Avian Influenza Virus Prevalence and Subtype Diversity in Wild Birds in Shanghai, China, 2016–2018
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement and Biosafety
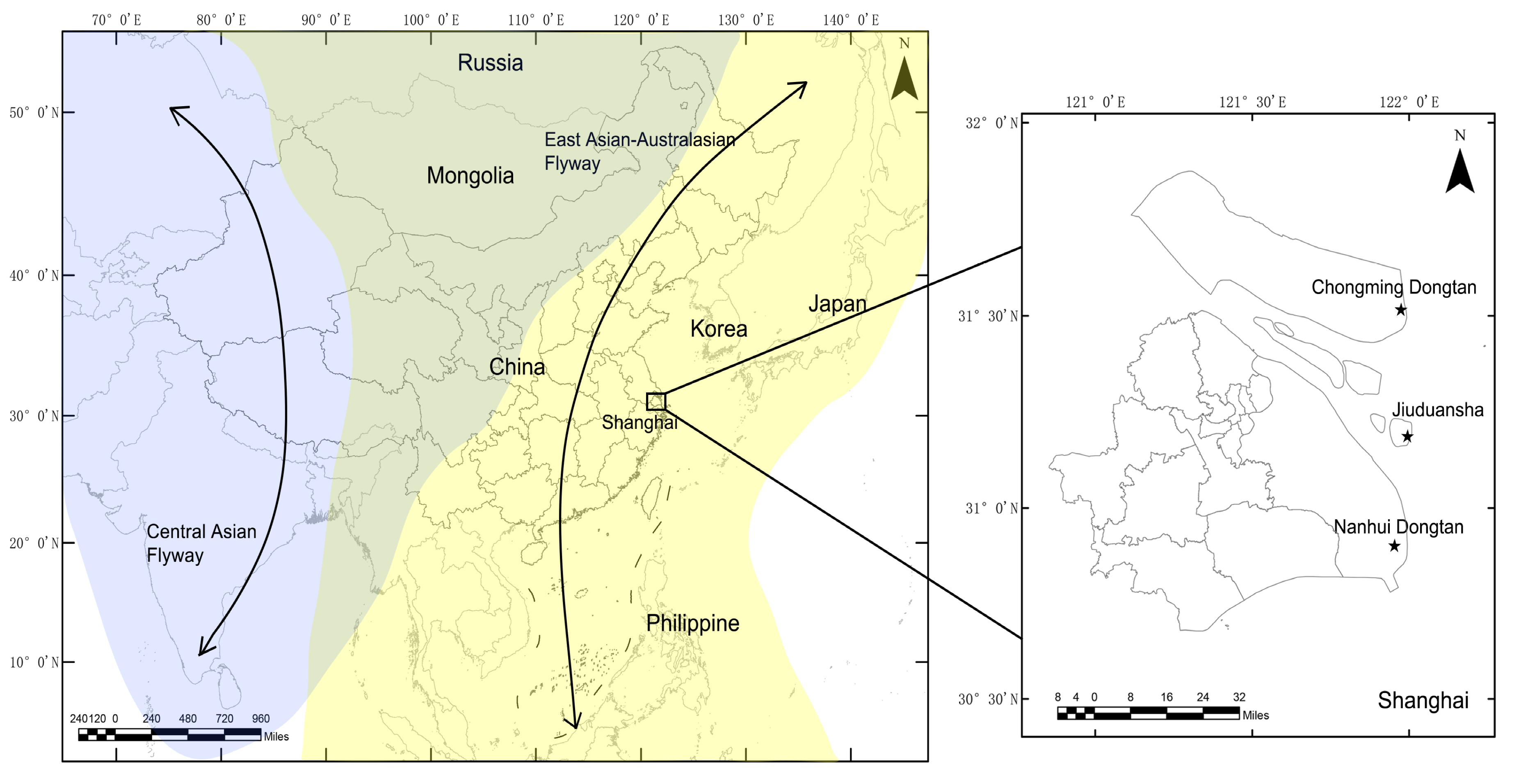
2.2. Sampling Sites
2.3. Sample Collection
2.4. Virus Detection
2.5. Sequence Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Sampling Overview
3.2. Prevalence Overview
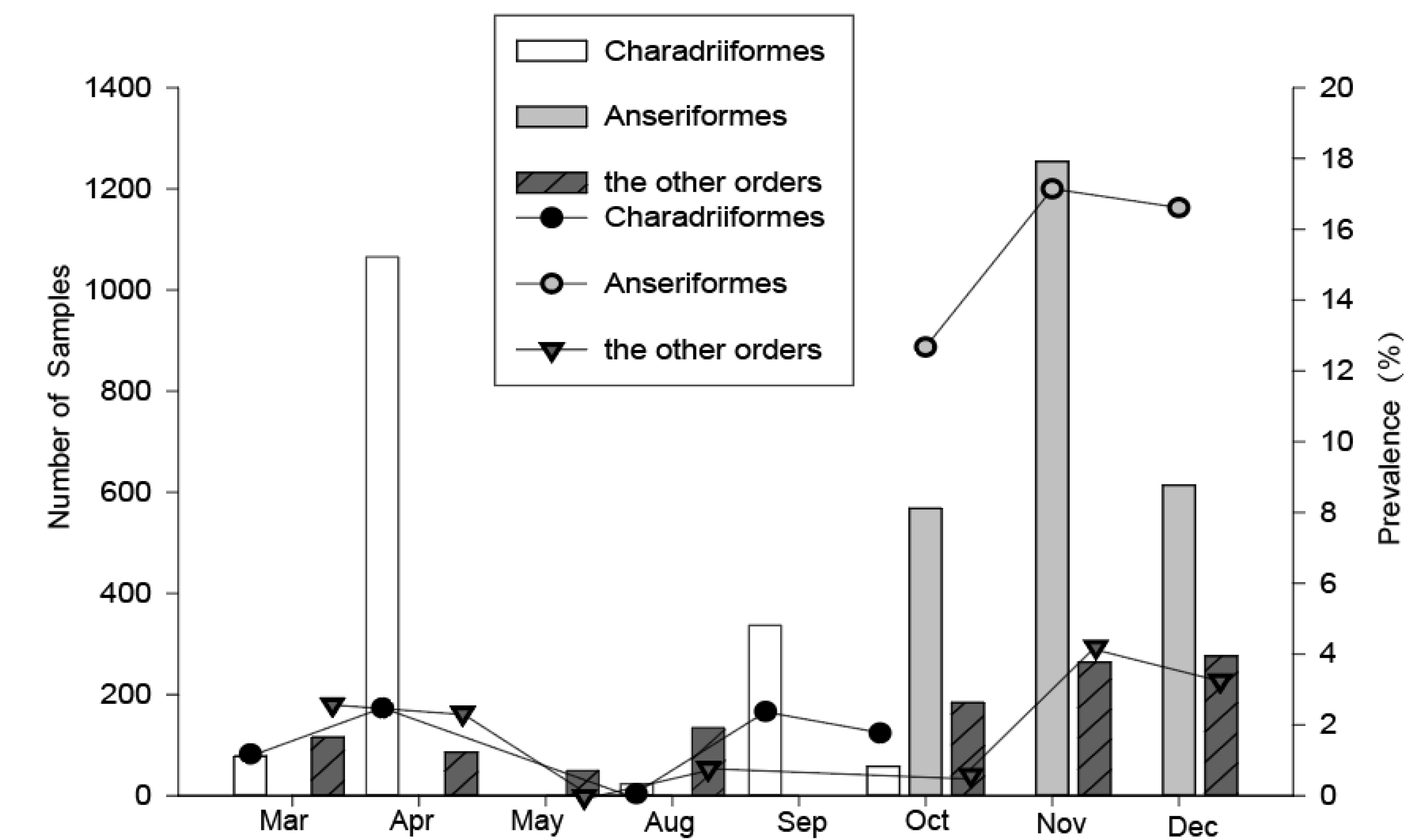
3.3. Seasonal Variation
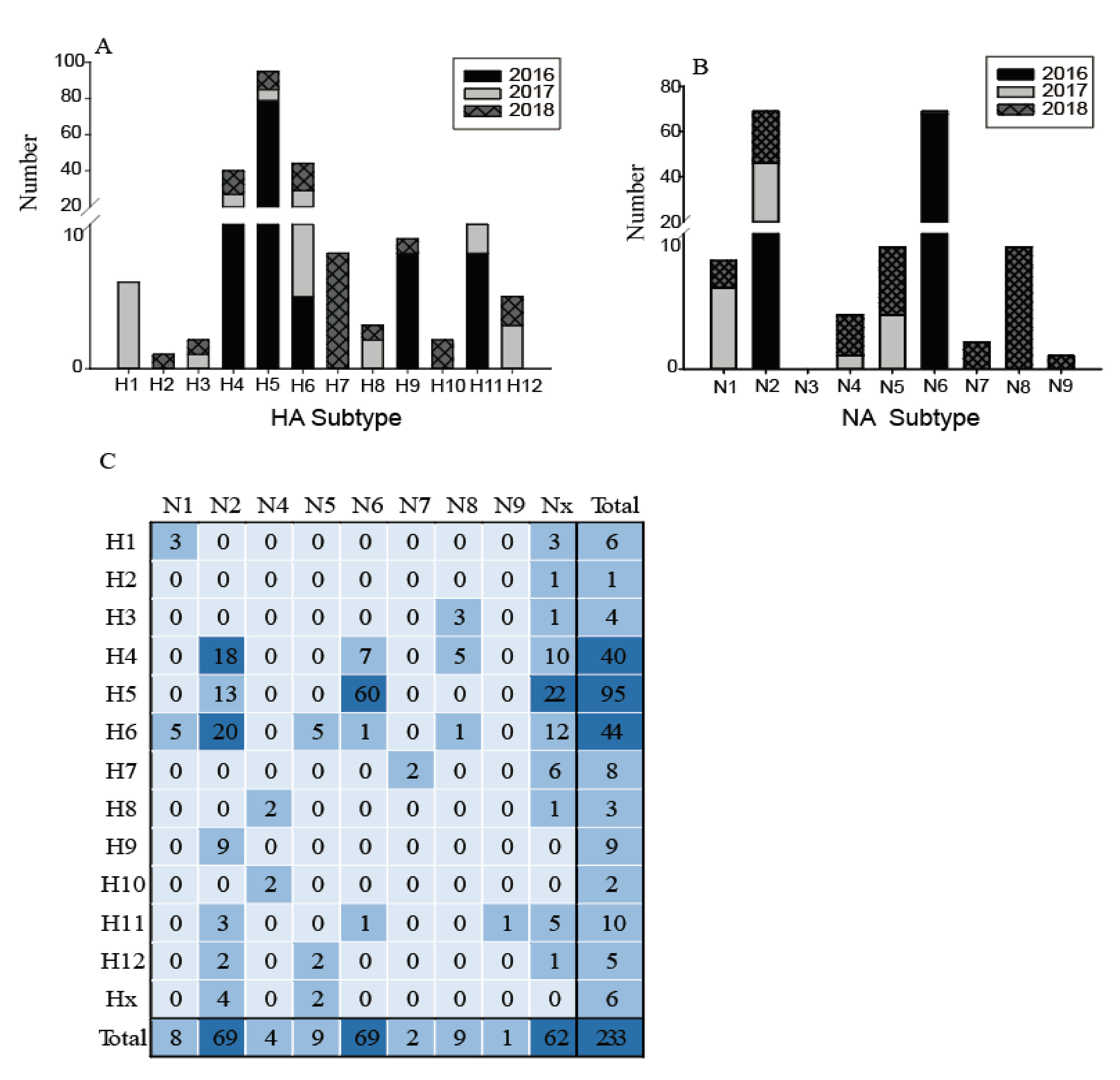
3.4. Subtype Diversity
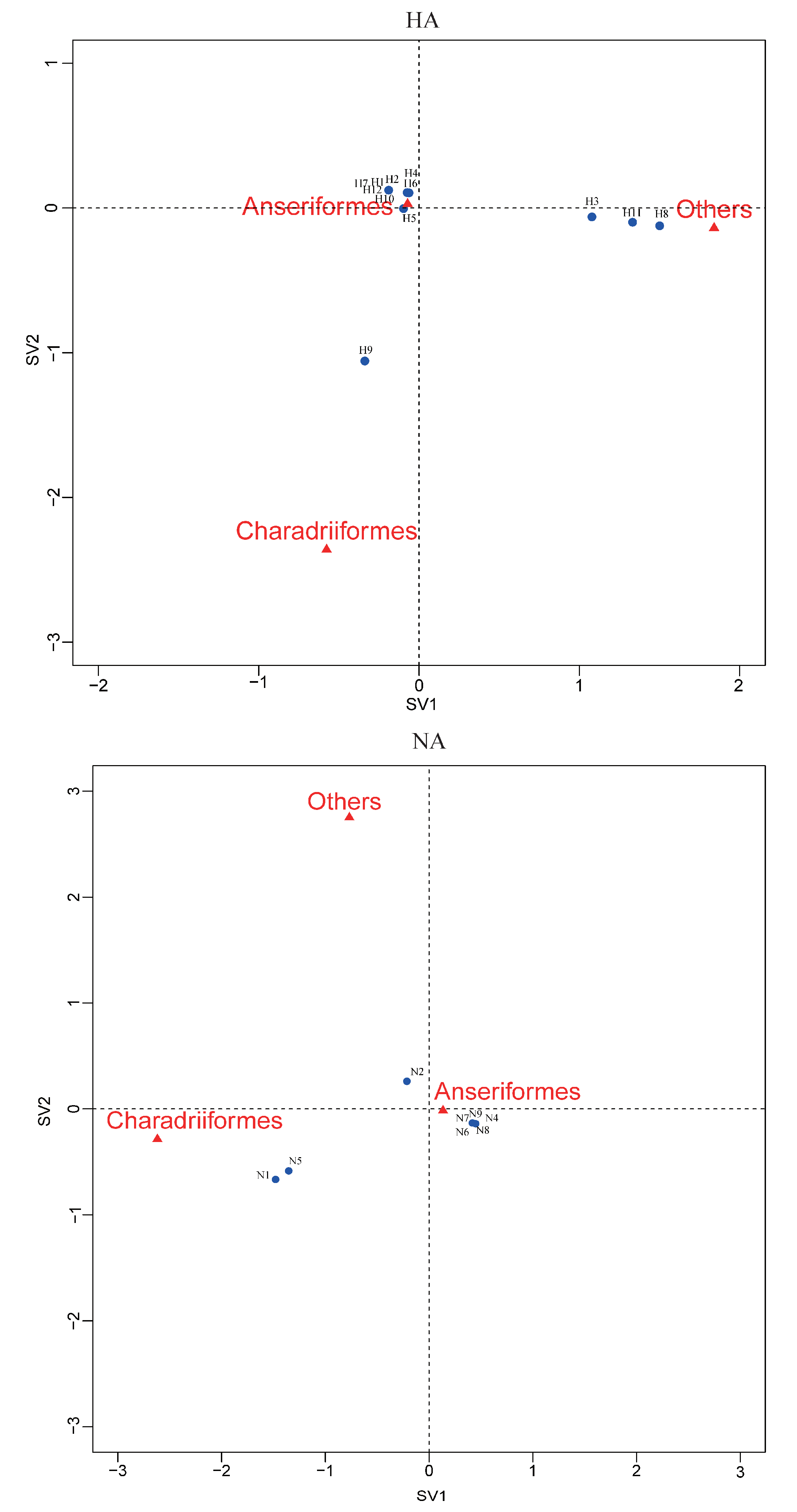
3.5. Species Differences
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olsen, B.; Munster, V.J.; Wallensten, A.; Waldenström, J.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Global patterns of influenza a virus in wild birds. Science 2006, 312, 384–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, R.G.; Yakhno, M.; Hinshaw, V.S.; Bean, W.J.; Copal Murti, K. Intestinal influenza: Replication and characterization of influenza viruses in ducks. Virology 1978, 84, 268–278. [Google Scholar] [CrossRef]
- Gaidet, N.; Cappelle, J.; Takekawa, J.Y.; Prosser, D.J.; Iverson, S.A.; Douglas, D.C.; Perry, W.M.; Mundkur, T.; Newman, S.H. Potential spread of highly pathogenic avian influenza H5N1 by wildfowl: Dispersal ranges and rates determined from large-scale satellite telemetry. J. Appl. Ecol. 2010, 47, 1147–1157. [Google Scholar] [CrossRef]
