Phylogenetic and Geospatial Evidence of Canine Parvovirus Transmission between Wild Dogs and Domestic Dogs at the Urban Fringe in Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wild-Dog Sample Collection
2.2. DNA Extraction and Conventional PCR
2.3. Real-Time PCR
2.4. Conventional PCR and Sequence Analysis
2.5. Wild-Dog Sample Data and Owned-Dog CPV Case Occurrence Data
2.6. Geospatial Analysis of Wild-Dog Data and Owned-Dog Data
2.7. Statistical Analysis of Domestic-Dog CPV Cases and Association with Wild-Dog Infection
3. Results
3.1. Wild-Dog Sampling
3.2. DNA Detection and Quantification
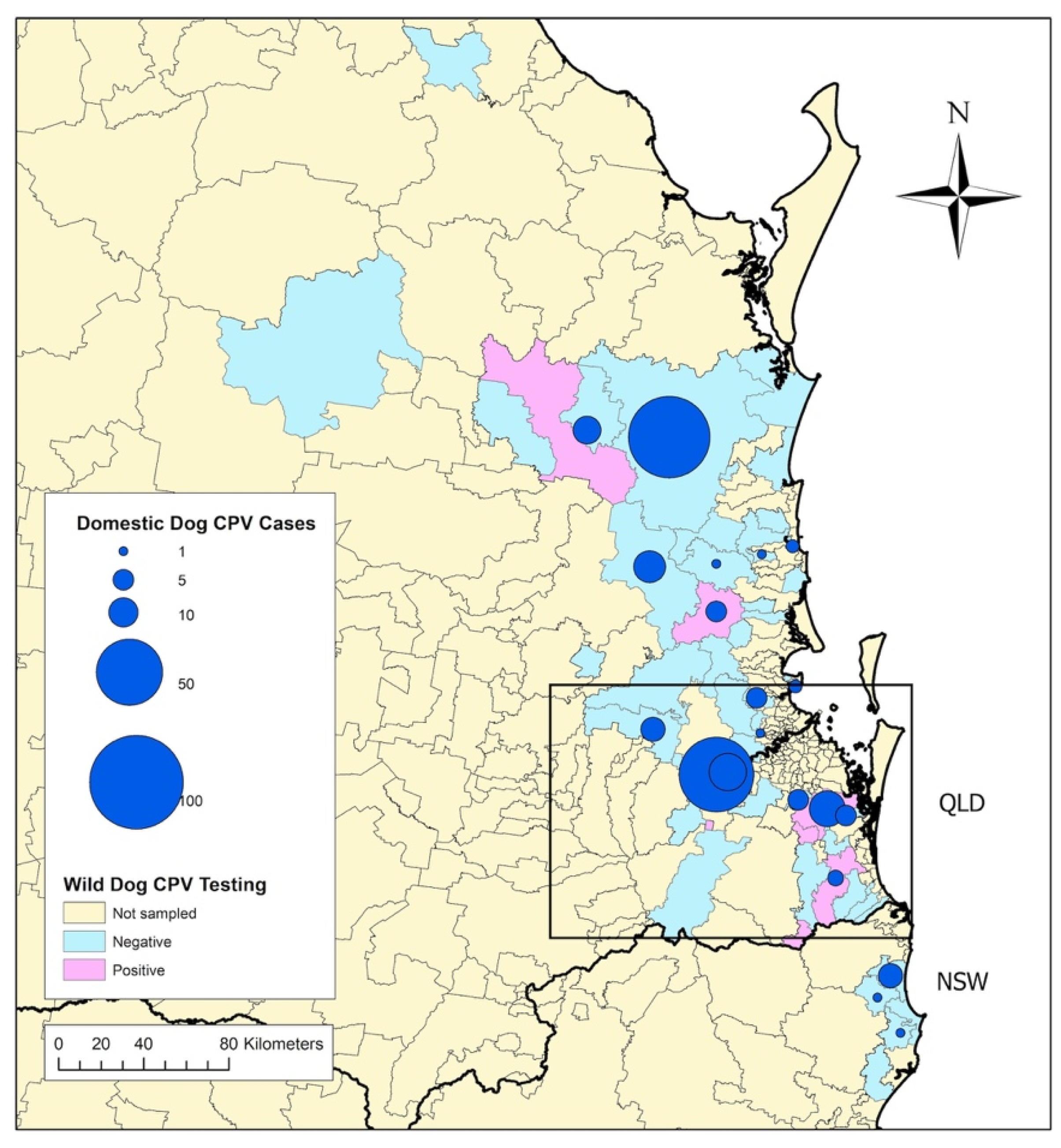
3.3. Geography and Prevalence of Wild-Dog Exposure to CPV
3.4. Association between CPV Exposure in Wild Dogs and CPV Cases in Owned Dogs
3.5. Wild-Dog CPV VP2 Sequencing and Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.-M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Pénzes, J.J.; et al. ICTV Virus Taxonomy Profile: Parvoviridae. J. Gen. Virol. 2019, 100, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Behdenna, A.; Lembo, T.; Calatayud, O.; Cleaveland, S.; Halliday, J.E.B.; Packer, C.; Lankester, F.; Hampson, K.; Craft, M.E.; Czupryna, A.; et al. Transmission ecology of canine parvovirus in a multi-host, multi-pathogen system. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calatayud, O.; Esperón, F.; Cleaveland, S.; Biek, R.; Keyyu, J.; Eblate, E.; Neves, E.; Lembo, T.; Lankester, F. Carnivore Parvovirus Ecology in the Serengeti Ecosystem: Vaccine Strains Circulating and New Host Species Identified. J. Virol. 2019, 93, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calatayud, O.; Esperón, F.; Velarde, R.; Oleaga, Á.; Llaneza, L.; Ribas, A.; Negre, N.; Torre, A.; Rodríguez, A.; Millán, J. Genetic characterization of Carnivore Parvoviruses in Spanish wildlife reveals domestic dog and cat-related sequences. Transbound. Emerg. Dis. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Chang, A.-M.; Wada, T.; Chen, M.-T.; Tu, Y.-S. Distribution of Carnivore protoparvovirus 1 in free-living leopard cats (Prionailurus bengalensis chinensis) and its association with domestic carnivores in Taiwan. PLoS ONE 2019, 14, e0221990. [Google Scholar] [CrossRef] [Green Version]
- Gese, E.M.; Schultz, R.D.; Rongstad, O.J.; Andersen, D.E. Prevalence of Antibodies against Canine Parvovirus and Canine Distemper Virus in Wild Coyotes in Southeastern Colorado. J. Wildl. Dis. 1991, 27, 320–323. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Mochizuki, M.; Naito, R.; Nakamura, K.; Miyazawa, T.; Mikami, T.; Takahashi, E. Predominance of Canine Parvovirus (CPV) in Unvaccinated Cat Populations and Emergence of New Antigenic Types of CPVs in Cats. Virology 2000, 278, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Steinel, A.; Munson, L.; van Vuuren, M.; Truyen, U. Genetic characterization of feline parvovirus sequences from various carnivores. J. Gen. Virol. 2000, 81, 345–350. [Google Scholar] [CrossRef]
