Co-Acquired Nanovirus and Geminivirus Exhibit a Contrasted Localization within Their Common Aphid Vector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Agro-Inoculation
2.2. Aphid Rearing
2.3. Plant and Insect Dissections
2.4. Fluorescent in Situ Hybridization (FISH)
2.5. Viral DNA Extraction
2.6. Quantitative Real-Time PCR (qPCR) Detection
2.7. Transmission Experiments
2.8. Leaf-Disc Inoculation Assay and Nested-PCR
2.9. Statistical Analysis
3. Results
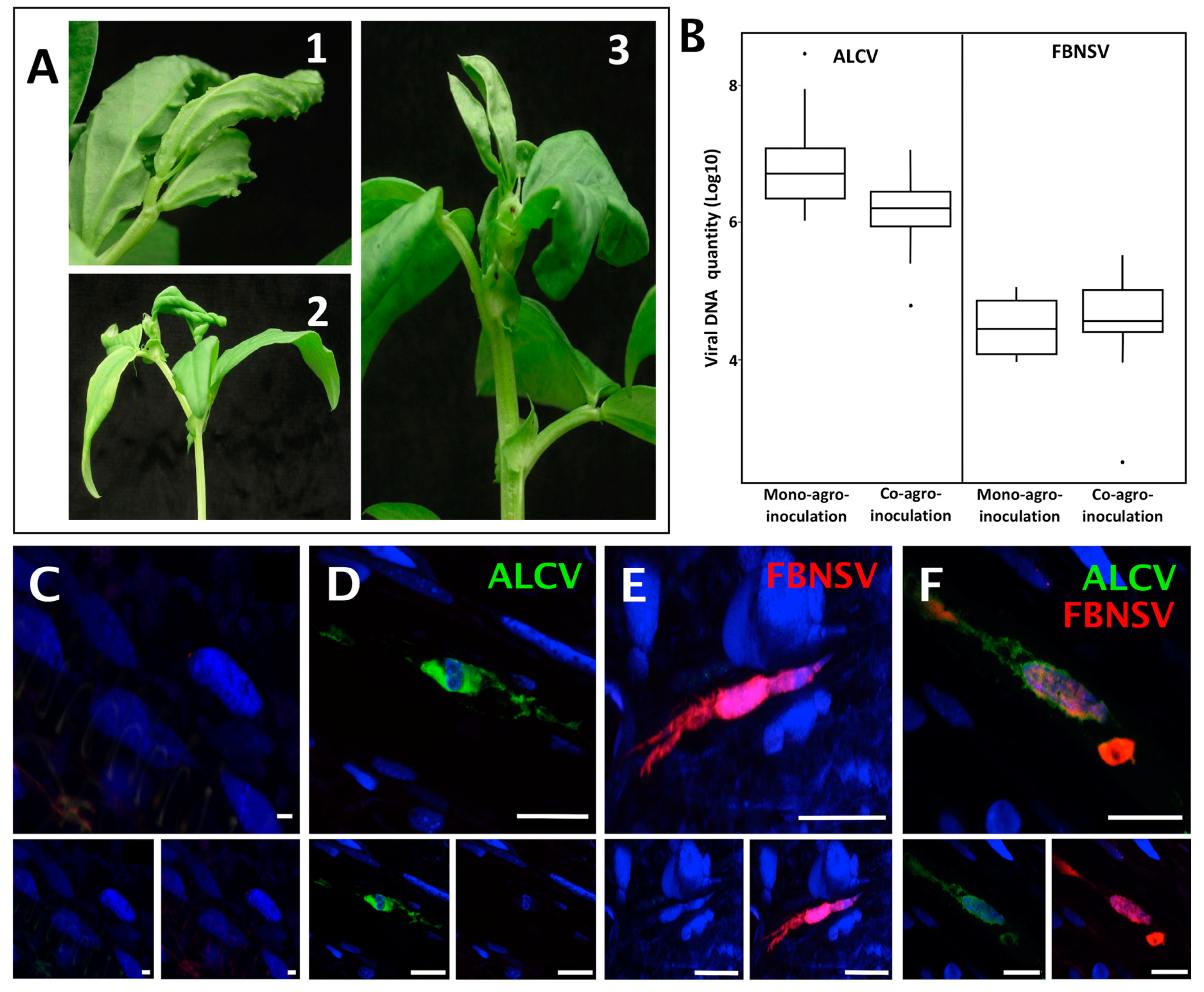
3.1. FBNSV and ALCV Can Co-Infect Plants and Cells within These Plants
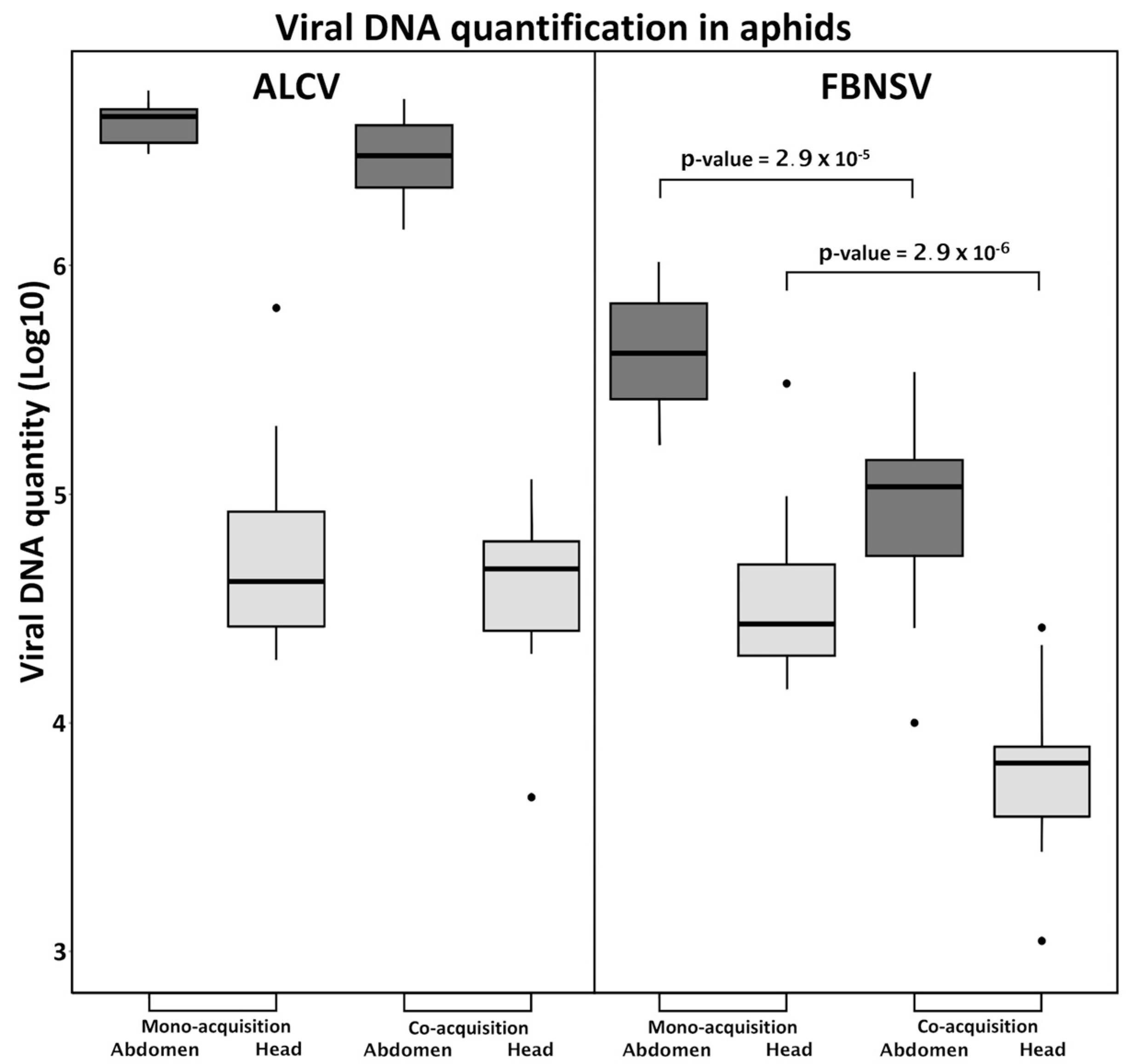
3.2. FBNSV and ALCV Are Co-Acquired from Co-Infected Plants
3.3. FBNSV and ALCV Accumulate in Distinct Cytoplasmic Aggregates in Aphid Gut Cells
3.4. FBNSV and ALCV Are Both Released Together with the Aphid Saliva
3.5. FBNSV Antagonizes ALCV at the Onset of Infection after Co-Transmission by Aphids
4. Discussion
4.1. Co-Infection of Plants by a Nanovirus and a Geminivirus
4.2. Possible Interference between ALCV and FBNSV
4.3. Distinct Intracellular Pathways for Nanovirus and Capulavirus in the Insect Vector
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hogenhout, S.A.; El Ammar, D.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanc, S.; Drucker, M.; Uzest, M. Localizing viruses in their insect vectors. Annu. Rev. Phytopathol. 2014, 52, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Ammar, E.D.; Gargani, D.; Lett, J.M.; Peterschmitt, M. Large accumulations of maize streak virus in the filter chamber and midgut cells of the leafhopper vector Cicadulina mbila. Arch. Virol. 2009, 154, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Bressan, A. Tropism, compartmentalization and retention of banana bunchy top virus (Nanoviridae) in the aphid vector Pentalonia nigronervosa. J. Gen. Virol. 2013, 94, 209–219. [Google Scholar] [CrossRef]