- Tian, H.; Zhou, S.; Dong, L.; Van Boeckel, T.M.; Cui, Y.; Newman, S.H.; Takekawa, J.Y.; Prosser, D.J.; Xiao, X.; Wu, Y.; et al. Avian influenza H5N1 viral and bird migration networks in Asia. Proc. Natl. Acad. Sci. USA 2015, 112, 172–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, D.L.; Liu, Y.; Low, B.W.; Espanola, C.P.; Choi, C.; Kawakami, K. Migratory songbirds in the East Asian-Australasian Flyway: A review from a conservation perspective. Bird Conserv. Int. 2015, 25, 1–37. [Google Scholar] [CrossRef]
- Cai, Y.; Tang, S.; Yuan, X.; Wang, J.; Ma, Z. Checklist and change of birds in Shanghai. J. Fudan Univ. (Nat. Sci.) 2011, 50, 334–343. [Google Scholar]
- Latorre-Margalef, N.; Tolf, C.; Grosbois, V.; Avril, A.; Bengtsson, D.; Wille, M.; Osterhaus, A.D.M.E.; Fouchier, R.A.M.; Olsen, B.J.; Waldenström, J. Long-term variation in influenza A virus prevalence and subtype diversity in migratory mallards in northern Europe. Proc. R. Soc. B 2014, 281, 20140098. [Google Scholar] [CrossRef] [Green Version]
- Bevins, S.N.; Pedersen, K.; Lutman, M.W.; Baroch, J.A.; Schmit, B.S.; Kohler, D.; Gidlewski, T.; Nolte, D.L.; Swafford, S.R.; Deliberto, T.J. Large-scale avian influenza surveillance in wild birds throughout the United States. PLoS ONE 2014, 9, e104360. [Google Scholar] [CrossRef] [Green Version]
- Hansbro, P.M.; Warner, S.; Tracey, J.P.; Edla Arzey, K.; Selleck, P.; O’Riley, K.; Beckett, E.L.; Bunn, C.; Kirkland, P.D.; Vijaykrishna, D.; et al. Surveillance and analysis of avian influenza viruses, Australia. Emerg. Infect. Dis. 2010, 16, 1896–1904. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, X.; Zhu, W.; Teng, Z.; Yu, X.; Gao, Y.; Wu, D.; Pei, E.; Yuan, Z.; Yang, L.; et al. Novel avian influenza A (H7N9) virus in tree sparrow, Shanghai, China, 2013. Emerg. Infect. Dis. 2013, 20, 850–853. [Google Scholar] [CrossRef]