- Truyen, U.; Platzer, G.; Parrish, C.R. Antigenic type distribution among canine parvoviruses in dogs and cats in Germany. Vet. Rec. 1996, 138, 365–366. [Google Scholar] [CrossRef]
- Van Arkel, A.; Kelman, M.; West, P.; Ward, M.P. The relationship between reported domestic canine parvovirus cases and wild canid distribution. Heliyon 2019, 5, e02511. [Google Scholar] [CrossRef]
- Zarnke, R.L.; Ballard, W.B. Serologic survey for selected microbial pathogens of wolves in Alaska, 1975–1982. J. Wildl. Dis. 1987, 23, 77–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, A.B.; Kohler, D.J.; Fox, K.A.; Brown, J.D.; Gerhold, R.W.; Shearn-Bochsler, V.I.; Dubovi, E.J.; Parrish, C.R.; Holmes, E.C. Frequent Cross-Species Transmission of Parvoviruses among Diverse Carnivore Hosts. J. Virol. 2013, 87, 2342–2347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shackelton, L.A.; Parrish, C.R.; Truyen, U.; Holmes, E.C. High rate of viral evolution associated with the emergence of carnivore parvovirus. Proc. Natl. Acad. Sci. USA 2005, 102, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Mylonakis, M.; Kalli, I.; Rallis, T. Canine parvoviral enteritis: an update on the clinical diagnosis, treatment, and prevention. Vet. Med. Res. Rep. 2016, 7, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, A.B.; Kohler, D.J.; Ortega, A.; Hoover, E.A.; Grove, D.M.; Holmes, E.C.; Parrish, C.R. Host-Specific Parvovirus Evolution in Nature Is Recapitulated by In Vitro Adaptation to Different Carnivore Species. Plos Pathog. 2014, 10, e1004475. [Google Scholar] [CrossRef]
- Allison, A.B.; Harbison, C.E.; Pagan, I.; Stucker, K.M.; Kaelber, J.T.; Brown, J.D.; Ruder, M.G.; Keel, M.K.; Dubovi, E.J.; Holmes, E.C.; et al. Role of Multiple Hosts in the Cross-Species Transmission and Emergence of a Pandemic Parvovirus. J. Virol. 2012, 86, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Steinel, A.; Parrish, C.R.; Bloom, M.E.; Truyen, U. Parvovirus infections in wild carnivores. J. Wildl. Dis. 2001, 37, 594–607. [Google Scholar] [CrossRef]
- Truyen, U.; Gruenberg, A.; Chang, S.-F.; Obermaier, B.; Veijalainen, P.; Parrish, C.R. Evolution of the Feline-Subgroup Parvoviruses and the Control of Canine Host Range In Vivo. J. Virol. 1995, 69, 9. [Google Scholar] [CrossRef] [Green Version]
- Truyen, U.; Evermann, J.F.; Vieler, E.; Parrish, C.R. Evolution of canine parvovirus involved loss and gain of feline host range. Virology 1996, 215, 186–189. [Google Scholar] [CrossRef] [Green Version]