- Vetten, H.J.; Dale, J.L.; Grigoras, I.; Gronenborn, B.; Harding, R.; Randles, J.W.; Thomas, J.E.; Timchenko, T.; Yeh, H.H. Virus taxonomy: The classification and nomenclature of viruses: The 9th report of the ICTV. In Nanoviridae; King, A.M.Q., Lefkowitz, E.J., Adams, M.J., Carstens, E.B., Eds.; Elsevier-AP Press: Amsterdam, The Netherlands, 2011; pp. 395–404. [Google Scholar]
- Czosnek, H.; Ghanim, M. Back to Basics: Are Begomoviruses Whitefly Pathogens? J. Integr. Agric. 2012, 11, 225–234. [Google Scholar] [CrossRef]
- Varsani, A.; Navas-Castillo, J.; Moriones, E.; Hernandez-Zepeda, C.; Idris, A.; Brown, J.K.; Murilo Zerbini, F.; Martin, D.P. Establishment of three new genera in the family Geminiviridae: Becurtovirus, Eragrovirus and Turncurtovirus. Arch. Virol. 2014, 159, 2193–2203. [Google Scholar] [CrossRef]
- Bejerman, N. Geminivirus–Vector Relationship; Springer: Cham, Switzerland, 2019; pp. 137–145. [Google Scholar] [CrossRef]
- Wang, L.L.; Wang, X.R.; Wei, X.M.; Huang, H.; Wu, J.X.; Chen, X.X.; Liu, S.S.; Wang, X.W. The autophagy pathway participates in resistance to tomato yellow leaf curl virus infection in whiteflies. Autophagy 2016, 12, 1560–1574. [Google Scholar] [CrossRef] [Green Version]
- Zerbini, F.M.; Briddon, R.W.; Idris, A.; Martin, D.P.; Moriones, E.; Navas-Castillo, J.; Rivera-Bustamante, R.; Roumagnac, P.; Varsani, A.; Ictv Report, C. ICTV Virus Taxonomy Profile: Geminiviridae. J. Gen. Virol. 2017, 98, 131–133. [Google Scholar] [CrossRef]
- Roumagnac, P.; Granier, M.; Bernardo, P.; Deshoux, M.; Ferdinand, R.; Galzi, S.; Fernandez, E.; Julian, C.; Abt, I.; Filloux, D.; et al. Alfalfa Leaf Curl Virus: An Aphid-Transmitted Geminivirus. J. Virol. 2015, 89, 9683–9688. [Google Scholar] [CrossRef] [Green Version]
- Ryckebusch, F.; Sauvion, N.; Granier, M.; Roumagnac, P.; Peterschmitt, M. Alfalfa leaf curl virus is transmitted by Aphis craccivora in a highly specific circulative manner. BioRxiv 2019. (Under Review). [Google Scholar] [CrossRef]
- Ryckebusch, F.; Peterschmitt, M.; Granier, M.; Sauvion, N. Alfalfa leaf curl virus is efficiently acquired by its aphid vector Aphis craccivora but inefficiently transmitted. BioRxiv 2020. (Under Review). [Google Scholar]
