Ecology 1935, 16, 403–422. [Google Scholar] [CrossRef]
- Runkle, J.R. Patterns of disturbance in some old-growth mesic forests of eastern North America. Ecology 1982, 63, 1533–1546. [Google Scholar] [CrossRef]
- Brisson, J.; Bouchard, A. In the past two centuries, human activities have caused major changes in the tree species composition of southern Québec, Canada. Écoscience 2003, 10, 236–246. [Google Scholar]
- Booth, J.D. Timber utilization on the agricultural frontier in southern Québec. J. Eastern Twonships Stud. 1994, 4, 15–30. [Google Scholar]
- Kesteman, J.-P.; Southam, P.; Saint-Pierre, D. Histoire des Cantons-de-l’Est; Les Presses de l’Université Laval: Ste-Foy, QC, Canada, 1998. [Google Scholar]
- Marie-Victorin, F.; Rouleau, E.; Brouillet, L.; Hay, S.G.; Goulet, I. Flore Laurentienne—3e Édition; Gaëtan Morin Éditeur Ltée: Montréal, QC, Canada, 2002; p. 1093. [Google Scholar]
- Abrams, M.D. Fire and the development of oak forests. BioScience 1992, 42, 346–353. [Google Scholar] [CrossRef]
- Weyenberg, S.A.; Frelich, L.E.; Reich, P.B. Logging versus fire: How does disturbance type influence the abundance of Pinus strobus regeneration? Silva Fenn. 2004, 38, 179–194. [Google Scholar] [CrossRef]
- MRNF. Le Plan de Gestion du Cerf de Virginie au Québec 2010–2017; Direction de l'expertise sur la faune et ses habitats, Ministère des ressources naturelles et de la faune: Québec, QC, Canada, 2010.
- Kittredge, D.B.; Ashton, P.M.S. Impact of deer browsing on regeneration in mixed stands in southern New England. North. J. Appl. For. 1995, 12, 115–120. [Google Scholar]
- Rooney, T.P. Deer impacts on forest ecosystems: A North American perspective. Forestry 2001, 74, 201–208. [Google Scholar] [CrossRef]
- Boulfroy, E.; Forget, E.; Hofmeyer, P.V.; Kenefi, L.S.; Larouche, C.; Lessard, G.; Lussier, J.-M.; Pinto, F.; Ruel, J.-C.; Weiskittel, A. Silvicultural Guide for Northern White-Cedar (Eastern White Cedar); Gen. Tech. Rep. NRS-98; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2012; p. 74. [Google Scholar]
- Villeneuve, N.; Brisson, J. Old-growth forests in the temperate deciduous zone of Quebec: Identification and evaluation for conservation and research purposes. For. Chron. 2003, 79, 559–569. [Google Scholar] [CrossRef]
- COSEWIC. Butternut Juglans cinerea. Available online: http://www.cosewic.gc.ca/eng/sct1/searchdetail_e.cfm?id=793&StartRow=1&boxStatus=All&boxTaxonomic=All&location=All&change=All&board=All&commonName=butternut&scienceName=&returnFlag=0&Page=1 (accessed on 22 May 2015).
- Tanguay, C. Distribution, Abondance et État de Santé du Noyer Cendré (Juglans cinerea) en Relation avec les Gradients Écologiques dans les Cantons-de-l’Est. Master’s Thesis, Université du Québec à Montréal, Montréal, QC, Canada, 2011. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Dale, V.H.; Joyce, L.A.; McNulty, S.; Neilson, R.P.; Ayres, M.P.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J.; et al. Climate change and forest disturbances. BioScience 2001, 51, 723–734. [Google Scholar] [CrossRef]
- Gauthier, S.; Bernier, P.; Kuuluvainen, T.; Shvidenko, A.Z.; Schepaschenko, D.G. Boreal forest health and global change. Science 2015, 349, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Hanson, P.J.; Weltzin, J.F. Drought disturbance from climate change: Response of United States forests. Sci. Tot. Environ. 2000, 262, 205–220. [Google Scholar] [CrossRef]
- Truax, B.; Gagnon, D.; Fortier, J.; Lambert, F. Yield in 8 year-old hybrid poplar plantations on abandoned farmland along climatic and soil fertility gradients. For. Ecol. Manag. 2012, 267, 228–239. [Google Scholar] [CrossRef]
- Fortier, J.; Truax, B.; Gagnon, D.; Lambert, F. Plastic allometry in coarse root biomass of mature hybrid poplar plantations. BioEnergy Res. 2015, 1–14. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 1.17–11. Available online: http://CRAN.R-project.org/package=vegan. (accessed on 4 December 2011).