- Kelman, M.; Ward, M.P.; Barrs, V.R.; Norris, J.M. The geographic distribution and financial impact of canine parvovirus in Australia. Transbound. Emerg. Dis. 2019, 66, 299–311. [Google Scholar] [CrossRef] [Green Version]
- Zourkas, E.; Ward, M.P.; Kelman, M. Canine parvovirus in Australia: A comparative study of reported rural and urban cases. Vet. Microbiol. 2015, 181, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Mulley, R.C.; Claxton, P.D.; Feilen, C.P. A serological survey of some infectious diseases in the red fox (Vulpes vulpes) in New South Wales. Proc. 4th Int. Conf. Wildl. Dis. Assoc. 1981, 44–46. [Google Scholar]
- Gabriele-Rivet, V.; Arsenault, J.; Wilhelm, B.; Brookes, V.J.; Newsome, T.M.; Ward, M.P. A Scoping Review of Dingo and Wild-Living Dog Ecology and Biology in Australia to Inform Parameterisation for Disease Spread Modelling. Front. Vet. Sci. 2019, 6, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harriott, L. Prevalence, Risk Factors, and Geographical Distribution of Zoonotic Pathogens Carried by Peri-urban Wild Dogs. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2018. [Google Scholar]
- Jackson, S.M.; Groves, C.P.; Fleming, P.J.S.; Aplin, K.P.; Eldridge, M.D.B.; Gonzalez, A.; Helgen, K.M. The Wayward Dog: Is the Australian native dog or Dingo a distinct species? Zootaxa 2017, 4317, 201. [Google Scholar] [CrossRef] [Green Version]
- Kinnear, J.E.; Sumner, N.R.; Onus, M.L. The red fox in Australia—an exotic predator turned biocontrol agent. Biol. Conserv. 2002, 108, 335–359. [Google Scholar] [CrossRef]
- Glen, A.S.; Dickman, C.R. Complex interactions among mammalian carnivores in Australia, and their implications for wildlife management. Biol. Rev. 2005, 80, 387. [Google Scholar] [CrossRef] [Green Version]
- Brailey, J.; Jenkins, D.; Aghazadeh, M.; Slapeta, J.; Beatty, J.A.; Barrs, V.R. Feral carnivores are reservoirs of Carnivore protoparvovirus 1 in Australia. Ecvim-Ca Congr. St Julian’smalta 2017. [Google Scholar]
- Haynes, S.; Holloway, S. Identification of parvovirus in the bone marrow of eight cats. Aust. Vet. J. 2012, 90, 136–139. [Google Scholar] [CrossRef]
- Van Brussel, K.; Carrai, M.; Lin, C.; Kelman, M.; Setyo, L.; Aberdein, D.; Brailey, J.; Lawler, M.; Maher, S.; Plaganyi, I.; et al. Distinct Lineages of Feline Parvovirus Associated with Epizootic Outbreaks in Australia, New Zealand and the United Arab Emirates. Viruses 2019, 11, 1155. [Google Scholar] [CrossRef] [Green Version]
- Laurenson, K.; Sillero-Zubiri, C.; Thompson, H.; Shiferaw, F.; Thirgood, S.; Malcolm, J. Disease as a threat to endangered species: Ethiopian wolves, domestic dogs and canine pathogens. Anim. Conserv. 1998, 1, 273–280. [Google Scholar] [CrossRef]