- Ma, Z.; Tang, S.; Lu, F.; Chen, J.K. Chongming island: A less important shorebird stopover site during southward migration? Stilt 2002, 41, 35–37. [Google Scholar]
- Zheng, S.; Choi, C.; Gan, X.; Ma, Z.; Tang, S.M.; Zhu, J. Shorebird numbers at the Jiuduansha wetlands during the 2005 southward migration. Stilt 2006, 50, 58–61. [Google Scholar]
- Huang, Y.; Khan, M.I.; Măndoiu, I. Neuraminidase Subtyping of Avian Influenza viruses with PrimerHunter-Designed Primers and Quadruplicate primer Pools. PLoS ONE 2013, 8, e81842. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Ashizawa, H.; Nakanishi, K.; Kaji, N.; Suzuki, K.; Okamatsu, M.; Yamaguchi, S.; Mase, M. Subtyping of avian influenza viruses H1 to H15 on the basis of hemagglutinin genes by PCR assay and molecular determination of pathogenic potential. J. Clin. Microbiol. 2008, 46, 3048–3055. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Chai, H.; Fan, Z.; Wang, X.; Yao, Q.; Ma, J.; Chen, S.; Hua, Y.; Deng, G.; Chen, H. New H6 influenza virus reassortment strains isolated from Anser fabalis in Anhui Province, China. Virol. J. 2017, 14, 36. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Xiong, C.; Chen, J.; Chen, G.; Zhang, J.; Li, Y.; Xiong, Y.; Wang, R.L.; Cao, Y.; Chen, Q.; et al. Two genetically diverse H7N7 avian influenza viruses isolated from migratory birds in central China. Emerg. Microbes Infect. 2018, 7, 1–12. [Google Scholar] [CrossRef]