- Savignac, C.; Desrochers, A.; Huot, J. Habitat use by Pileated Woodpeckers at two spatial scales in eastern Canada. Can. J. Zool. 2000, 78, 219–225. [Google Scholar] [CrossRef]
- Trudeau, C.; Imbeau, L.; Drapeau, P.; Mazerolle, M.J. Site occupancy and cavity use by the northern flying squirrel in the boreal forest. J. Wildlife Manag. 2011, 75, 1646–1656. [Google Scholar] [CrossRef]
- Brisson, J.; Bergeron, Y.; Bouchard, A.; Leduc, A. Beech-maple dynamics in an old-growth forest in southern Québec, Canada. Écoscience 1994, 1, 40–46. [Google Scholar]
- Décamps, H.; Pinay, G.; Naiman, R.J.; Petts, G.E.; McClain, M.E.; Hillbricht-Ilkowska, A.; Hanley, T.A.; Holmes, R.M.; Quinn, J.; Gilbert, J.; et al. Riparian zone: Where biogeochemistry meets biodiversity in management practice. Policy J. Ecol. 2004, 52, 3–18. [Google Scholar]
- Pollock, M.M.; Beechie, T.J. Does riparian forest restoration thinning enhance biodiversity? The ecological importance of large wood. JAWRA 2014, 50, 543–559. [Google Scholar] [CrossRef]
- MDDELCC. Liste des Plantes Vasculaires Vulnérables; Ministère du Développement Durable, de l’Environnement et de la Lutte aux Changements Climatiques du Québec: Québec, QC, Canada, 2014; p. 1.
- Nault, A.; Gagnon, D. Ramet demography of Allium tricoccum, a spring ephemeral, perennial forest herb. J. Ecol. 1993, 81, 101–119. [Google Scholar] [CrossRef]
- Mazurek, M.J.; Zielinski, W.J. Individual legacy trees influence vertebrate wildlife diversity in commercial forests. For. Ecol. Manag. 2004, 193, 321–334. [Google Scholar] [CrossRef]
- Remm, J.; Lõhmus, A. Tree cavities in forests—The broad distribution pattern of a keystone structure for biodiversity. For. Ecol. Manag. 2011, 262, 579–585. [Google Scholar] [CrossRef]
- Gauthier, S.; Vaillancourt, M.-A.; Leduc, A.; de Grandpré, L.; Kneeshaw, D.D.; Morin, H.; Drapeau, P.; Bergeron, Y. Aménagement Écosystémique en Forêt Boréale; Presses de l’Université du Québec: Québec, QC, Canada, 2008; p. 568. [Google Scholar]
- Hunter, M.L. Natural fire regimes as spatial models for managing boreal forests. Biol. Conserv. 1993, 65, 115–120. [Google Scholar] [CrossRef]
- MacKay, D. Un Patrimoine en Péril—La Crise des Forêts Canadiennes; Les Publications du Québec: Québec, QC, Canada, 1986. [Google Scholar]
- Kasson, M.T.; Livingston, W.H. Relationships among beech bark disease, climate, radial growth response and mortality of American beech in northern Maine, USA. For. Pathol. 2012, 42, 199–212. [Google Scholar] [CrossRef]
- Crow, T.R. Reproductive mode and mechanisms for self-replacement of northern red oak (Quercus rubra)—A review. For. Sci. 1988, 34, 19–40. [Google Scholar]
- Farrar, J.L. Les Arbres du Canada; Fides et le Service Canadien des Forêts, Ressources Naturelles Canada: St-Laurent, QC, Canada, 2006; p. 502. [Google Scholar]
