Influence of Irradiance and Temperature on the Virus MpoV-45T Infecting the Arctic Picophytoplankter Micromonas polaris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species and Culturing Conditions
2.2. One-Step Viral Infection Experiments
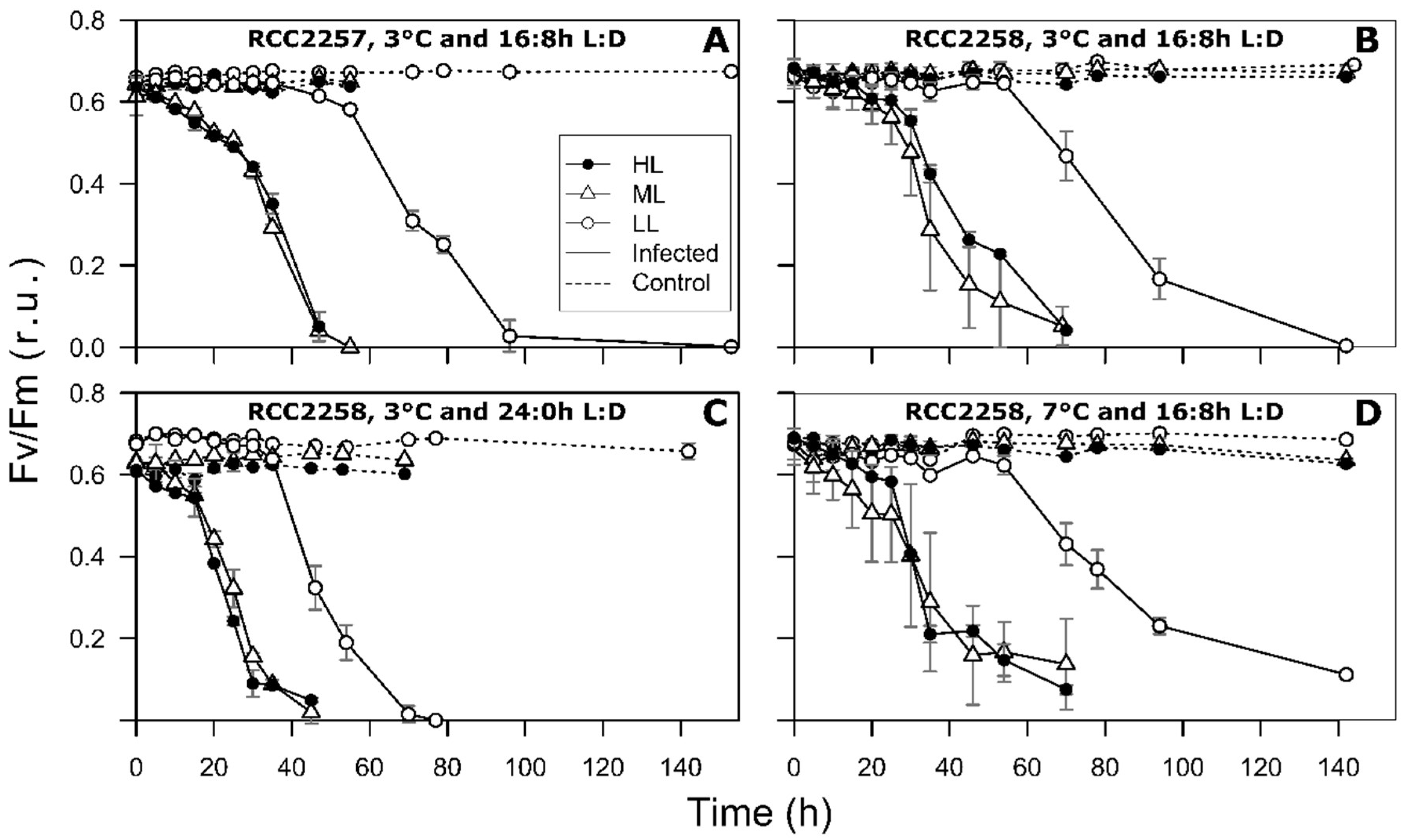
2.3. Photosynthetic Efficiency
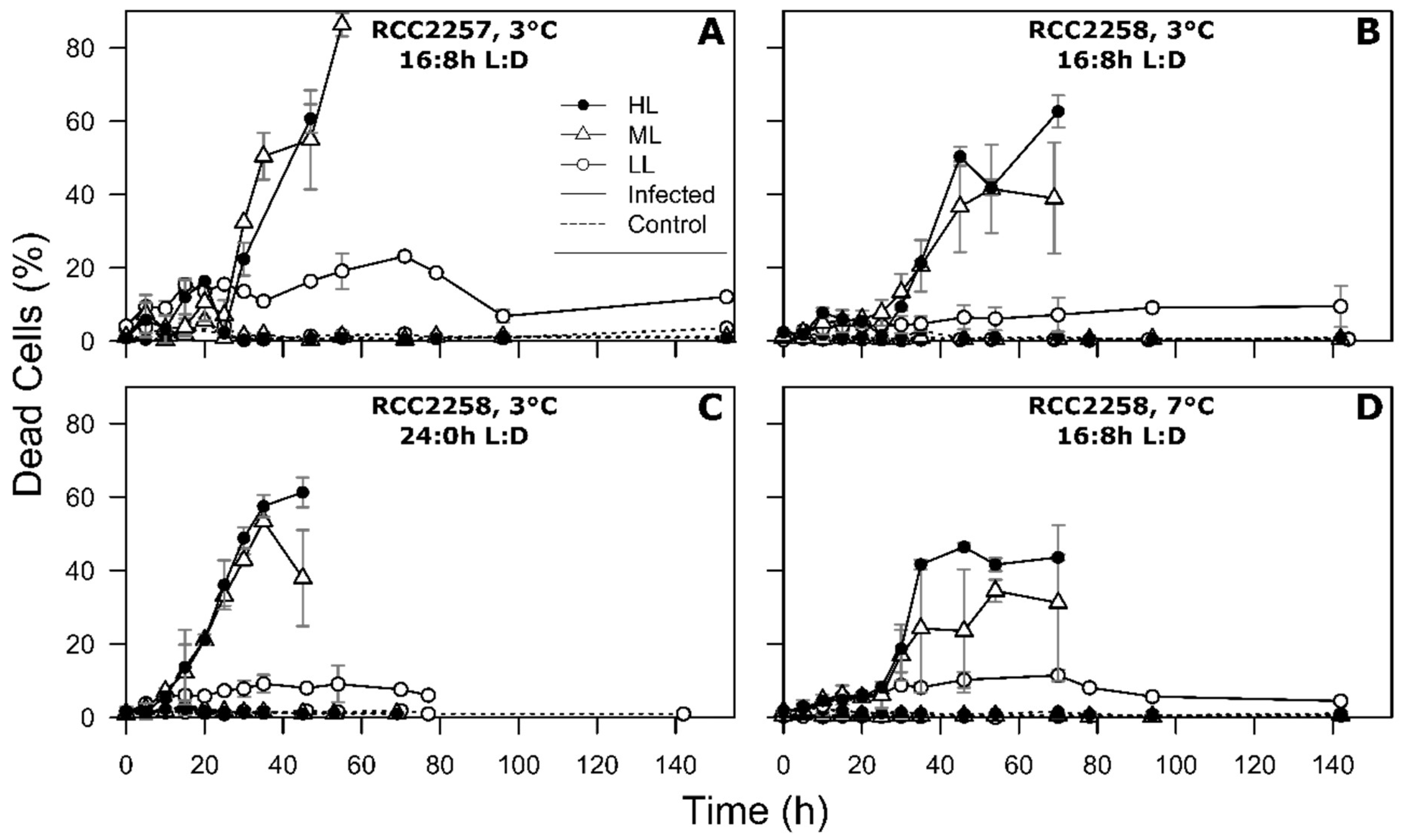
2.4. Flow Cytometry
2.5. Statistical Analyses
3. Results
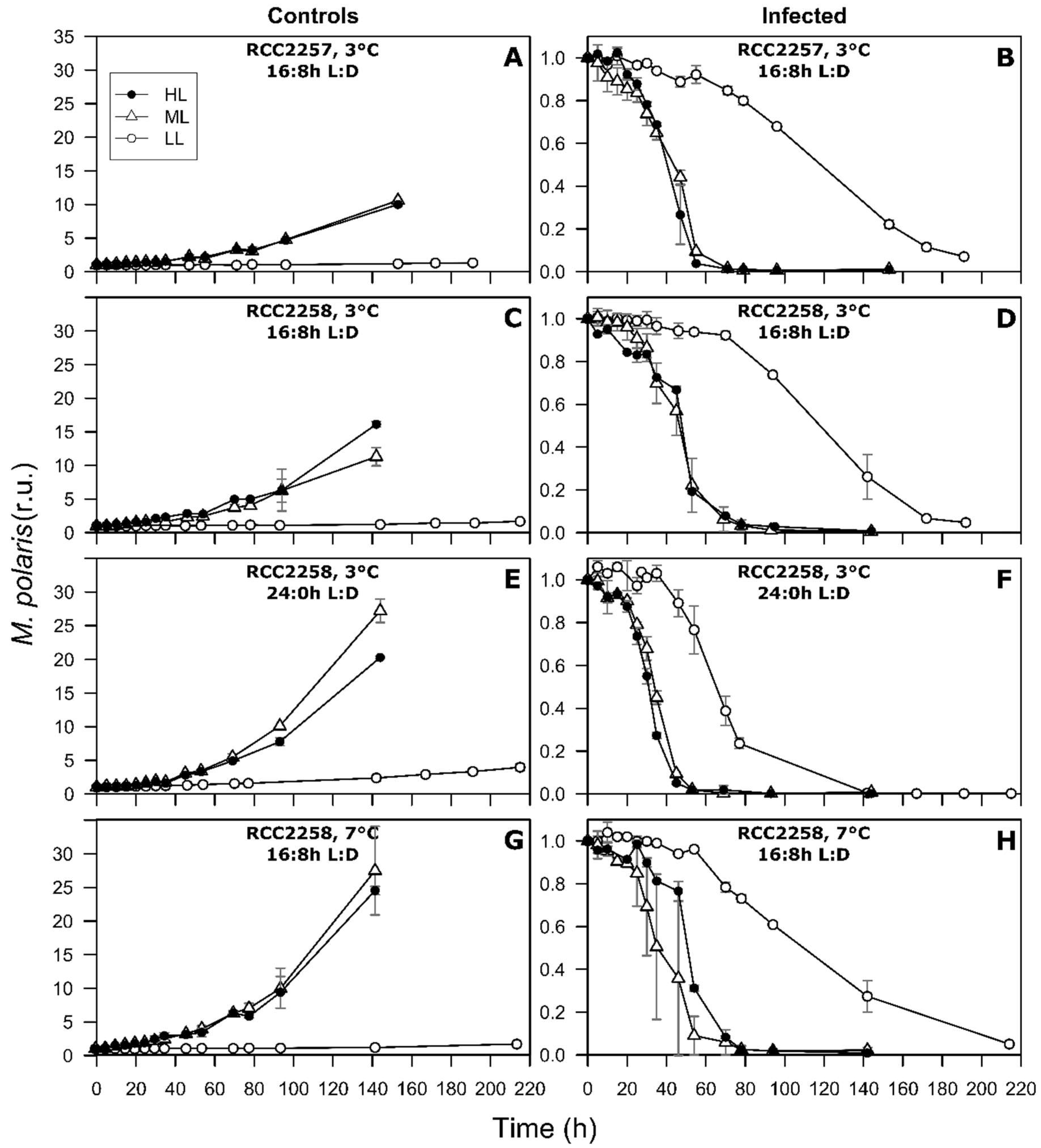
3.1. Non-Infected Controls
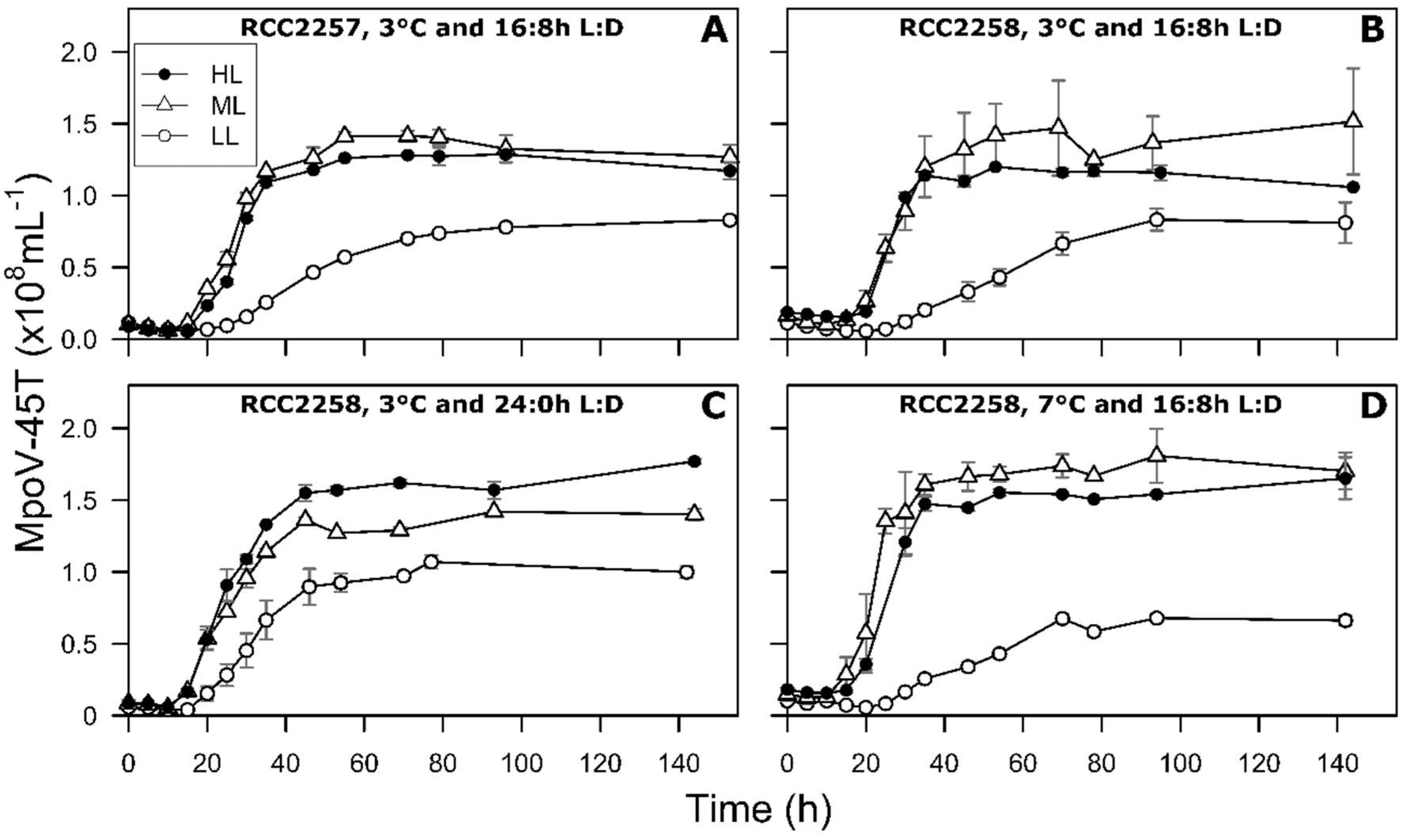
3.2. Infection Dynamics at Different Light Intensities
3.3. Effects of Light Period on Viral Infection Dynamics
3.4. Effect of Temperature on Viral Infection Dynamics
4. Discussion
4.1. Reduced Virus Proliferation Under LL
4.2. Continuous Light Enhances Virus Proliferation
4.3. MpoV-45T Propagation is Temperature Dependent
4.4. Ecological Implications
