Clostridium perfringens Virulent Bacteriophage CPS2 and Its Thermostable Endolysin LysCPS2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, and Growth Conditions
2.2. Isolation and Propagation of Bacteriophage CPS2
2.3. Transmission Electron Microscopy (TEM) Analysis
2.4. DNA Purification and Whole Genome Sequencing of Bacteriophage CPS2
2.5. Cloning, Expression, and Purification of LysCPS2
2.6. Lytic Activity Assay
2.7. CBD Binding and Fluorescence Microscopy
3. Results and Discussion
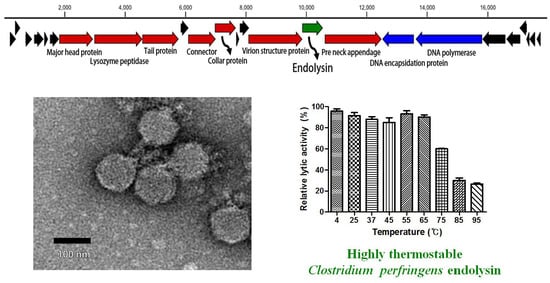
3.1. Morphology of Phage CPS2
3.2. Antibacterial Properties of Phage CPS2
3.3. Genomic Analysis of CPS2
3.4. Identification and Expression of the LysCPS2 Endolysin
3.5. Antimicrobial Spectrum of LysCPS2
3.6. pH, Temperature, NaCl, and Metal Effects on LysCPS2 Activity
3.7. Determination of Thermal Stability
3.8. Binding Activity of LysCPS2_CBD
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Canard, B.; Saint-Joanis, B.; Cole, S. Genomic diversity and organization of virulence genes in the pathogenic anaerobe Clostridium perfringens. Mol. Microbiol. 1992, 6, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.S.; Rasko, D.A.; Cheung, J.K.; Ravel, J.; Seshadri, R.; DeBoy, R.T.; Ren, Q.; Varga, J.; Awad, M.M.; Brinkac, L.M. Skewed genomic variability in strains of the toxigenic bacterial pathogen, Clostridium perfringens. Genome Res. 2006, 16, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.C.; Shrestha, A.; McClane, B.A. Clostridium perfringens enterotoxin: Action, genetics, and translational applications. Toxins 2016, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; van Immerseel, F. Necrotic enteritis in broilers: An updated review on the pathogenesis. Avian Pathol. 2011, 40, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef] [PubMed]
- Borysowski, J.; Weber-Dąbrowska, B.; Górski, A. Bacteriophage endolysins as a novel class of antibacterial agents. Exp. Biol. Med. 2006, 231, 366–377. [Google Scholar] [CrossRef]
- Nariya, H.; Miyata, S.; Tamai, E.; Sekiya, H.; Maki, J.; Okabe, A. Identification and characterization of a putative endolysin encoded by episomal phage phiSM101 of Clostridium perfringens. Appl. Microbiol. Biotechnol. 2011, 90, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, M.; Vukov, N.; Scherer, S.; Loessner, M.J. The murein hydrolase of the bacteriophage φ3626 dual lysis system is active against all tested Clostridium perfringens strains. Appl. Environ. Microbiol. 2002, 68, 5311–5317. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, T.; Horn, N.; Wegmann, U.; Dugo, G.; Narbad, A.; Mayer, M.J. Expression and delivery of an endolysin to combat Clostridium perfringens. Appl. Microbiol. Biotechnol. 2014, 98, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Seal, B.S. Characterization of bacteriophages virulent for Clostridium perfringens and identification of phage lytic enzymes as alternatives to antibiotics for potential control of the bacterium1. Poult. Sci. 2013, 92, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Seal, B.S.; Fouts, D.E.; Simmons, M.; Garrish, J.K.; Kuntz, R.L.; Woolsey, R.; Schegg, K.M.; Kropinski, A.M.; Ackermann, H.-W.; Siragusa, G.R. Clostridium perfringens bacteriophages ΦCP39O and ΦCP26F: Genomic organization and proteomic analysis of the virions. Arch. Virol. 2011, 156, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Kirby, K. A new method for the isolation of ribonucleic acids from mammalian tissues. Biochem. J. 1956, 64, 405. [Google Scholar] [CrossRef] [PubMed]