- Chen, L.; Lin, X.; Guo, W.; Tian, J.; Wang, W.; Ying, X.; Wang, M.; Yu, B.; Yang, Z.; Shi, M.; et al. Diversity and evolution of avian influenza viruses in live poultry markets, free-range poultry and wild wetland birds in China. J. Gener. Virol. 2016, 97, 844–854. [Google Scholar] [CrossRef]
- Lee, C.W.; Suarez, D.L. Application of real-time RT-PCR for the quantitation and competitive replication study of H5 and H7 subtype avian influenza virus. J. Virol. Methods 2004, 119, 151–158. [Google Scholar] [CrossRef]
- He, G.; Zhou, L.; Zhu, C.; Shi, H.; Li, X.; Wu, D.; Liu, J.; Lv, J.; Hu, C.; Li, Z.; et al. Identification of two novel avian influenza A (H5N6) viruses in wild birds, Shanghai, in 2016. Vet. Microbiol. 2017, 28, 53–57. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, J.; Pei, E.; Xue, W.; Lyu, J.; Cai, Y.; Wu, D.; Wu, W.; Liu, Y.; Jin, H.; et al. Novel avian influenza A (H5N8) viruses in migratory birds, China, 2013–2014. Emerg. Infect. Dis. 2016, 22, 1121. [Google Scholar] [CrossRef] [Green Version]
- Grillo, V.; Arzey, K.; Hansbro, P.; Hurt, A.; Warner, S.; Bergfeld, J.; Burgess, G.W.; Cookson, B.; Dickason, C.J.; Ferenczi, M.; et al. Avian influenza in Australia: A summary of 5 years of wild bird surveillance. Aust. Vet. J. 2015, 93, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Gan, X.; Choi, C.; Jing, K.; Tang, S.; Li, B.; Chen, J. Watering bird communities in newly-formed wetland in the Yangtze River estuary. Ecol. Res. 2007, 22, 115–124. [Google Scholar] [CrossRef]