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brussaard, C.P.D.; Kuipers, B.; Veldhuis, M.J.W. A mesocosm study of Phaeocystis globosa population dynamics: I. Regulatory role of viruses in bloom control. Harmful Algae 2005, 4, 859–874. [Google Scholar] [CrossRef]
- Mojica, K.D.A.; Huisman, J.; Wilhelm, S.W.; Brussaard, C.P.D. Latitudinal variation in virus-induced mortality of phytoplankton across the North Atlantic Ocean. ISME J. 2016, 10, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, M.T.; Suttle, C.A. Dynamics of lytic virus infecting the photosynthetic marine picoflagellate Micromonas pusilla. Limnol. Oceanogr. 1995, 40, 730–739. [Google Scholar] [CrossRef]
- Brussaard, C.P.D.; Bratbak, G.; Baudoux, A.C.; Ruardij, P. Phaeocystis and its interaction with viruses. In Phaeocystis, Major Link in the Biogeochemical Cycling of Climate-Relevant Elements; Springer: Dordrecht, The Netherlands, 2007; pp. 201–215. ISBN 9781402062131. [Google Scholar]
- Lehahn, Y.; Koren, I.; Schatz, D.; Frada, M.; Sheyn, U.; Boss, E.; Efrati, S.; Rudich, Y.; Trainic, M.; Sharoni, S.; et al. Decoupling physical from biological processes to assess the impact of viruses on a mesoscale algal bloom. Curr. Biol. 2014, 24, 2041–2046. [Google Scholar] [CrossRef] [PubMed]
- Baudoux, A.C.; Noordeloos, A.A.M.; Veldhuis, M.J.W.; Brussaard, C.P.D. Virally induced mortality of Phaeocystis globosa during two spring blooms in temperate coastal waters. Aquat. Microb. Ecol. 2006, 44, 207–217. [Google Scholar] [CrossRef]
- Baudoux, A.C.; Veldhuis, M.J.W.; Witte, H.J.; Brussaard, C.P.D. Viruses as mortality agents of picophytoplankton in the deep chlorophyll maximum layer during IRONAGES III. Limnol. Oceanogr. 2007, 52, 2519–2529. [Google Scholar] [CrossRef] [Green Version]
- Suttle, C.A. Marine viruses—Major players in the global ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Brussaard, C.P.D.; Wilhelm, S.W.; Thingstad, F.; Weinbauer, M.G.; Bratbak, G.; Heldal, M.; Kimmance, S.A.; Middelboe, M.; Nagasaki, K.; Paul, J.H.; et al. Global-scale processes with a nanoscale drive: The role of marine viruses. ISME J. 2008, 2, 575–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojica, K.D.A.; Brussaard, C.P.D. Factors affecting virus dynamics and microbial host-virus interactions in marine environments. FEMS Microbiol. Ecol. 2014, 89, 495–515. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.E. Intensified warming of the Arctic: Causes and impacts on middle latitudes. Glob. Planet. Chang. 2014, 117, 52–63. [Google Scholar] [CrossRef]
- Timmermans, M.L.; Ladd, C.; Wood, K. Sea Surface Temperature. Available online: https://www.arctic.noaa.gov/Report-Card/Report-Card-2017/ArtMID/7798/ArticleID/698/Sea-Surface-Temperature (accessed on 20 August 2018).