- López-Pérez, A.M.; Moreno, K.; Chaves, A.; Ibarra-Cerdeña, C.N.; Rubio, A.; Foley, J.; List, R.; Suzán, G.; Sarmiento, R.E. Carnivore Protoparvovirus 1 at the Wild–Domestic Carnivore Interface in Northwestern Mexico. EcoHealth 2019, 16, 502–511. [Google Scholar] [CrossRef]
- Mainka, S.A.; Xianmeng, Q.; Tingmei, H.; Appel, M.J. Serologic Survey of Giant Pandas (Ailuropoda melanoleuca), and Domestic Dogs and Cats in the Wolong Reserve, China. J. Wildl. Dis. 1994, 30, 86–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodroffe, R.; Prager, K.C.; Munson, L.; Conrad, P.A.; Dubovi, E.J.; Mazet, J.A.K. Contact with Domestic Dogs Increases Pathogen Exposure in Endangered African Wild Dogs (Lycaon pictus). PLoS ONE 2012, 7, e30099. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Jamett, G.; Cunningham, A.A.; Bronsvoort, B.D.; Cleaveland, S. Serosurvey of canine distemper virus and canine parvovirus in wild canids and domestic dogs at the rural interface in the Coquimbo Region, Chile. Eur. J. Wildl. Res. 2015, 61, 329–332. [Google Scholar] [CrossRef]
- Truyen, U.; Müller, T.; Heidrich, R.; Tackmann, K.; Carmichael, L.E. Survey on viral pathogens in wild red foxes (Vulpes vulpes) in Germany with emphasis on parvoviruses and analysis of a DNA sequence from a red fox parvovirus. Epidemiol. Infect. 1998, 121, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Ikeda, Y.; Nakamura, K.; Naito, R.; Mochizuki, M.; Tohya, Y.; Vu, D.; Mikami, T.; Takahashi, E. Isolation of Feline Parvovirus from Peripheral Blood Mononuclear Cells of Cats in Northern Vietnam. Microbiol. Immunol. 1999, 43, 609–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norja, P.; Hokynar, K.; Aaltonen, L.-M.; Chen, R.; Ranki, A.; Partio, E.K.; Kiviluoto, O.; Davidkin, I.; Leivo, T.; Eis-Hubinger, A.M.; et al. Bioportfolio: Lifelong persistence of variant and prototypic erythrovirus DNA genomes in human tissue. Proc. Natl. Acad. Sci. USA 2006, 103, 7450–7453. [Google Scholar] [CrossRef] [Green Version]
- Peters, I.R.; Peeters, D.; Helps, C.R.; Day, M.J. Development and application of multiple internal reference (housekeeper) gene assays for accurate normalisation of canine gene expression studies. Vet. Immunol. Immunopathol. 2007, 117, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Elia, G.; Martella, V.; Desario, C.; Campolo, M.; Trani, L.D.; Tarsitano, E.; Tempesta, M.; Buonavoglia, C. A real-time PCR assay for rapid detection and quantitation of canine parvovirus type 2 in the feces of dogs. Vet. Microbiol. 2005, 105, 19–28. [Google Scholar] [CrossRef]