- Ostry, M.E.; Laflamme, G.; Katovich, S.A. Silvicultural approaches for management of eastern white pine to minimize impacts of damaging agents. For. Pathol. 2010, 40, 332–346. [Google Scholar] [CrossRef]
- Anagnostakis, S.L. Chestnut Blight: The Classical Problem of an Introduced Pathogen. Mycologia 1987, 79, 23–37. [Google Scholar] [CrossRef]
- Diskin, M.; Steiner, K.C.; Hebard, F.V. Recovery of American chestnut characteristics following hybridization and backcross breeding to restore blight-ravaged Castanea dentata. For. Ecol. Manag. 2006, 223, 439–447. [Google Scholar] [CrossRef]
- Jacobs, D.F. Toward development of silvical strategies for forest restoration of American chestnut (Castanea dentata) using blight-resistant hybrids. Biol. Conserv. 2007, 137, 497–506. [Google Scholar] [CrossRef]
- Clark, S.; McNab, H.; Loftis, D.; Zarnoch, S. American Chestnut Growth and Survival Five Years after Planting in Two Silvicultural Treatments in the Southern Appalachians, USA. Forests 2012, 3, 1017–1033. [Google Scholar] [CrossRef]
- Larouche, C.; Ruel, J.-C. Development of northern white-cedar regeneration following partial cutting, with and without deer browsing. Forests 2015, 6, 344–359. [Google Scholar] [CrossRef]
- Côté, S.D.; Rooney, T.P.; Tremblay, J.-P.; Dussault, C.; Waller, D.M. Ecological impacts of deer overabundance. Ann. Rev. Ecol. Evol. Syst. 2004, 35, 113–147. [Google Scholar] [CrossRef]
- Stanturf, J.A.; Palik, B.J.; Dumroese, R.K. Contemporary forest restoration: A review emphasizing function. For. Ecol. Manag. 2014, 331, 292–323. [Google Scholar] [CrossRef]
- Coates, K.D.; Burton, P.J. A gap-based approach for development of silvicultural systems to address ecosystem management objectives. For. Ecol. Manag. 1997, 99, 337–354. [Google Scholar] [CrossRef]
- Messier, C.; Bigué, B.; Bernier, L. Using fast-growing plantations to promote forest ecosystem protection in Canada. Unasylva 2003, 54, 59–63. [Google Scholar]
- Fortier, J.; Truax, B.; Gagnon, D.; Lambert, F. Hybrid poplar yields in Québec: Implications for a sustainable forest zoning management system. For. Chron. 2012, 88, 391–407. [Google Scholar] [CrossRef]
- Czapowskyj, M.M.; Safford, L.O. Site preparation, fertilization, and 10-year yields of hybrid poplar on a clearcut forest site in eastern Maine, USA. New For. 1993, 7, 331–344. [Google Scholar]
- Gelhaye, D.; Ranger, J.; Bonneau, M. Biomass and nutrient content of a short rotation coppice of Beaupre poplars planted on a non-alluvial acidic soil improved by fertilization. Ann. For. Sci. 1997, 54, 649–665. [Google Scholar] [CrossRef]
- Nelson, A.S.; Saunders, M.R.; Wagner, R.G.; Weiskittel, A.R. Early stand production of hybrid poplar and white spruce in mixed and monospecific plantations in eastern Maine. New For. 2012, 43, 519–534. [Google Scholar] [CrossRef]