- ACIA. Chapter 9: Marine Systems. In Arctic Climate Impact Assessment; Cambridge University Press: Cambridge, UK, 2005; pp. 453–538. 1042p, ISBN 0 521 86509 3. [Google Scholar]
- Carmack, E.C.; Macdonald, R.W.; Jasper, S. Phytoplankton productivity on the Canadian Shelf of the Beaufort Sea. Mar. Ecol. Prog. Ser. 2004, 277, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Stroeve, J.C.; Markus, T.; Boisvert, L.; Miller, J.; Barrett, A. Changes in Arctic melt season and implications for sea ice loss. Geophys. Res. Lett. 2014, 41, 1216–1225. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, F.A.; Carmack, E.C. Deepening of the nutricline and chlorophyll maximum in the Canada Basin interior, 2003–2009. Geophys. Res. Lett. 2010, 37, 24. [Google Scholar] [CrossRef]
- Slagstad, D.; Wassmann, P.F.J.; Ellingsen, I. Physical constrains and productivity in the future Arctic Ocean. Front. Mar. Sci. 2015, 2, 85. [Google Scholar] [CrossRef] [Green Version]
- Li, W.K.W.; McLaughlin, F.A.; Lovejoy, C.; Carmack, E.C. Smallest algae thrive as the arctic ocean freshens. Science 2009, 326, 539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; He, J.; Lin, L.; Jin, H. Dominance of picophytoplankton in the newly open surface water of the central Arctic Ocean. Polar Biol. 2015, 38, 1081–1089. [Google Scholar] [CrossRef]
- Brussaard, C.P.D.; Noordeloos, A.A.M.; Witte, H.; Collenteur, M.C.J.; Schulz, K.; Ludwig, A.; Riebesell, U. Arctic microbial community dynamics influenced by elevated CO2 levels. Biogeosciences 2013, 10, 719–731. [Google Scholar] [CrossRef] [Green Version]
- Bach, L.T.; Alvarez-Fernandez, S.; Hornick, T.; Stuhr, A.; Riebesell, U. Simulated ocean acidification reveals winners and losers in coastal phytoplankton. PLoS ONE 2017, 12, e0188198. [Google Scholar] [CrossRef] [PubMed]
- Kilias, E.S.; Nöthig, E.M.; Wolf, C.; Metfies, K. Picoeukaryote plankton composition off West Spitsbergen at the entrance to the Arctic Ocean. J. Eukaryot. Microbiol. 2014, 61, 569–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovejoy, C.; Vincent, W.F.; Bonilla, S.; Roy, S.; Martineau, M.J.; Terrado, R.; Potvin, M.; Massana, R.; Pedrós-Alió, C. Distribution, phylogeny, and growth of cold-adapted picoprasinophytes in Arctic seas. J. Phycol. 2007, 43, 78–89. [Google Scholar] [CrossRef]
- Terrado, R.; Lovejoy, C.; Massana, R.; Vincent, W.F. Microbial food web responses to light and nutrients beneath the coastal Arctic Ocean sea ice during the winter-spring transition. J. Mar. Syst. 2008, 74, 964–977. [Google Scholar] [CrossRef]
- Coello-Camba, A.; Agustí, S.; Vaqué, D.; Holding, J.; Arrieta, J.M.; Wassmann, P.; Duarte, C.M. Experimental Assessment of Temperature Thresholds for Arctic Phytoplankton Communities. Estuaries Coasts 2015, 38, 873–885. [Google Scholar] [CrossRef]
- Balzano, S.; Marie, D.; Gourvil, P.; Vaulot, D. Composition of the summer photosynthetic pico and nanoplankton communities in the Beaufort Sea assessed by T-RFLP and sequences of the 18S rRNA gene from flow cytometry sorted samples. ISME J. 2012, 6, 1480–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maat, D.S.; Biggs, T.; Evans, C.; van Bleijswijk, J.D.L.; van Der Wel, N.N.; Dutilh, B.E.; Brussaard, C.P.D. Characterization and Temperature Dependence of Arctic Micromonas polaris Viruses. Viruses 2017, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A.; Geider, R.J. Temperature and algal growth. New Phytol. 1988, 110, 441–461. [Google Scholar] [CrossRef] [Green Version]