- Decaro, N.; Desario, C.; Elia, G.; Martella, V.; Mari, V.; Lavazza, A.; Nardi, M.; Buonavoglia, C. Evidence for immunisation failure in vaccinated adult dogs infected with canine parvovirus type 2c. Microbiol. Q. J. Microbiol. Sci. 2008, 31, 125–130. [Google Scholar]
- Kwan, E.; Carrai, M.; Lanave, G.; Hill, J.; Parry, K.; Kelman, M.; Meers, J.; Decaro, N.; Beatty, J.; Martella, V.; et al. Analysis of canine parvoviruses circulating in Australia reveals predominance of variant 2b strains and identifies feline parvovirus-like mutations in the capsid proteins. Transbound. Emerg. Dis. 2020. under review. [Google Scholar]
- Ward, M.P.; Kelman, M. Companion animal disease surveillance: A new solution to an old problem? Spat. Spatio-Temporal Epidemiol. 2011, 2, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics 1270.0.55.003—Australian Statistical Geography Standard (ASGS): Volume 3—Non ABS Structures, July 2016. Available online: https://www.abs.gov.au/AUSSTATS/[email protected]/DetailsPage/1270.0.55.003July%202016?OpenDocument (accessed on 18 June 2020).
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meers, J.; Kyaw-Tanner, M.; Bensink, Z.; Zwijnenberg, R. Genetic analysis of canine parvovirus from dogs in Australia. Aust. Vet. J. 2007, 85, 392–396. [Google Scholar] [CrossRef]
- Wang, J.; Lin, P.; Zhao, H.; Cheng, Y.; Jiang, Z.; Zhu, H.; Wu, H.; Cheng, S. Continuing evolution of canine parvovirus in China: Isolation of novel variants with an Ala5Gly mutation in the VP2 protein. Infect. Genet. Evol. 2016, 38, 73–78. [Google Scholar] [CrossRef]
- Frölich, K.; Streich, W.J.; Fickel, J.; Jung, S.; Truyen, U.; Hentschke, J.; Dedek, J.; Prager, D.; Latz, N. Epizootiologic Investigations of Parvovirus Infections in Free-ranging Carnivores from Germany. J. Wildl. Dis. 2005, 41, 231–235. [Google Scholar] [CrossRef]
- Knobel, D.L.; Butler, J.R.A.; Lembo, T.; Critchlow, R.; Gompper, M.E. Dogs, disease, and wildlife. In Free-Ranging Dogs and Wildlife Conservation; Gompper, M.E., Ed.; Oxford University Press: Oxford, UK, 2013; pp. 144–169. ISBN 978-0-19-966321-7. [Google Scholar]
- Nelson, B.; Hebblewhite, M.; Ezenwa, V.; Shury, T.; Merrill, E.H.; Paquet, P.C.; Schmiegelow, F.; Seip, D.; Skinner, G.; Webb, N. Prevalence of antibodies to canine parvovirus and distemper virus in wolves in the Canadian Rocky Mountains. J. Wildl. Dis. 2012, 48, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Courtenay, O.; Quinnell, R.J.; Chalmers, W.S.K. Contact rates between wild and domestic canids: no evidence of parvovirus or canine distemper virus in crab-eating foxes. Vet. Microbiol. 2001, 81, 9–19. [Google Scholar] [CrossRef]
