Changes in Species Composition in Alder Swamp Forest Following Forest Dieback
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Vegetation Sampling
2.3. Data Processing and Analysis
3. Results
4. Discussion
4.1. Alder Carrs Dynamics and Related Floristic Changes
4.2. Knowledge Gaps Related to Biodiversity Dynamics in Alder Carrs
4.3. Implication for Resurvey Studies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Whitmore, T.C. Canopy gaps and the two major groups of forest trees. Ecology 1989, 70, 536–538. [Google Scholar] [CrossRef]
- Yamamoto, S.-I. Forest gap dynamics and tree regeneration. J. For. Res. 2000, 5, 223–229. [Google Scholar] [CrossRef]
- Holeksa, J.; Jaloviar, P.; Kucbel, S.; Saniga, M.; Svoboda, M.; Szewczyk, J.; Szwagrzyk, J.; Zielonka, T.; Żywiec, M. Models of disturbance driven dynamics in the West Carpathian spruce forests. For. Ecol. Manag. 2017, 388, 79–89. [Google Scholar] [CrossRef]
- Barthelmes, A.; Gerloff, D.; Klerk, P.; Joosten, H. Short-term vegetation dynamics of Alnus dominated peatlands: A high resolution palaeoecological case study from Western Pomerania (NE Germany). Folia Geobot. 2010, 45, 279–302. [Google Scholar] [CrossRef]
- Pokorný, P.; Klimešová, J.; Klimeš, L. Late holocene history and vegetation dynamics of a floodplain alder carr: A case study from Eastern Bohemia, Czech Republic. Folia Geobot. 2000, 35, 43–58. [Google Scholar] [CrossRef]
- Natlandsmyr, B.; Hjelle, K.L. Long-term vegetation dynamics and land-use history: Providing a baseline for conservation strategies in protected Alnus glutinosa swamp woodlands. For. Ecol. Manag. 2016, 372, 78–92. [Google Scholar] [CrossRef]
- Saarse, L.; Niinemets, E.; Poska, A.; Veski, S. Is there a relationship between crop farming and the Alnus decline in the eastern Baltic region? Veg. History Archaeob. 2010, 19, 17–28. [Google Scholar] [CrossRef]
- Muller, S.D.; Miramont, C.; Bruneton, H.; Carré, M.; Sottocornola, M.; Court-Picon, M.; de Beaulieu, J.-L.; Nakagawa, T.; Schevin, P. A palaeoecological perspective for the conservation and restoration of wetland plant communities in the central French Alps, with particular emphasis on alder carr vegetation. Rev. Palaeobot. Palynol. 2012, 171, 124–139. [Google Scholar] [CrossRef]