- Baudoux, A.C.; Brussaard, C.P.D. Influence of irradiance on virus-algal host interactions. J. Phycol. 2008, 44, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Maat, D.S.; de Blok, R.; Brussaard, C.P.D. Combined Phosphorus Limitation and Light Stress Prevent Viral Proliferation in the Phytoplankton Species Phaeocystis globosa, but Not in Micromonas pusilla. Front. Mar. Sci. 2016, 3, 160. [Google Scholar] [CrossRef]
- Marie, D.; Partensky, F.; Vaulot, D.; Brussaard, C. Enumeration of Phytoplankton, Bacteria, and Viruses in Marine Samples. In Current Protocols in Cytometry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; Volume 10, ISBN 1934-9300. [Google Scholar]
- Suttle, C.A. Enumeration and Isolation of Viruses. In Handbook of Methods in Aquatic Microbial Ecology; JLewis Publishers: Boca Raton, FL, USA, 1993; pp. 121–137. ISBN 0873715640. [Google Scholar]
- Passmore, R.; Hsu, J.; Liu, R.X.; Tam, E.; Cai, Y.; Su, W.; Frasca, J.; Brigden, S.M.; Comeau, A.M.; Ortmann, A.C. MPN Assay Analyzer. Available online: http://www.webcitation.org/6ogxAqLbE (accessed on 20 August 2018).
- Brussaard, C.P.D. Optimization of Procedures for Counting Viruses by Flow Cytometry. Appl. Environ. Microbiol. 2004, 70, 1506–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genty, B.; Harbinson, J.; Briantais, J.M.; Baker, N.R. The relationship between non-photochemical quenching of chlorophyll fluorescence and the rate of photosystem 2 photochemistry in leaves. Photosynth. Res. 1990, 25, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.M. Parameters and probes. In Practical Flow Cytometry; Wiley-Liss: Hoboken, NJ, USA, 2003; pp. 275–276. ISBN 9780471722731. [Google Scholar]
- DuRand, M.D.; Green, R.E.; Sosik, H.M.; Olson, R.J. Diel variations in optical properties of Micromonas pusilla (Prasinophyceae). J. Phycol. 2002, 38, 1132–1142. [Google Scholar] [CrossRef]
- Brussaard, C.P.D.; Marie, D.; Thyrhaug, R.; Bratbak, G. Flow cytometric analysis of phytoplankton viability following viral infection. Aquat. Microb. Ecol. 2001, 26, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Evans, C.; Malin, G.; Mills, G.P.; Wilson, W.H. Viral infection of Emiliania huxleyi (Prymnesiophyceae) leads to elevated production of reactive oxygen species. J. Phycol. 2006, 42, 1040–1047. [Google Scholar] [CrossRef]
- Raven, J.A. The twelfth Tansley Lecture. Small is beautiful: The picophytoplankton. Funct. Ecol. 1998, 12, 503–513. [Google Scholar] [CrossRef]
- Laney, S.R.; Krishfield, R.A.; Toole, J.M. The euphotic zone under Arctic Ocean sea ice: Vertical extents and seasonal trends. Limnol. Oceanogr. 2017, 62, 1910–1934. [Google Scholar] [CrossRef] [Green Version]
- Johnsen, G.; Norli, M.; Moline, M.; Robbins, I.; von Quillfeldt, C.; Sørensen, K.; Cottier, F.; Berge, J. The advective origin of an under-ice spring bloom in the Arctic Ocean using multiple observational platforms. Polar Biol. 2018, 41, 1197–1216. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.M.; Campbell, D.A.; Lawrence, J.E. Resource dynamics during infection of Micromonas pusilla by virus MpV-Sp1. Environ. Microbiol. 2007, 9, 2720–2727. [Google Scholar] [CrossRef] [PubMed]