- Brochet, A.; Guillemain, M.; Lebarbenchon, C.; Simon, G.; Fritz, H.; Green, A.J.; Renaud, F.; Thomas, F.; Gauthier-Clerc, M. The potential distance of highly pathogenic avian influenza virus dispersal by mallard, common teal and Eurasian pochard. Ecohealth 2009, 6, 449–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Li, X.; Hu, C.; Zhu, C.; Li, A.; Wu, D.; Wang, T.; He, G. Isolation of H8N4 avian influenza virus from wild birds in Shanghai, China. Acta Virol. 2019, 63, 121–125. [Google Scholar] [CrossRef]
- Hu, C.; Li, X.; Zhu, C.; Zhou, F.; Tang, W.; Wu, D.; Li, Z.; Zhou, L.; Liu, J.; Wei, X.; et al. Co-circulation of multiple reassortant H6 subtype avian influenza viruses in wild birds in eastern China, 2016–2017. Virol. J. 2020, 17, 62. [Google Scholar] [CrossRef]
| Sampling Sites † | Orders | 2016 | 2017 | 2018 | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Samples | No. of Positive | Prevalence (95% CI, %) | No. of Samples | No. of Positive | Prevalence (95% CI, %) | No. of Samples | No. of Positive | Prevalence (95% CI, %) | No. of Samples | No. of Positive | Prevalence (95% CI, %) | ||
| CM | Anseriformes ‡ | 114 | 30 | 26.3 (17.8–34.8) | 114 | 30 | 26.3 (17.8–34.8) | ||||||
| Charadriiformes | 336 | 7 | 2.1 (0.4–3.8) | 519 | 12 | 2.3 (0.9–3.7) | 709 | 17 | 2.4 (1.2–3.6) | 1564 | 36 | 2.3 (2.1–2.4) | |
| Other orders | 584 | 10 | 1.7 (0.6–2.9) | 460 | 17 | 3.7 (1.9–5.5) | 68 | 0 | 0.0 (0.0–5.3) | 1112 | 27 | 2.4 (0.0–3.9) | |
| Total | 920 | 17 | 1.8 (1.6–2.2) | 979 | 29 | 3.0 (1.9–4.1) | 891 | 47 | 5.3 (0.0–26.0) | 2790 | 93 | 3.3 (1.4–5.4) | |
| JDS | Anseriformes | 219 | 45 | 20.5 (15.0–26.1) | 240 | 42 | 17.5 (12.5–22.5) | 403 | 65 | 16.1 (12.4–19.8) | 862 | 152 | 17.6 (15.5–20.6) |