- Douda, J.; Čejková, A.; Douda, K.; Kochánková, J. Development of alder carr after the abandonment of wet grasslands during the last 70 years. Ann. For. Sci. 2009, 66, 712. [Google Scholar] [CrossRef]
- Stenger, J. Erlenbruchwälder–Dynamik in Raum und Zeit. Konsequenzen für den Prozesschutz in einer Waldgesellschaft. Nat. Landsch. 2000, 32, 262–270. [Google Scholar]
- Reda, P. Rozmieszczenie lasów i parków oraz zmiany w składzie dendroflory po powodzi w 1997 roku w dolinie zalewowej Odry we Wrocławiu. In Praca Doktorska; Uniwersytet Wrocławski: Wrocław, Poland, 2002. [Google Scholar]
- Gorzelak, A. Auswirkungen von überschwemmungen auf die flora am beispiel des oderhochwassers 1997. Beitr. zur Forstwirtsch. Landsch. 2000, 34, 8–11. [Google Scholar]
- McVean, D.N. Ecology of Alnus glutinosa (L.) Gaertn: III. Seedling establishment. J. Ecol. 1956, 44, 195–218. [Google Scholar] [CrossRef]
- Pancer-Kotejowa, E.; Zarzycki, K. Zarys ekologii. In Olsze Alnus Mill; Białobok, S., Ed.; PWN: Warszaw, Poland, 1980; Volume 8, pp. 229–257. [Google Scholar]
- McVean, D.N. Ecology of Alnus glutinosa (L.) Gaertn: V. Notes on some british alder populations. J. Ecol. 1956, 44, 321–330. [Google Scholar] [CrossRef]
- Solinska-Górnicka, B. Alder (Alnus glutinosa) carr in Poland. Tuexenia 1987, 7, 329–346. [Google Scholar]
- Slezák, M.; Hrivnák, R.; Petrášová, A.; Dítě, D. Variability of alder-dominated forest vegetation along a latitudinal gradient in Slovakia. Acta Soc. Bot. Pol. 2013, 82, 25–35. [Google Scholar] [CrossRef]
- McVean, D.N. Ecology of Alnus glutinosa (L.) Gaertn: IV. Root system. J. Ecol. 1956, 44, 219–225. [Google Scholar] [CrossRef]
- Czerwiński, A.; Matowicka, B. Contact dynamic zones between plant communities in river valley and moraine upland. Phytocenosis (Supplementum Cartographie Geobotanicae) 1991, 3, 235–241. [Google Scholar]
- Slezák, M.; Hrivnák, R.; Petrášová, A. Syntaxonomy and ecology of black alder vegetation in the southern part of Central Slovakia. Hacquetia 2011, 10, 119–136. [Google Scholar] [CrossRef]
- Piotrowska, H. Lasy południowo-wschodniego Uznamu. Bad. Fizjogr. nad Polską Zach. 1960, 6, 69–158. [Google Scholar]
- Kazda, M. Changes in alder fens following a decrease in the ground water table: Results of a geographical information system application. J. Appl. Ecol. 1995, 32, 100–110. [Google Scholar] [CrossRef]