- Demory, D.; Arsenieff, L.; Simon, N.; Six, C.; Rigaut-Jalabert, F.; Marie, D.; Ge, P.; Bigeard, E.; Jacquet, S.; Sciandra, A.; et al. Temperature is a key factor in Micromonas-virus interactions. ISME J. 2017, 11, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Puxty, R.J.; Millard, A.D.; Evans, D.J.; Scanlan, D.J. Shedding new light on viral photosynthesis. Photosynth. Res. 2015, 126, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Hadas, H.; Einav, M.; Fishov, I.; Zaritsky, A. Bacteriophage T4 development depends on the physiology of its host Escherichia coli. Microbiology 1997, 143, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T.; Herschler, T.D.; Stopar, D. Bacteriophage latent-period evolution as a response to resource availability. Appl. Environ. Microbiol. 2001, 67, 4233–4241. [Google Scholar] [CrossRef] [PubMed]
- Brussaard, C.P.; Thyrhaug, R.; Marie, D.; Bratbak, G. Flow cytometry analyses of viral infection in two marine phytoplankton species, Micromonas pusilla (Prasinophyceae) and Phaeocystis pouchetii (prymnesiophyceae). J. Phycol. 1999, 35, 941–948. [Google Scholar] [CrossRef]
- Franklin, D.J.; Brussaard, C.P.D.; Berges, J.A. What is the role and nature of programmed cell death in phytoplankton ecology? Eur. J. Phycol. 2006, 41, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sheyn, U.; Rosenwasser, S.; Ben-Dor, S.; Porat, Z.; Vardi, A. Modulation of host ROS metabolism is essential for viral infection of a bloom-forming coccolithophore in the ocean. ISME J. 2016, 10, 1742–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vardi, A.; Haramaty, L.; Van Mooy, B.A.S.; Fredricks, H.F.; Kimmance, S.A.; Larsen, A.; Bidle, K.D. Host-virus dynamics and subcellular controls of cell fate in a natural coccolithophore population. Proc. Natl. Acad. Sci. USA 2012, 109, 19327–19332. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Diaz, J.M.; Plummer, S. Production of extracellular reactive oxygen species by phytoplankton: Past and future directions. J. Plankton Res. 2018, 40, 655–666. [Google Scholar] [CrossRef]
- Evans, C.; Wilson, W.H. Preferential grazing of Oxyrrhis marina on virus-infected Emiliania huxleyi. Limnol. Oceanogr. 2008, 53, 2035–2040. [Google Scholar] [CrossRef]
- Coello-Camba, A.; Agustí, S. Thermal Thresholds of Phytoplankton Growth in Polar Waters and Their Consequences for a Warming Polar Ocean. Front. Mar. Sci. 2017, 4, 168. [Google Scholar] [CrossRef]
- Hoppe, C.J.M.; Flintrop, C.M.; Rost, B. The Arctic picoeukaryote Micromonas pusilla benefits synergistically from warming and ocean acidification. Biogeosciences 2018, 15, 4353–4365. [Google Scholar] [CrossRef]
- Maat, D.S.; Brussaard, C.P.D. Both phosphorus- and nitrogen limitation constrain viral proliferation in marine phytoplankton. Aquat. Microb. Ecol. 2016, 77, 87–97. [Google Scholar] [CrossRef]
- Maat, D.S.; van Bleijswijk, J.D.L.; Witte, H.J.; Brussaard, C.P.D. Virus production in phosphorus-limited Micromonas pusilla stimulated by a supply of naturally low concentrations of different phosphorus sources, far into the lytic cycle. FEMS Microbiol. Ecol. 2016, 92, fiw136. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.E.; Chan, A.T. Micromonas pusilla Virus: The Virus Growth Cycle and Associated Physiological Events Within the Host Cells; Host Range Mutation. J. Gen. Virol. 1982, 63, 199–206. [Google Scholar] [CrossRef]