- Zeugin, J.A.; Hartley, J.L. Ethanol precipitation of DNA. Focus 1985, 7, 1–2. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M. The RAST Server: Rapid annotations using subsystems technology. BMC Genomics 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Kim, M.; Ryu, S. Characterization of a novel endolysin LysSA11 and its utility as a potent biocontrol agent against Staphylococcus aureus on food and utensils. Food Microbiol. 2017, 68, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Walmagh, M.; Boczkowska, B.; Grymonprez, B.; Briers, Y.; Drulis-Kawa, Z.; Lavigne, R. Characterization of five novel endolysins from Gram-negative infecting bacteriophages. Appl. Microbiol. Biotechnol. 2013, 97, 4369–4375. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Shin, J.H.; Heu, S.; Park, J.-K.; Ryu, S. Lateral flow assay-based bacterial detection using engineered cell wall binding domains of a phage endolysin. Biosens. Bioelectron. 2017, 96, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Sim, J.; Kang, T.; Nguyen, H.H.; Park, H.K.; Chung, B.H.; Ryu, S. A novel and highly specific phage endolysin cell wall binding domain for detection of Bacillus cereus. Eur. Biophys. J. 2015, 44, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.; Cherniak, R. Structure of the capsular polysaccharide of Clostridium perfringens Hobbs 10 determined by NMR spectroscopy. Carbohydr. Res. 1997, 305, 65–72. [Google Scholar] [CrossRef]
- Kretzer, J.W.; Lehmann, R.; Schmelcher, M.; Banz, M.; Kim, K.-P.; Korn, C.; Loessner, M.J. Use of high-affinity cell wall-binding domains of bacteriophage endolysins for immobilization and separation of bacterial cells. Appl. Environ. Microbiol. 2007, 73, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Volozhantsev, N.V.; Oakley, B.B.; Morales, C.A.; Verevkin, V.V.; Bannov, V.A.; Krasilnikova, V.M.; Popova, A.V.; Zhilenkov, E.L.; Garrish, J.K.; Schegg, K.M. Molecular characterization of podoviral bacteriophages virulent for Clostridium perfringens and their comparison with members of the Picovirinae. PLoS ONE 2012, 7, e38283. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Schuch, R.; Zhu, S.; Tscherne, D.M.; Fischetti, V.A. Genomic sequence of C1, the first streptococcal phage. J. Bacteriol. 2003, 185, 3325–3332. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.J.; Narbad, A.; Gasson, M.J. Molecular characterization of a Clostridium difficile bacteriophage and its cloned biologically active endolysin. J. Bacteriol. 2008, 190, 6734–6740. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, K.H.; Kandler, O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 1972, 36, 407–477. [Google Scholar] [PubMed]
- Son, B.; Yun, J.; Lim, J.-A.; Shin, H.; Heu, S.; Ryu, S. Characterization of LysB4, an endolysin from the Bacillus cereus-infecting bacteriophage B4. BMC Microbiol. 2012, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, D.G.; Dong, S.; Baker, J.R.; Engler, J.A. The bifunctional peptidoglycan lysin of Streptococcus agalactiae bacteriophage B30. Microbiology 2004, 150, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Khakhum, N.; Yordpratum, U.; Boonmee, A.; Tattawasart, U.; Rodrigues, J.L.; Sermswan, R.W. Cloning, expression, and characterization of a peptidoglycan hydrolase from the Burkholderia pseudomallei phage ST79. AMB Express 2016, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Mikoulinskaia, G.V.; Odinokova, I.V.; Zimin, A.A.; Lysanskaya, V.Y.; Feofanov, S.A.; Stepnaya, O.A. Identification and characterization of the metal ion-dependent l-alanoyl-d-glutamate peptidase encoded by bacteriophage T5. FEBS J. 2009, 276, 7329–7342. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, R.; Briers, Y.; Hertveldt, K.; Robben, J.; Volckaert, G. Identification and characterization of a highly thermostable bacteriophage lysozyme. Cell. Mol. Life Sci. CMLS 2004, 61, 2753–2759. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Waldherr, F.; Loessner, M.J. Listeria bacteriophage peptidoglycan hydrolases feature high thermoresistance and reveal increased activity after divalent metal cation substitution. Appl. Microbiol. Biotechnol. 2012, 93, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhu, C.; Chen, J.; Ye, X.; Huang, Y.-P. Antibacterial Activity of Stenotrophomonas maltophilia Endolysin P28 against both Gram-positive and Gram-negative Bacteria. Front. Microbiol. 2015, 6, 1299. [Google Scholar] [CrossRef] [PubMed]
- Swift, S.M.; Seal, B.S.; Garrish, J.K.; Oakley, B.B.; Hiett, K.; Yeh, H.-Y.; Woolsey, R.; Schegg, K.M.; Line, J.E.; Donovan, D.M. A thermophilic phage endolysin fusion to a Clostridium perfringens-specific cell wall binding domain creates an anti-Clostridium antimicrobial with improved thermostability. Viruses 2015, 7, 3019–3034. [Google Scholar] [CrossRef] [PubMed]
- Loessner, M.J. Bacteriophage endolysins—Current state of research and applications. Curr. Opin. Microbiol. 2005, 8, 480–487. [Google Scholar] [CrossRef] [PubMed]





| Bacterial Strain | CPS2 Plaque Formation | Lysis Zone Formation by LysCPS2 | Binding Activity of LysCPS2_CBD | Reference or Source |
|---|---|---|---|---|
| Clostridium strains | ||||
| C. perfringens H3 | Lysis from without | + | + | [20] |
| C. perfringens ATCC 3624 | Lysis from without | + | + | ATCC a |
| C. perfringens ATCC 13124 | + | + | + | ATCC |
| C. perfringens FORC25 | − | + | + | This study |
| C. perfringens human stool isolate 1 | − | + | + | This study |
| C. perfringens human stool isolate 2 | − | + | + | This study |
| C. perfringens human stool isolate 3 | + | + | + | This study |
| C. perfringens human stool isolate 4 | Lysis from without | + | + | This study |
| C. histolyticum ATCC 19401 | − | − | − | ATCC |
| C. indolis ATCC 25771 | − | − | − | ATCC |
| Other Gram-positive bacteria | ||||
| Bacillus cereus ATCC 10987 | − | − | − | ATCC |
| Bacillus subilis ATCC 23857 | − | − | − | ATCC |
| Listeria monocytogenes EGD-e | − | − | − | [21] |
| Staphylococcus aureus RN4220 | − | − | − | [16] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, E.; Son, B.; Ryu, S. Clostridium perfringens Virulent Bacteriophage CPS2 and Its Thermostable Endolysin LysCPS2. Viruses 2018, 10, 251. https://0-doi-org.brum.beds.ac.uk/10.3390/v10050251
Ha E, Son B, Ryu S. Clostridium perfringens Virulent Bacteriophage CPS2 and Its Thermostable Endolysin LysCPS2. Viruses. 2018; 10(5):251. https://0-doi-org.brum.beds.ac.uk/10.3390/v10050251
Chicago/Turabian StyleHa, Eunsu, Bokyung Son, and Sangryeol Ryu. 2018. "Clostridium perfringens Virulent Bacteriophage CPS2 and Its Thermostable Endolysin LysCPS2" Viruses 10, no. 5: 251. https://0-doi-org.brum.beds.ac.uk/10.3390/v10050251