- Kazda, M.; Verbücheln, G.; Brans, S.; Luwe, M. Mapping of vegetation and soil changes in an alder carr affected by a decrease of water-table height. Phytocenosis (Supplementum Cartographie Geobotanicae) 1991, 3, 243–250. [Google Scholar]
- Kopeć, D.; Ratajczyk, N.; Wolańska-Kamińska, A.; Walisch, M.; Kruk, A. Floodplain forest vegetation response to hydroengineering and climatic pressure—A five decade comparative analysis in the Bzura river valley (Central Poland). For. Ecol. Manag. 2014, 314, 120–130. [Google Scholar] [CrossRef]
- Wittig, R.; Michels, C.; Wetzstein Sunke, C. Effects of irrigations on swamp forests drained by lignite mining. In Nature Conservation; Gafta, D., Akeroyd, J., Eds.; Spring: Berlin/Heidelberg, Germany, 2006; pp. 402–416. [Google Scholar]
- Dajdok, Z.; Pielech, R.; Raj, A.; Szczęśniak, E.; Wieniawska-Raj, B.; Zając, K. Rezerwaty Przyrody Województwa Dolnośląskiego; Regionalna Dyrekcja Ochrony Środowiska we Wrocławiu: Wrocław, Poland, 2017; p. 151. [Google Scholar]
- Dajdok, Z.; Klink, A.; Polechońska, L.; Dambiec, M.; Pielech, R. Abundance of Coleanthus subtilis in relation to nutrient concentrations in pond soils—A case study of localities in Poland. Flora 2017, 235, 41–50. [Google Scholar] [CrossRef]
- Magnuszewski, P.; Sendzimir, J.; Kronenberg, J. Conceptual modeling for adaptive environmental assessment and management in the Barycz valley, Lower Silesia, Poland. Int. J. Environ. Res. Public Health 2005, 2, 194–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wuczyński, A.; Smyk, B.; Kołodziejczyk, P.; Lenkiewicz, W.; Orłowski, G.; Pola, A. Long-term changes in numbers of geese stopping over and wintering in south-western Poland. Central Eur. J. Biol. 2012, 7, 495–506. [Google Scholar] [CrossRef] [Green Version]
- Marek, S. Biologia i Stratygrafia Torfowisk Olszynowych w Polsce; PWRiL: Warszawa, Poland, 1965; Volume 57, p. 264. [Google Scholar]
- Piasecki, J. Temperatura powietrza (1951–1980). In Atlas Śląska Dolnego i Opolskiego; Pawlak, W., Ed.; Uniwersytet Wrocławski, Pracownia Atlasu Dolnego Śląska: Wrocław, Poland, 1997; p. 47. [Google Scholar]
- Bac-Bronowicz, J. Opady atmosferyczne (1951–1980). In Atlas Śląska Dolnego i Opolskiego; Pawlak, W., Ed.; Uniwersytet Wrocławski, Pracownia Atlasu Dolnego Śląska: Wrocław, Poland, 1997; p. 43. [Google Scholar]
- Anioł-Kwiatkowska, J.; Pender, K. Plan ochrony leśnego rezerwatu przyrody “Olszyny Niezgodzkie”. Unpublished work. 1997. [Google Scholar]