| NH | Anseriformes | 483 | 60 | 12.4 (9.4–15.5) | 430 | 84 | 19.5 (15.7–23.4) | 547 | 63 | 11.5 (8.8–14.3) | 1460 | 207 | 14.2 (9.5–19.4) |
| Total | 1622 | 122 | 7.5 (1.0–22.2) | 1649 | 155 | 9.4 (3.1–23.5) | 1841 | 175 | 9.5 (4.8–17.1) | 5112 | 452 | 8.8 (7.5–10.1) | |
| Orders. | Common Name | Scientific Name | No. Samples Collected in 2016–2018 | No. AIV Positive | Prevalence (95% CI, %) | HA Subtypes (n) | NA Subtypes (n) | Shannon Entropy Values of the Subtype Diversity (HA, NA) |
|---|---|---|---|---|---|---|---|---|
| Anseriformes | Common teal | Anas crecca | 1852 | 283 | 15.3 (13.6–16.9) | H1 (6), H2 (1), H3(1), H4 (25), H5 (71), H6 (29), H7 (8), H8 (2), H9 (3), H10 (1), H11 (6), H12 (3) | N1 (6), N2 (35), N4 (3), N5 (5), N6 (57), N7(2), N8 (8), N9 (1) | 0.73, 0.60 |
| Spot-billed duck | Anas poecilorhyncha | 160 | 42 | 26.3 (19.1–33.4) | H4 (13), H5 (9), H6 (7) | N2 (10), N5 (1) | 0.46, 0.13 | |
| Northern pintail | Anas acuta | 50 | 5 | 10.0 (0.4–19.3) | H3 (1), H9 (1) | N2 (2) | 0.30, 0 | |
| Mallard | Anas platyrhynchos | 62 | 15 | 24.2 (12.7–35.7) | H3 (1), H5 (2), H10 (1), H12 (2) | N4 (1), N6 (3) | 0.58, 0.24 | |
| Eurasian wigeon | Anas penelope | 74 | 9 | 12.2 (4.0–20.3) | H5 (4), H6 (1), H11(1) | N2 (3), N6 (3) | 0.38, 0.30 | |
| Northern shoveler | Anas clypeata | 116 | 17 | 14.7 (7.8–21.5) | H4 (1), H5 (4), H6 (4), H9 (2) | N2 (5), N6 (5) | 0.55, 0.30 | |
| Common pochard | Aythya ferina | 28 | 4 | 14.3 (0.0–29.0) | H5 (1) | N2 (1) | 0, 0 | |
| Falcated teal | Anas falcata | 34 | 6 | 17.7 (3.4–31.9) | H6 (1), H9 (2) | N2(2) | 0.28, 0 | |