- Hipper, C.; Brault, V.; Ziegler-Graff, V.; Revers, F. Viral and cellular factors involved in phloem transport of plant viruses. Front. Plant Sci. 2013, 4, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, W.B.; Hiebert, E.; Webb, S.E.; Tsai, J.H.; Polston, J.E. Location of Geminiviruses in the Whitefly Bemisia tabaci (Homoptera: Aleyrodidae). Plant Dis. 1998, 82, 1147–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bressan, A.; Watanabe, S. Immunofluorescence localisation of Banana bunchy top virus (family Nanoviridae) within the aphid vector, Pentalonia nigronervosa, suggests a virus tropism distinct from aphid-transmitted luteoviruses. Virus Res. 2011, 155, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, J.J.; Zhang, T.; Li, F.F.; Ghanim, M.; Zhou, X.P.; Ye, G.Y.; Liu, S.S.; Wang, X.W. Specific cells in the primary salivary glands of the whitefly Bemisia tabaci control retention and transmission of begomoviruses. J. Virol. 2014, 88, 13460–13468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briddon, R.W.; Pinner, M.S.; Stanley, J.; Markham, P.G. Geminivirus coat protein gene replacement alters insect specificity. Virology 1990, 177, 85–94. [Google Scholar] [CrossRef]
- Azzam, O.; Frazer, J.; de la Rosa, D.; Beaver, J.S.; Ahlquist, P.; Maxwell, D.P. Whitefly transmission and efficient ssDNA accumulation of bean golden mosaic geminivirus require functional coat protein. Virology 1994, 204, 289–296. [Google Scholar] [CrossRef]
- Franz, A.W.; van der Wilk, F.; Verbeek, M.; Dullemans, A.M.; van den Heuvel, J.F. Faba bean necrotic yellows virus (genus Nanovirus) requires a helper factor for its aphid transmission. Virology 1999, 262, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Grigoras, I.; Vetten, H.J.; Commandeur, U.; Ziebell, H.; Gronenborn, B.; Timchenko, T. Nanovirus DNA-N encodes a protein mandatory for aphid transmission. Virology 2018, 522, 281–291. [Google Scholar] [CrossRef]