- Douda, J.; Boublík, K.; Slezák, M.; Biurrun, I.; Nociar, J.; Havrdová, A.; Doudová, J.; Aćić, S.; Brisse, H.; Brunet, J.; et al. Vegetation classification and biogeography of European floodplain forests and alder carrs. Appl. Veg. Sci. 2016, 19, 147–163. [Google Scholar] [CrossRef]
- Ellenberg, H. Vegetation Ecology of Central Europe; Cambridge University Press: Cambridge, UK, 2009; p. 756. [Google Scholar]
- Döring-Mederake, U. Alnion forests in Lower Saxony (FRG), their ecological requirements, classification and position within Carici elongatae-Alnetum of Northern Central Europe. Vegetatio 1990, 89, 107–119. [Google Scholar] [CrossRef]
- Kopecký, M.; Macek, M. Vegetation resurvey is robust to plot location uncertainty. Divers. Distrib. 2015, 21, 322–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapfer, J.; Hédl, R.; Jurasinski, G.; Kopecký, M.; Schei, F.H.; Grytnes, J.-A. Resurveying historical vegetation data—opportunities and challenges. Appl. Veg. Sci. 2017, 20, 164–171. [Google Scholar] [CrossRef]
- Ross, L.C.; Woodin, S.J.; Hester, A.; Thompson, D.B.A.; Birks, H.J.B. How important is plot relocation accuracy when interpreting re-visitation studies of vegetation change? Plant Ecol. Divers. 2010, 3, 1–8. [Google Scholar] [CrossRef]
- Kent, M.; Coker, P. Vegetation Description and Analysis: A practical Approach; John Wiley & Sons: Chichester, UK; New York, NY, USA; Brisbane, Australia; Toronto, Japan; Singapore, 1992; p. 363. [Google Scholar]
- Pielech, R.; Baran, J.; Bodziarczyk, J.; Kucharzyk, S.; Malicki, M.; Smoczyk, M.; Wilczek, Z.; Zarzycki, W.; Zięba, A. Forest database of Southern Poland. Phytocoenologia 2018, in press. [Google Scholar]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulißen, D. Zeigerwerte von Pflanzen in Mitteleuropa. Scr. Geobot. 1992, 18, 1–258. [Google Scholar]
- Diekmann, M. Species indicator values as an important tool in applied plant ecology—A review. Basic Appl. Ecol. 2003, 4, 493–506. [Google Scholar] [CrossRef]
- Pielech, R.; Zając, K.; Kadej, M.; Malicki, M.; Malkiewicz, A.; Tarnawski, D. Ellenberg’s indicator values support prediction of suitable habitat for pre-diapause larvae of endangered butterfly Euphydryas aurinia. PLoS ONE 2017, 12, e0179026. [Google Scholar] [CrossRef] [PubMed]