- Christersson, L. Wood production potential in poplar plantations in Sweden. Biomass Bioenergy 2010, 34, 1289–1299. [Google Scholar] [CrossRef]
- Christersson, L. Poplar plantations for paper and energy in the south of Sweden. Biomass Bioenergy 2008, 32, 997–1000. [Google Scholar] [CrossRef]
- Hartley, M.J. Rationale and methods for conserving biodiversity in plantation forests. For. Ecol. Manag. 2002, 155, 81–95. [Google Scholar] [CrossRef]
- Thompson, I.D.; Baker, J.A.; Ter-Mikaelian, M. A review of the long-term effects of post-harvest silviculture on vertebrate wildlife, and predictive models, with an emphasis on boreal forests in Ontario, Canada. For. Ecol. Manag. 2003, 177, 441–469. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Laudon, H.; Sponseller, R.; Lucas, R.; Futter, M.; Egnell, G.; Bishop, K.; Ågren, A.; Ring, E.; Högberg, P. Consequences of more intensive forestry for the sustainable management of forest soils and waters. Forests 2011, 2, 243–260. [Google Scholar] [CrossRef]
- Ministère des Forêts, de la Faune et des Parcs. Entente d’Attribution de Biomasse Forestière (EABF) et Permis pour la Récolte de bois aux fins d’Approvisionner une Usine de Transformation du bois (PRAU); Ministère des Forêts, de la Faune et des Parcs, Gouvernement du Québec: Québec, QC, Canada, 2015.
- Achat, D.L.; Deleuze, C.; Landmann, G.; Pousse, N.; Ranger, J.; Augusto, L. Quantifying consequences of removing harvesting residues on forest soils and tree growth—A meta-analysis. For. Ecol. Manag. 2015, 348, 124–141. [Google Scholar] [CrossRef]
- Hesselink, T.P. Increasing pressures to use forest biomass: A conservation viewpoint. For. Chron. 2010, 86, 28–35. [Google Scholar] [CrossRef]
- Fortier, J.; Gagnon, D.; Truax, B.; Lambert, F. Biomass and volume yield after 6 years in multiclonal hybrid poplar riparian buffer strips. Biomass Bioenergy 2010, 34, 1028–1040. [Google Scholar] [CrossRef]
- Fortier, J.; Gagnon, D.; Truax, B.; Lambert, F. Nutrient accumulation and carbon sequestration in 6 year-old hybrid poplars in multiclonal agricultural riparian buffer strips. Agric. Ecosyst. Environ. 2010, 137, 276–287. [Google Scholar] [CrossRef]
- Simavi, M.A. Effet de Plantations de Bandes Riveraines D’arbres sur L’abondance et la Répartition de la Faune Aquatique dans des Ruisseaux Dégradés de Milieux Agricoles dans les Cantons-de-l’Est. Master’s Thesis, Université du Québec à Montréal, Montréal, QC, Canada, 2012. [Google Scholar]
- Périnet, P.; Gagnon, H.; Morin, S. Liste des Clones Recommandés de Peuplier Hybride par Sous-Région Écologique au Québec (Mise à Jour Octobre 2010); Direction de la Recherche Forestière, MRN: Québec, QC, Canada, 2010; p. 1.