- Suttle, C.A. The Ecological, Evolutionary and Geochemical Consequences of Viral Infection of Cyanobacteria and Eukaryotic Algae. Viral Ecol. 2000, 1, 247–296. [Google Scholar]
- McKie-Krisberg, Z.M.; Sanders, R.W. Phagotrophy by the picoeukaryotic green alga Micromonas: Implications for Arctic Oceans. ISME J. 2014, 8, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- McKie-Krisberg, Z.M.; Sanders, R.W.; Gast, R.J. Evaluation of Mixotrophy-Associated Gene Expression in Two Species of Polar Marine Algae. Front. Mar. Sci. 2018, 5, 273. [Google Scholar] [CrossRef]
- Joli, N.; Monier, A.; Logares, R.; Lovejoy, C. Seasonal patterns in Arctic prasinophytes and inferred ecology of Bathycoccus unveiled in an Arctic winter metagenome. ISME J. 2017, 11, 1372–1385. [Google Scholar] [CrossRef] [PubMed]

| Host Strain | Temp | Light Cycle | Light Int. | Growth | Fv/Fm | FSC | SSC | RFL | ROS | Dead Cells |
|---|---|---|---|---|---|---|---|---|---|---|
| RCC2257 | 3 | 16:8 | LL | 0.06 | 0.67 | 414 | 23 | 200 | 77 | 2.2 |
| ML | 0.48 | 0.64 | 597 | 25 | 96 | 54 | 1.1 | |||
| HL | 0.49 | 0.64 | 679 | 23 | 65 | 59 | 1.1 | |||
| RCC2258 | 3 | 16:8 | LL | 0.09 | 0.66 | 458 | 23 | 381 | 76 | 0.5 |
| ML | 0.48 | 0.67 | 735 | 27 | 177 | 50 | 0.7 | |||
| HL | 0.49 | 0.67 | 789 | 27 | 136 | NA | 1.4 | |||
| RCC2258 | 3 | 24:0 | LL | 0.16 | 0.69 | 550 | 24 | 317 | NA | 1.4 |
| ML | 0.58 | 0.64 | 892 | 29 | 144 | NA | 1.5 | |||
| HL | 0.60 | 0.61 | 955 | 28 | 108 | NA | 1.5 | |||
| RCC2258 | 7 | 16:8 | LL | 0.11 | 0.67 | 571 | 25 | 330 | 49 | 0.3 |
| ML | 0.70 | 0.67 | 771 | 27 | 148 | 61 | 0.5 | |||
| HL | 0.69 | 0.67 | 797 | 27 | 137 | NA | 1.6 |
| Host Strain | Temp. | Light Cycle | Light Intensity | Latent Period | Max. Prod. | Burst Size |
|---|---|---|---|---|---|---|
| LL | 20–25 | 1.6 ± 0.0 | 146 ± 1 | |||
| RCC2257 | 3 | 16:8 | ML | 10–15 | 5.6 ± 0.3 | 237 ± 4 |
| HL | 10–15 | 5.3 ± 0.1 | 251 ± 5 | |||
| LL | 20–25 | 1.4 ± 0.2 | 125 ± 8 | |||
| RCC2258 | 3 | 16:8 | ML | 15–20 | 5.5 ± 0.5 | 235 ± 44 |
| HL | 15–20 | 6.6 ± 0.0 | 214 ± 16 | |||
| LL | 15–20 | 3.6 ± 0.3 | 211 ± 1 | |||
| RCC2258 | 3 | 24:0 | ML | 10–15 | 5.1 ± 2.0 | 238 ± 6 |
| HL | 10–15 | 5.8 ± 0.0 | 250 ± 2 | |||
| LL | 20–25 | 1.3 ± 0.0 | 137 ± 15 | |||
| RCC2258 | 7 | 16:8 | ML | 10–15 | 8.3 ± 1.6 | 270 ± 7 |
| HL | 10–15 | 7.6 ± 0.1 | 275 ± 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piedade, G.J.; Wesdorp, E.M.; Montenegro-Borbolla, E.; Maat, D.S.; Brussaard, C.P.D. Influence of Irradiance and Temperature on the Virus MpoV-45T Infecting the Arctic Picophytoplankter Micromonas polaris. Viruses 2018, 10, 676. https://0-doi-org.brum.beds.ac.uk/10.3390/v10120676
Piedade GJ, Wesdorp EM, Montenegro-Borbolla E, Maat DS, Brussaard CPD. Influence of Irradiance and Temperature on the Virus MpoV-45T Infecting the Arctic Picophytoplankter Micromonas polaris. Viruses. 2018; 10(12):676. https://0-doi-org.brum.beds.ac.uk/10.3390/v10120676
Chicago/Turabian StylePiedade, Gonçalo J., Ella M. Wesdorp, Elena Montenegro-Borbolla, Douwe S. Maat, and Corina P. D. Brussaard. 2018. "Influence of Irradiance and Temperature on the Virus MpoV-45T Infecting the Arctic Picophytoplankter Micromonas polaris" Viruses 10, no. 12: 676. https://0-doi-org.brum.beds.ac.uk/10.3390/v10120676