- Helm, N.; Essl, F.; Mirtl, M.; Dirnböck, T. Multiple environmental changes drive forest floor vegetation in a temperate mountain forest. Ecol. Evolut. 2017, 7, 2155–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelený, D.; Schaffers, A.P. Too good to be true: Pitfalls of using mean Ellenberg indicator values in vegetation analyses. J. Veg. Sci. 2012, 23, 419–431. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-1. 2018. Available online: https://cran.r-project.org/web/packages/vegan/ (accessed on 17 May 2018).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Spring: New York, NY, USA, 2009. [Google Scholar]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.-P.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G.; et al. Vegetation of europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19, 3–264. [Google Scholar] [CrossRef]
- Martin, P.A.; Newton, A.C.; Cantarello, E.; Evans, P. Stand dieback and collapse in a temperate forest and its impact on forest structure and biodiversity. For. Ecol. Manag. 2015, 358, 130–138. [Google Scholar] [CrossRef]
- Evans, P.M.; Newton, A.C.; Cantarello, E.; Martin, P.; Sanderson, N.; Jones, D.L.; Barsoum, N.; Cottrell, J.E.; A’Hara, S.W.; Fuller, L. Thresholds of biodiversity and ecosystem function in a forest ecosystem undergoing dieback. Sci. Rep. 2017, 7, 6775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffer, M.; Carpenter, S.R.; Lenton, T.M.; Bascompte, J.; Brock, W.; Dakos, V.; van de Koppel, J.; van de Leemput, I.A.; Levin, S.A.; van Nes, E.H.; et al. Anticipating critical transitions. Science 2012, 338, 344–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffer, M.; Carpenter, S.R. Catastrophic regime shifts in ecosystems: Linking theory to observation. Trends Ecol. Evolut. 2003, 18, 648–656. [Google Scholar] [CrossRef]
- Lõhmus, K.; Paal, T.; Liira, J. Long-term colonization ecology of forest-dwelling species in a fragmented rural landscape—Dispersal versus establishment. Ecol. Evolut. 2014, 4, 3113–3126. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Von Oheimb, G. Migration of vascular plants to secondary woodlands in southern Sweden. J. Ecol. 1998, 86, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Dzwonko, Z. Relations between the floristic composition of isolated young woods and their proximity to ancient woodland. J. Veg. Sci. 1993, 4, 693–698. [Google Scholar] [CrossRef]