- Woodfin, S.L.; Wright, L.L.; Curtin, D.T. SRIC: Integration of Production and Harvesting System Costs. Proceedings of the IEA/BA Task II Workshop: Economic Evaluation of Short-Rotation Biomass Energy Systems; Duluth, MN, USA, 11–13 August 1987, Lothner, D.C., Bradley, D.P., Gambles, R.L., Eds.; USDA Forest Service: Duluth, MN, USA, 1988; pp. 115–150. [Google Scholar]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Gladstone, W.T.; Thomas Ledig, F. Reducing pressure on natural forests through high-yield forestry. For. Ecol. Manag. 1990, 35, 69–78. [Google Scholar] [CrossRef]
- Simončič, T.; Spies, T.; Deal, R.; Bončina, A. A conceptual framework for characterizing forest areas with high societal values: Experiences from the Pacific Northwest of USA and Central Europe. Environ. Manag. 2015, 56, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Boothroyd-Roberts, K.; Gagnon, D.; Truax, B. Can hybrid poplar plantations accelerate the restoration of forest understory attributes on abandoned fields? For. Ecol. Manag. 2013, 287, 77–89. [Google Scholar] [CrossRef]
- Weih, M.; Karacic, A.; Munkert, H.; Verwijst, T.; Diekmann, M. Influence of young poplar stands on floristic diversity in agricultural landscapes (Sweden). Basic Appl. Ecol. 2003, 4, 149–156. [Google Scholar] [CrossRef]
- Brockerhoff, E.; Jactel, H.; Parrotta, J.; Quine, C.; Sayer, J. Plantation forests and biodiversity: Oxymoron or opportunity? Biodivers. Conserv. 2008, 17, 925–951. [Google Scholar] [CrossRef]
- Knapp, B.; Insam, H. Recycling of biomass ashes: Current technologies and future research needs. In Recycling of Biomass Ashes; Insam, H., Knapp, B.A., Eds.; Springer Berlin: Heidelberg, Germany, 2011; pp. 1–16. [Google Scholar]
- Keith, H.; Mackey, B.G.; Lindenmayer, D.B. Re-evaluation of forest biomass carbon stocks and lessons from the world’s most carbon-dense forests. PNAS 2009, 106, 11635–11640. [Google Scholar] [CrossRef] [PubMed]
- Pregitzer, K.S.; Euskirchen, E.S. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob. Change Biol. 2004, 10, 2052–2077. [Google Scholar] [CrossRef]
- Rheinhardt, R.; Brinson, M.; Meyer, G.; Miller, K. Carbon storage of headwater riparian zones in an agricultural landscape. Carbon Balance Manag. 2012, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Davies, O.; Kerr, G. Comparing the costs and revenues of transformation to continuous cover forestry for sitka spruce in Great Britain. Forests 2015, 6, 2424–2449. [Google Scholar] [CrossRef]
- Agence de mise en valeur de la forêt privée de l’Estrie. Liste des activités de mise en valeur admissibles à une aide financière. Available online: http://www.agenceestrie.qc.ca/programme.htm (accessed on 25 August 2015).
- MAPAQ. Prime-Vert—Volet 1. Interventions en agroenvironnement par une exploitation agricole. Available online: http://www.mapaq.gouv.qc.ca/fr/Productions/md/programmesliste/agroenvironnement/sous-volet/Pages/PrimeVertvolet1.aspx (accessed on 25 August 2015).
- Gouvernement du Québec. La Conservation au Volontaire: Vous Pouvez Faire une Différence. Principales Options de Conservation Légales pour les Propriétaires de Terrains Privés; Gouvernement du Québec: Québec, QC, Canada, 2014; p. 11.
- Vose, J.M.; Wear, D.N.; Mayfield Iii, A.E.; Dana Nelson, C. Hemlock woolly adelgid in the southern Appalachians: Control strategies, ecological impacts, and potential management responses. For. Ecol. Manag. 2013, 291, 209–219. [Google Scholar] [CrossRef]
- Goldman, R.L.; Thompson, B.H.; Daily, G.C. Institutional incentives for managing the landscape: Inducing cooperation for the production of ecosystem services. Ecol. Econ. 2007, 64, 333–343. [Google Scholar] [CrossRef]
- Leitch, Z.J.; Lhotka, J.M.; Stainback, G.A.; Stringer, J.W. Private landowner intent to supply woody feedstock for bioenergy production. Biomass Bioenergy 2013, 56, 127–136. [Google Scholar] [CrossRef]
- Tittler, R.; Filotas, É.; Kroese, J.; Messier, C. Maximizing conservation and production with intensive forest management: It’s all about location. Environ. Manag. 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lautenschlager, R.A. Can intensive silviculture contribute to sustainable forest management in northern ecosystems? For. Chron. 2000, 76, 283–295. [Google Scholar] [CrossRef]
- Bureau du forestier en chef. Succès des Plantations. Avis du Forestier en Chef. FEC-AVIS-04-2015; Bureau du forestier en chef: Roberval, QC, Canada, 2015; p. 22.