- Di Mattia, J.; Vernerey, M.S.; Yvon, M.; Pirolles, E.; Villegas, M.; Gaafar, Y.; Ziebell, H.; Michalakis, Y.; Zeddam, J.L.; Blanc, S. Route of a multipartite (nano)virus across the body of its aphid vector. J. Virol. 2020. [Google Scholar] [CrossRef]
- Grigoras, I.; Timchenko, T.; Katul, L.; Grande-Perez, A.; Vetten, H.J.; Gronenborn, B. Reconstitution of authentic nanovirus from multiple cloned DNAs. J. Virol. 2009, 83, 10778–10787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicard, A.; Yvon, M.; Timchenko, T.; Gronenborn, B.; Michalakis, Y.; Gutierrez, S.; Blanc, S. Gene copy number is differentially regulated in a multipartite virus. Nat. Commun. 2013, 4, 2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boissinot, S.; Pichon, E.; Sorin, C.; Piccini, C.; Scheidecker, D.; Ziegler-Graff, V.; Brault, V. Systemic propagation of a fluorescent infectious clone of a polerovirus following inoculation by agrobacteria and aphids. Viruses 2017, 9, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernerey, M.S.; Pirolles, E.; Sicard, A.; Blanc, S. Localizing Genome Segments and Protein Products of a Multipartite Virus in Host Plant Cells. Bio-Protocol 2019, 9. [Google Scholar] [CrossRef]
- Sicard, A.; Pirolles, E.; Gallet, R.; Vernerey, M.S.; Yvon, M.; Urbino, C.; Peterschmitt, M.; Gutierrez, S.; Michalakis, Y.; Blanc, S. A multicellular way of life for a multipartite virus. eLife 2019, 8, e43599. [Google Scholar] [CrossRef]
- Ghanim, M.; Brumin, M.; Popovski, S. A simple, rapid and inexpensive method for localization of Tomato yellow leaf curl virus and Potato leafroll virus in plant and insect vectors. J. Virol. Methods 2009, 159, 311–314. [Google Scholar] [CrossRef]
- Gallet, R.; Fabre, F.; Michalakis, Y.; Blanc, S. The number of target molecules of the amplification step limits accuracy and sensitivity in ultra deep sequencing viral population studies. J. Virol. 2017, 91, e00561-17. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://www.r-project.org (accessed on 14 December 2019).