- Orczewska, A. Colonization capacity of herb woodland species in fertile, recent alder woodlanda adjacent to ancient forest sites. Pol. J. Ecol. 2010, 58, 297–310. [Google Scholar]
- Hermy, M.; Honnay, O.; Firbank, L.; Grashof-Bokdam, C.; Lawesson, J.E. An ecological comparison between ancient and other forest plant species of Europe, and the implications for forest conservation. Biol. Conserv. 1999, 91, 9–22. [Google Scholar] [CrossRef]
- Hermy, M.; Verheyen, K. Legacies of the past in the present-day forest biodiversity: A review of past land-use effects on forest plant species composition and diversity. Ecol. Res. 2007, 22, 361–371. [Google Scholar] [CrossRef]
- Dzwonko, Z. Wskaźnikowe gatunki roślin starych lasów i ich znaczenie dla ochrony przyrody i kartografii roślinności. Prace Geogr. 2001, 178, 120–132. [Google Scholar]
- Stańska, M.; Stański, T.; Gladzka, A.; Bartos, M. Spider assemblages of hummocks and hollows in a primeval alder carr in the Bialowieza National Park - effect of vegetation structure and soil humidity. Pol. J. Ecol. 2016, 64, 564–577. [Google Scholar] [CrossRef]
- Verheyen, K.; De Frenne, P.; Baeten, L.; Waller, D.M.; Hédl, R.; Perring, M.P.; Blondeel, H.; Brunet, J.; Chudomelová, M.; Decocq, G.; et al. Combining biodiversity resurveys across regions to advance global change research. BioScience 2017, 67, 73–83. [Google Scholar] [CrossRef]
- Tingley, M.W.; Beissinger, S.R. Detecting range shifts from historical species occurrences: New perspectives on old data. Trends Ecol. Evol. 2009, 24, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Kent, M. Vegetation Description and Data Analysis: A Practical Approach; Wiley-Blackwell: Hoboken, NJ, USA, 2012; p. 414. [Google Scholar]
- Chytrý, M.; Hennekens, S.M.; Jiménez-Alfaro, B.; Knollová, I.; Dengler, J.; Jansen, F.; Landucci, F.; Schaminée, J.H.J.; Aćić, S.; Agrillo, E.; et al. European Vegetation Archive (EVA): An integrated database of European vegetation plots. Appl. Veg. Sci. 2016, 19, 173–180. [Google Scholar] [CrossRef]
- Dengler, J.; Team, S.C. Splot: The first global vegetation-plot database and opportunities to contribute in IAVS. IAVS Bull. 2014, 34–37. [Google Scholar]
- Dengler, J.; Jansen, F.; Glöckler, F.; Peet, R.K.; De Cáceres, M.; Chytrý, M.; Ewald, J.; Oldeland, J.; Lopez-Gonzalez, G.; Finckh, M.; et al. The Global Index of Vegetation-plot Databases (GIVD): A new resource for vegetation science. J. Veg. Sci. 2011, 22, 582–597. [Google Scholar] [CrossRef]