- Dancause, A. Le Reboisement au Québec; Les Publications du Québec: Québec, QC, Canada, 2008; p. 177. [Google Scholar]
- Labrecque, M.; Teodorescu, T.I. Field performance and biomass production of 12 willow and poplar clones in short-rotation coppice in southern Quebec (Canada). Biomass Bioenergy 2005, 29, 1–9. [Google Scholar] [CrossRef]
- Bentrup, G. Conservation Buffers: Design Guidelines for Buffers, Corridors, and Greenways; Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2008; p. 110.
- McKinley, D.C.; Ryan, M.G.; Birdsey, R.A.; Giardina, C.P.; Harmon, M.E.; Heath, L.S.; Houghton, R.A.; Jackson, R.B.; Morrison, J.F.; Murray, B.C.; et al. A synthesis of current knowledge on forests and carbon storage in the United States. Ecol. Appl. 2011, 21, 1902–1924. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W. Choosing the appropriate scale of reserves for conservation. Ann. Rev. Ecol. Syst. 1999, 30, 83–108. [Google Scholar] [CrossRef]
- Humphrey, J.; Watts, K.; Fuentes-Montemayor, E.; Macgregor, N.; Peace, A.; Park, K. What can studies of woodland fragmentation and creation tell us about ecological networks? A literature review and synthesis. Landsc. Ecol. 2015, 30, 21–50. [Google Scholar] [CrossRef]
- Ma, Z.; Kittredge, D.B. How family forest owners consider timber harvesting, land sale, and conservation easement decisions: Insights from Massachusetts, USA. Int. J. For. Res. 2011. [Google Scholar] [CrossRef]
- Côté, M.-A.; Gilbert, D.; Nadeau, S. Characterizing the profiles, motivations and behaviour of Quebec’s forest owners. For. Policy Econ. 2015, 59, 83–90. [Google Scholar] [CrossRef]
- Boothroyd-Roberts, K.; Gagnon, D.; Truax, B. Hybrid poplar plantations are suitable habitat for reintroduced forest herbs with conservation status. SpringerPlus 2013, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lust, N.; Kongs, T.; Nachtergale, L.; De Keersmaeker, L. Spontaneous ingrowth of tree species in poplar plantations in Flanders. Ann. For. Sci. 2001, 58, 861–868. [Google Scholar] [CrossRef]
- Perron, J.-Y. Inventaire forestier. In Manuel de Foresterie; Ordre des Ingénieurs Forestiers du Québec, Ed.; Les Presses de l’Université Laval: Ste-Foy, QC, Canada, 1996; pp. 390–473. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truax, B.; Gagnon, D.; Lambert, F.; Fortier, J. Multiple-Use Zoning Model for Private Forest Owners in Agricultural Landscapes: A Case Study. Forests 2015, 6, 3614-3664. https://0-doi-org.brum.beds.ac.uk/10.3390/f6103614
Truax B, Gagnon D, Lambert F, Fortier J. Multiple-Use Zoning Model for Private Forest Owners in Agricultural Landscapes: A Case Study. Forests. 2015; 6(10):3614-3664. https://0-doi-org.brum.beds.ac.uk/10.3390/f6103614
Chicago/Turabian StyleTruax, Benoit, Daniel Gagnon, France Lambert, and Julien Fortier. 2015. "Multiple-Use Zoning Model for Private Forest Owners in Agricultural Landscapes: A Case Study" Forests 6, no. 10: 3614-3664. https://0-doi-org.brum.beds.ac.uk/10.3390/f6103614