- Houston, D.M.; Ribble, C.S.; Head, L.L. Risk factors associated with parvovirus enteritis in dogs: 283 cases (1982-1991). J. Am. Vet. Med. Assoc. 1996, 208, 542. [Google Scholar]
- Ling, M.; Norris, J.M.; Kelman, M.; Ward, M.P. Risk factors for death from canine parvoviral-related disease in Australia. Vet. Microbiol. 2012, 158, 280–290. [Google Scholar] [CrossRef]
- Mech, L.D.; Goyal, S.M.; Paul, W.J.; Newton, W.E. Demographic effects of canine parvovirus on a free-ranging wolf population over 30 years. J. Wildl. Dis. 2008, 44, 824–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeill, A.; Leung, L.; Goullet, M.; Gentle, M.; Allen, B. Dingoes at the Doorstep: Home Range Sizes and Activity Patterns of Dingoes and Other Wild Dogs around Urban Areas of North-Eastern Australia. Animals 2016, 6, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, B.L.; Goullet, M.; Allen, L.R.; Lisle, A.; Leung, L.K.-P. Dingoes at the doorstep: Preliminary data on the ecology of dingoes in urban areas. Landsc. Urban Plan. 2013, 119, 131–135. [Google Scholar] [CrossRef] [Green Version]
- Gordon, J.C.; Angrick, E.J. Canine parvovirus: environmental effects on infectivity. Am. J. Vet. Res. 1986, 47, 1464. [Google Scholar]
- Almberg, E.S.; Mech, L.D.; Smith, D.W.; Sheldon, J.W.; Crabtree, R.L. A Serological Survey of Infectious Disease in Yellowstone National Park’s Canid Community. PLoS ONE 2009, 4, e7042. [Google Scholar] [CrossRef]
- Santos, N.; Almendra, C.; Tavares, L. Serologic Survey for Canine Distemper Virus and Canine Parvovirus in Free-ranging Wild Carnivores from Portugal. J. Wildl. Dis. 2009, 45, 221–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobrino, R.; Arnal, M.C.; Luco, D.F.; Gortázar, C. Prevalence of antibodies against canine distemper virus and canine parvovirus among foxes and wolves from Spain. Vet. Microbiol. 2008, 126, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Assay | Primer/Probe | Sequence 5’ - 3’ | Polarity | Amplicon Size (bp) | Position † |
|---|---|---|---|---|---|
| Real Time Assay | CPV-For | AAACAGGAATTAACTATACTAATATATTTA | + | 93 | 4104–4135 |
| CPV-Rev | AAATTTGACCATTTGGATAAACT | − | 4176–4198 | ||
| CPV-Pb | FAM—TGGTCCTTTAACTGCATTAAATAATGTACC—TAMRA | + | 4143–4172 |
| Category | Variable | No. Dogs Sampled | Percentage | No. Dogs Negative | No. Dogs Positive | Chi2 | DF | p-Value |
|---|---|---|---|---|---|---|---|---|
| State | QLD | 146 | 85.4 | 136 | 8 | 1.05 | 1 | 0.3051 |
| NSW | 18 | 10.5 | 18 | 0 | ||||
| NR | 7 | 4.1 | ||||||
| Total | 171 | |||||||
| Sex | Male | 76 | 44.4 | 68 | 4 | 0.08 | 1 | 0.7833 |
| Female | 87 | 50.9 | 83 | 4 | ||||
| NR | 8 | 4.7 | ||||||
| Total | 171 | |||||||
| Age | <6 months | 44 | 25.7 | 42 | 2 | 3.86 | 4 | 0.425 |
| 6–12 months | 44 | 25.7 | 40 | 3 | ||||