- Woods, K.D. Predictability, contingency, and convergence in late succession: Slow systems and complex data-sets. J. Veg. Sci. 2007, 18, 543–554. [Google Scholar] [CrossRef]
- Král, K.; Daněk, P.; Janík, D.; Krůček, M.; Vrška, T. How cyclical and predictable are Central European temperate forest dynamics in terms of development phases? J. Veg. Sci. 2018, 29, 84–97. [Google Scholar] [CrossRef]
- Hédl, R.; Kopecký, M.; Komárek, J. Half a century of succession in a temperate oakwood: From species-rich community to mesic forest. Divers. Distrib. 2010, 16, 267–276. [Google Scholar] [CrossRef]
- Taverna, K.; Peet, R.K.; Phillips, L.C. Long-term change in ground-layer vegetation of deciduous forests of the North Carolina Piedmont, USA. J. Ecol. 2005, 93, 202–213. [Google Scholar] [CrossRef] [Green Version]

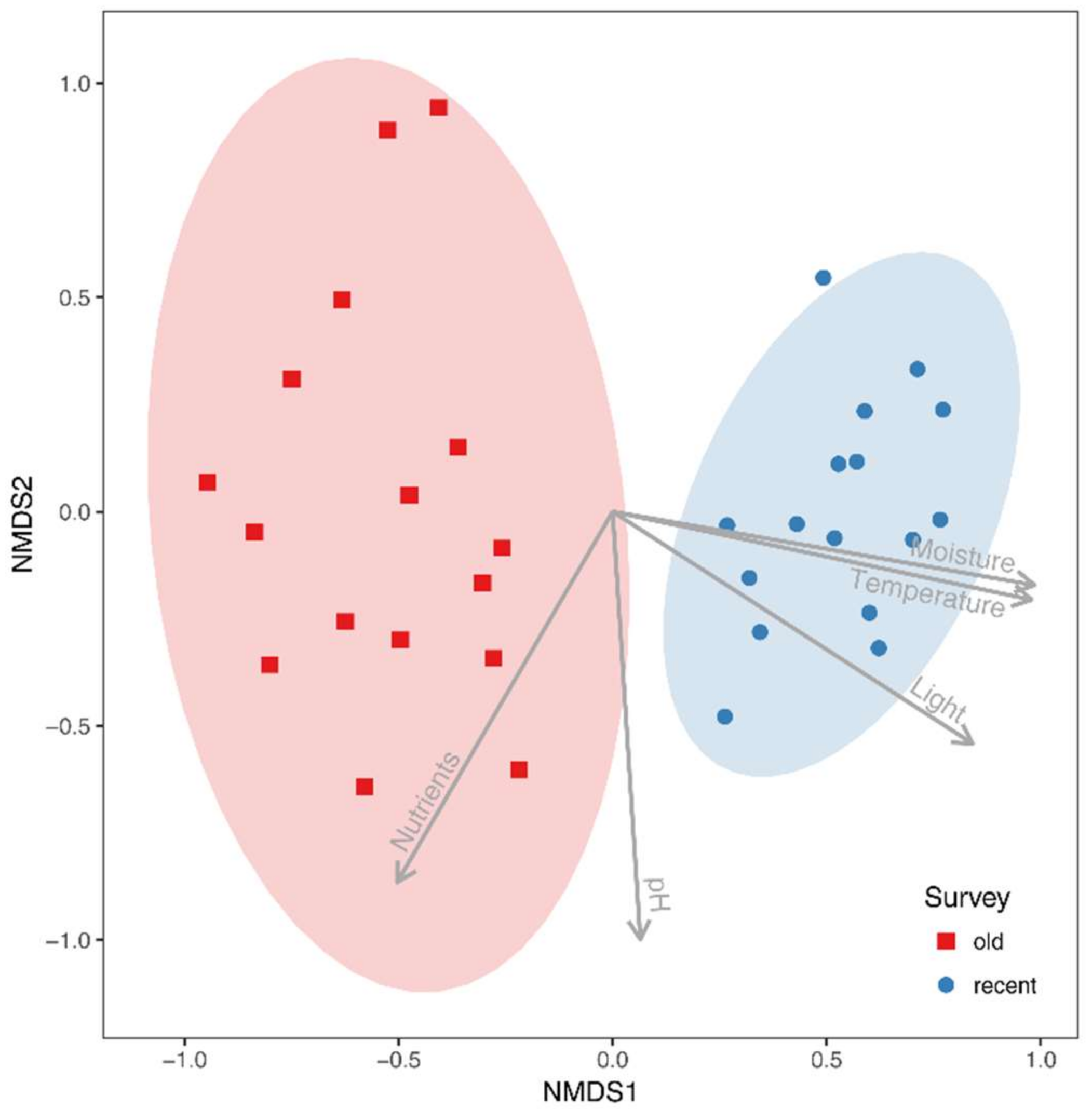
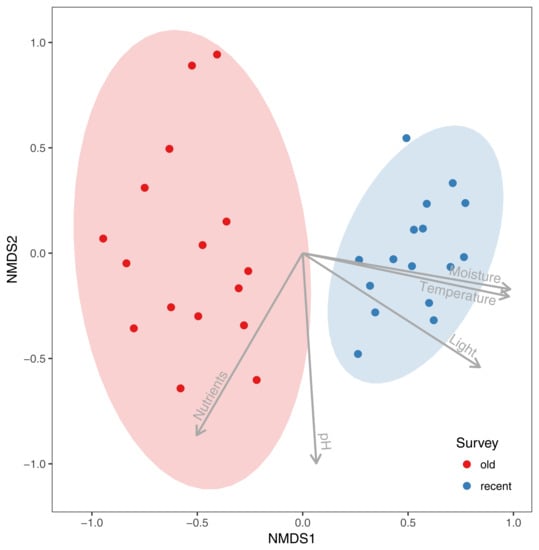
| Variable | NMDS1 | NMDS2 | R2 | p |
|---|---|---|---|---|
| Light | 0.841 | −0.541 | 0.391 | 0.004 |
| Temperature | 0.979 | −0.206 | 0.294 | 0.009 |
| Moisture | 0.985 | −0.171 | 0.816 | 0.001 |
| pH | 0.065 | −0.998 | 0.141 | 0.118 |
| Nutrients | −0.503 | −0.864 | 0.120 | 0.157 |
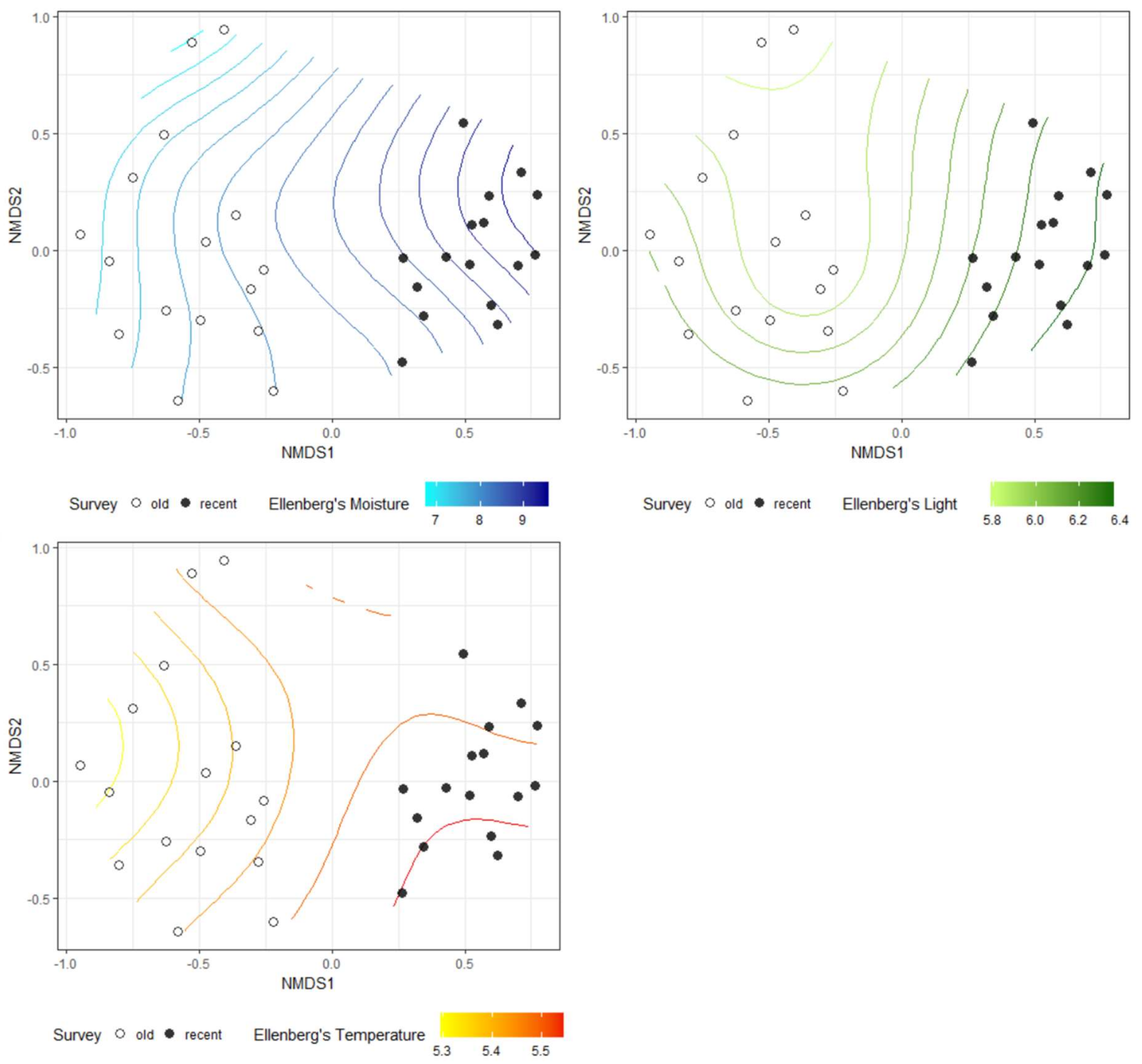
| EIVs | 1993 | 2013 | F-value | p | ||
|---|---|---|---|---|---|---|
| x− | SD | x− | SD | |||
| Light | 5.96 | 0.37 | 6.36 | 0.17 | 15.345 | 0.114 |
| Temperature | 5.44 | 0.17 | 5.56 | 0.09 | 5.947 | 0.330 |
| Moisture | 7.54 | 0.51 | 9.01 | 0.49 | 68.107 | 0.001 |
| pH | 5.91 | 0.49 | 5.78 | 0.28 | 0.852 | 0.706 |
| Nutrients | 6.18 | 0.56 | 5.79 | 0.35 | 5.622 | 0.348 |
| Forest Characteristics | 1993 | 2013 | p | ||
|---|---|---|---|---|---|
| x̄ | SD | x̄ | SD | ||
| Trees | 51.88 | 19.31 | 34.38 | 22.20 | 0.024 |
| Shrubs | 3.94 | 4.51 | 4.75 | 6.27 | 0.677 |
| Herbs | 82.19 | 21.83 | 86.25 | 9.57 | 0.503 |
| Evenness | 0.70 | 0.10 | 0.71 | 0.08 | 0.858 |
| Richness | 21.63 | 10.04 | 20.63 | 7.32 | 0.750 |
| Shannon | 2.12 | 0.57 | 2.12 | 0.40 | 0.989 |
| Simpson | 0.78 | 0.10 | 0.79 | 0.08 | 0.845 |
| Species | Frequency | χ2 | p | ||
|---|---|---|---|---|---|
| 1993 | 2013 | ||||
| Alnus glutinosa (L.) Gaertn. | 100.0% | 100.0% | - | - | |
| Carex elongata L. | ↗ | 31.3% | 93.8% | 10.800 | 0.001 |