- Franz, A.; Makkouk, K.M.; Vetten, H.J. Host range of faba bean necrotic yellows virus and potential yield loss in infected faba bean. Phytopathol. Mediterr. 1997, 36, 94–103. [Google Scholar]
- Gronenborn, B. Nanoviruses: Genome organisation and protein function. Vet. Microbiol. 2004, 98, 103–109. [Google Scholar] [CrossRef]
- Timchenko, T.; Bernardi, F. Les nanovirus, petits virus de plantes: Similitudes et différences avec les géminivirus. Virologie 2007, 11, 27–42. [Google Scholar] [CrossRef]
- Moreno, A.B.; Lopez-Moya, J.J. When viruses play team sports: Mixed infections in plants. Phytopathology 2020, 110, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.; Stanley, J. A nanovirus-like DNA component associated with yellow vein disease of Ageratum conyzoides: Evidence for interfamilial recombination between plant DNA viruses. Virology 1999, 264, 142–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansoor, S.; Khan, S.H.; Hussain, M.; Zafar, Y.; Pinner, M.S.; Briddon, R.W.; Stanley, J.; Markham, P.G. Association of a begomovirus and nanovirus-like molecule with Ageratum Yellow Vein Disease in Pakistan. Plant Dis. 2000, 84, 101. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J. Subviral DNAs associated with geminivirus disease complexes. Vet. Microbiol. 2004, 98, 121–129. [Google Scholar] [CrossRef]
- Simon, K.O.; Cardamone, J.J., Jr.; Whitaker-Dowling, P.A.; Youngner, J.S.; Widnell, C.C. Cellular mechanisms in the superinfection exclusion of vesicular stomatitis virus. Virology 1990, 177, 375–379. [Google Scholar] [CrossRef]
- Gray, S.; Cilia, M.; Ghanim, M. Circulative, “nonpropagative” virus transmission: An orchestra of virus-, insect-, and plant-derived instruments. Adv. Virus Res. 2014, 89, 141–199. [Google Scholar] [CrossRef]
- Sicard, A.; Zeddam, J.L.; Yvon, M.; Michalakis, Y.; Gutierrez, S.; Blanc, S. Circulative nonpropagative aphid transmission of nanoviruses: An oversimplified view. J. Virol. 2015, 89, 9719–9726. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Borthakur, D.; Bressan, A. Localization of Banana bunchy top virus and cellular compartments in gut and salivary gland tissues of the aphid vector Pentalonia nigronervosa. Insect Sci. 2016, 23, 591–602. [Google Scholar] [CrossRef]
- Pan, L.L.; Chen, Q.F.; Zhao, J.J.; Guo, T.; Wang, X.W.; Hariton-Shalev, A.; Czosnek, H.; Liu, S.S. Clathrin-mediated endocytosis is involved in Tomato yellow leaf curl virus transport across the midgut barrier of its whitefly vector. Virology 2017, 502, 152–159. [Google Scholar] [CrossRef]
- Xia, W.Q.; Liang, Y.; Chi, Y.; Pan, L.L.; Zhao, J.; Liu, S.S.; Wang, X.W. Intracellular trafficking of begomoviruses in the midgut cells of their insect vector. PLoS Pathog. 2018, 14, e1006866. [Google Scholar] [CrossRef] [Green Version]

| Detection | qPCR | Nested-PCR | qPCR | Nested-PCR | |
|---|---|---|---|---|---|
| 24 h | 48 h | ||||
| Mono-inoculation | ALCV a | 0/25 | 0/25 | 2/38 | 9/38 |
| FBNSV b | 6/6 | nt | 13/14 | nt | |
| Co-inoculation | ALCV c | 0/26 | 0/26 | 0/41 | 5/41 |
| FBNSV d | 24/26 | nt | 38/41 | nt | |
| ALCV + FBNSV e | 0/26 | 5/41 | |||
| Source Plants | Detection | Transmission Rate | |
|---|---|---|---|
| Test 1-4 | Test 5 | ||
| Mono-inoculation | ALCV a | 4/30 | 16/28 |
| FBNSV b | 19/20 | 5/7 | |
| Co-inoculation | ALCV c | 0/54 | 2/29 |
| FBNSV d | 49/54 | 27/29 | |
| ALCV + FBNSV e | 0/54 | 0/29 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mattia, J.; Ryckebusch, F.; Vernerey, M.-S.; Pirolles, E.; Sauvion, N.; Peterschmitt, M.; Zeddam, J.-L.; Blanc, S. Co-Acquired Nanovirus and Geminivirus Exhibit a Contrasted Localization within Their Common Aphid Vector. Viruses 2020, 12, 299. https://0-doi-org.brum.beds.ac.uk/10.3390/v12030299
Di Mattia J, Ryckebusch F, Vernerey M-S, Pirolles E, Sauvion N, Peterschmitt M, Zeddam J-L, Blanc S. Co-Acquired Nanovirus and Geminivirus Exhibit a Contrasted Localization within Their Common Aphid Vector. Viruses. 2020; 12(3):299. https://0-doi-org.brum.beds.ac.uk/10.3390/v12030299
Chicago/Turabian StyleDi Mattia, Jérémy, Faustine Ryckebusch, Marie-Stéphanie Vernerey, Elodie Pirolles, Nicolas Sauvion, Michel Peterschmitt, Jean-Louis Zeddam, and Stéphane Blanc. 2020. "Co-Acquired Nanovirus and Geminivirus Exhibit a Contrasted Localization within Their Common Aphid Vector" Viruses 12, no. 3: 299. https://0-doi-org.brum.beds.ac.uk/10.3390/v12030299