| Gadwall | Anas strepera | 26 | 2 | 7.7 (0.0–19.9) | ||||
| Mandarin | Aix galericulata | 32 | 6 | 18.8 (3.7–33.8) | H5 (1), H6 (1) | N2 (4), N5 (1), N6 (1) | 0.30, 0.38 | |
| Tufted duck | Aythya fuligula | 2 | ||||||
| Charadriiformes | Great knot | Calidris tenuirostris | 930 | 22 | 2.4 (1.3–3.4) | N1 (2), N2 (2), N5 (2) | 0, 0.48 | |
| Red knot | Calidris canutus | 124 | 4 | 3.2 (0.0–6.7) | N2 (1) | -, 0 | ||
| Dunlin | Calidris alpina | 114 | 1 | 0.9 (0.0–3.0) | H5 (1) | - | 0, - | |
| Terek sandpiper | Xenus cinereus | 86 | 1 | 1.2 (0.0–4.0) | H9 (1) | N2 (1) | 0.55, - | |
| Sharp-tailed Sandpiper | Calidris acuminata | 22 | 1 | 4.6 (0.0–15.5) | ||||
| Bar-tailed godwit | Limosa lapponica | 80 | 1 | 1.3 (0.0–4.3) | ||||
| Ruddy turnstone | Arenaria interpres | 24 | 2 | 8.3 (0.0–21.5) | ||||
| Whimbrel | Numenius phaeopus | 26 | 4 | 15.4 (0.0–31.2) | ||||
| Common snipe | Gallinago gallinago | 22 | ||||||
| Red-necked stint | Calidris ruficollis | 30 | ||||||
| Greenshank | Tringa nebularia | 24 | ||||||
| Sanderling | Calidris alba | 2 | ||||||
| Wood sandpiper | Tringa glareola | 18 | ||||||
| Golden plover | Pluvialis dominica | 14 | ||||||
| Mongolian plover | Charadrius mongolus | 6 | ||||||
| Calidris Ferruginea | Calidris ferruginea | 4 | ||||||
| Grey plover | Pluvialis squatarola | 20 | ||||||
| Crake | Metopidius indicus | 18 | ||||||
| Gruiformes | Peale | Fulica atra | 106 | 14 | 13.2 (6.3–20.1) | H3 (1), H5 (2), H8 (1), H11 (3) | N2 (3) | |
| Common moorhen | Gallinula chloropus | 82 | 7 | 8.5 (1.9–15.2) | H6 (1) | |||
| Galliformes | Pheasant | Phasianus colchicus | 2 | |||||