| Phragmites australis (Cav.) Trin. ex Steud. | 43.8% | 81.3% | 3.333 | 0.068 | |
| Urtica dioica L. | ↘ | 87.5% | 31.3% | 8.295 | 0.004 |
| Dryopteris carthusiana (Vill.) H.P. Fuchs | ↗ | 25.0% | 81.3% | 8.031 | 0.005 |
| Deschampsia cespitosa (L.) P.Beauv. | 68.8% | 37.5% | 2.008 | 0.156 | |
| Carex acutiformis Ehrh. | 43.8% | 62.5% | 0.502 | 0.479 | |
| Galium palustre L. | 37.5% | 62.5% | 1.125 | 0.289 | |
| Lemna minor L. | + | 0.0% | 100.0% | - | - |
| Iris pseudacorus L. | 31.3% | 62.5% | 2.008 | 0.156 | |
| Thelypteris palustris Schott | 31.3% | 56.3% | 1.143 | 0.285 | |
| Lycopus europaeus L. | ↗ | 12.5% | 75.0% | 10.286 | 0.001 |
| Calamagrostis canescens (Weber) Roth | 43.8% | 43.8% | 0.000 | 1.000 | |
| Solanum dulcamara L. | ↗ | 6.3% | 75.0% | 12.955 | 0.000 |
| Rubus idaeus L. | 56.3% | 25.0% | 2.073 | 0.150 | |
| Lemna trisulca L. | + | 0.0% | 75.0% | - | - |
| Lythrum salicaria L. | ↗ | 12.5% | 62.5% | 6.533 | 0.011 |
| Bidens frondosa L. | 18.8% | 50.0% | 2.216 | 0.136 | |
| Lysimachia vulgaris L. | + | 0.0% | 68.8% | - | - |
| Hydrocharis morsus-ranae L. | + | 0.0% | 62.5% | - | - |
| Ribes nigrum L. | ↘ | 56.3% | 6.3% | 7.127 | 0.008 |
| Prunus padus L. | 37.5% | 25.0% | 0.145 | 0.703 | |
| Lysimachia thyrsiflora L. | + | 0.0% | 56.3% | - | - |
| Sorbus aucuparia L. | 37.5% | 18.8% | 0.618 | 0.432 | |
| Peucedanum palustre (L.) Moench | 25.0% | 31.3% | 0.000 | 1.000 | |
| Galium aparine L. | – | 56.3% | 0.0% | - | - |
| Acer pseudoplatanus L. | 25.0% | 25.0% | 0.000 | 1.000 | |
| Humulus lupulus L. | ↘ | 43.8% | 6.3% | 4.167 | 0.041 |
| Stellaria aquatica (L.) Scop. | – | 50.0% | 0.0% | - | - |
| Scutellaria galericulata L. | 18.8% | 31.3% | 0.167 | 0.683 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pielech, R.; Malicki, M. Changes in Species Composition in Alder Swamp Forest Following Forest Dieback. Forests 2018, 9, 316. https://0-doi-org.brum.beds.ac.uk/10.3390/f9060316
Pielech R, Malicki M. Changes in Species Composition in Alder Swamp Forest Following Forest Dieback. Forests. 2018; 9(6):316. https://0-doi-org.brum.beds.ac.uk/10.3390/f9060316
Chicago/Turabian StylePielech, Remigiusz, and Marek Malicki. 2018. "Changes in Species Composition in Alder Swamp Forest Following Forest Dieback" Forests 9, no. 6: 316. https://0-doi-org.brum.beds.ac.uk/10.3390/f9060316