| 1–2 years | 34 | 19.9 | 33 | 0 | ||||
| 2–5 years | 14 | 8.2 | 13 | 1 | ||||
| >5 years | 17 | 9.9 | 14 | 2 | ||||
| NR | 18 | 10.5 | ||||||
| Total | 171 | |||||||
| Year captured | 2012 | 2 | 1.2 | 2 | 0 | 0.91 | 3 | 0.8227 |
| 2013 | 63 | 36.8 | 58 | 4 | ||||
| 2014 | 90 | 52.6 | 85 | 4 | ||||
| 2015 | 9 | 5.3 | 9 | 0 | ||||
| NR | 7 | 4.1 | ||||||
| Total | 171 |
| Location Captured | ||||||||
|---|---|---|---|---|---|---|---|---|
| GenBank Accession No. | Sample ID | Sex | Age | Date Captured | Region | Postcode | State Locality | Viral Copies per µL/DNA |
| MT447094 | WD29 | M | <6 months | 25/11/13 | Woodford | 4514 | South East QLD | 2.89 × 103 |
| MT447095 | WD46 | M | 6–12 months | 28/2/14 | The Gap | 4061 | Brisbane QLD | 2.05 × 104 |
| MT447096 | WD48 | F | >5 years | 5/2/13 | Tamborine | 4270 | South East QLD | 1.95 × 107 |
| MT447097 | WD49 | M | >5 years | 15/5/14 | Nerang | 4211 | Gold Coast QLD | 1.57 × 106 |
| MT447098 | WD50 | M | 2–5 years | 24/8/13 | Beenleigh | 4207 | South East QLD | 6.56 × 105 |
| MT447099 | WD51 | F | <6 months | 27/11/13 | Ipswich | 4305 | South East QLD | 2.19 × 104 |
| NA | WD58 | F | 6–12 months | 2/3/14 | Goomeri | 4601 | South East QLD | 4.08 × 101 |
| NA | WD59 | F | 6–12 months | 26/2/14 | The Gap | 4061 | Brisbane QLD | 3.41 × 101 |
| Domestic-Dog CPV Cases | Domestic-Dog CPV Case Occurrence | Wild-Dog CPV Status | ||||
|---|---|---|---|---|---|---|
| Positive | Negative | Total | Odds Ratio | p-value | ||
| In all years between 2009 and 2015 | Present | 5 | 14 | 19 | 6.43 | 0.0350 |
| Absent | 2 | 36 | 38 | |||
| Total | 7 | 50 | 57 | |||
| In same year as wild-dog sampling in postcode | Present | 3 | 4 | 7 | 8.63 | 0.0332 |
| Absent | 4 | 46 | 50 | |||
| Total | 7 | 50 | 57 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelman, M.; Harriott, L.; Carrai, M.; Kwan, E.; Ward, M.P.; Barrs, V.R. Phylogenetic and Geospatial Evidence of Canine Parvovirus Transmission between Wild Dogs and Domestic Dogs at the Urban Fringe in Australia. Viruses 2020, 12, 663. https://0-doi-org.brum.beds.ac.uk/10.3390/v12060663
Kelman M, Harriott L, Carrai M, Kwan E, Ward MP, Barrs VR. Phylogenetic and Geospatial Evidence of Canine Parvovirus Transmission between Wild Dogs and Domestic Dogs at the Urban Fringe in Australia. Viruses. 2020; 12(6):663. https://0-doi-org.brum.beds.ac.uk/10.3390/v12060663
Chicago/Turabian StyleKelman, Mark, Lana Harriott, Maura Carrai, Emily Kwan, Michael P. Ward, and Vanessa R. Barrs. 2020. "Phylogenetic and Geospatial Evidence of Canine Parvovirus Transmission between Wild Dogs and Domestic Dogs at the Urban Fringe in Australia" Viruses 12, no. 6: 663. https://0-doi-org.brum.beds.ac.uk/10.3390/v12060663