| Ciconiiformes | Spotted redshank | Tringa erythropus | 12 | |||||
| Little egret | Egretta | 110 | 5 | 4.6 (0.2–8.9) | H4 (1) | N8 (1) | ||
| Green-backed heron | Butorides striatus | 16 | ||||||
| Night heron | Nycticorax nycticorax | 14 | ||||||
| Podicipediformes | Little grebe | Tachybaptus ruficollis | 2 | |||||
| Strigiformes | Oriental scops owl | Otus sunia | 2 | |||||
| Columbiformes | Bead neck dove | Streptopelia chinensis | 118 | |||||
| Oriental turtle-dove | Streptopelia orientalis | 2 | ||||||
| Upupiformes | Eurasian hoopoe | Upupa epops | 2 | |||||
| Passeriformes | Yellow wagtai | Motacilla flava | 244 | |||||
| Gray wagtail | Motacilla cinerea | 30 | ||||||
| White wagtail | Motacilla alba | 4 | ||||||
| Chinese bulbul | Pycnonotus sinensis | 90 | 1 | 1.1 (0.0–3.8) | ||||
| Tree sparrow | Passer montanus | 148 | ||||||
| Blackbird | Turdus merula sowerbyi | 92 | ||||||
| Grey-backed thrush | Turdus hortulorum | 12 | ||||||
| Black-tailed hawfinch | Eophona migratoria | 12 | ||||||
| Crested myna | Acridotheres cristatellus | 6 | ||||||
| Long-tailed shrike | Lanius schach | 6 | ||||||
| Total | 5112 | 452 | 8.8 (8.1–9.6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Tang, W.; Li, X.; Hu, C.; Wu, D.; Wang, T.; He, G. Avian Influenza Virus Prevalence and Subtype Diversity in Wild Birds in Shanghai, China, 2016–2018. Viruses 2020, 12, 1031. https://0-doi-org.brum.beds.ac.uk/10.3390/v12091031
Tang L, Tang W, Li X, Hu C, Wu D, Wang T, He G. Avian Influenza Virus Prevalence and Subtype Diversity in Wild Birds in Shanghai, China, 2016–2018. Viruses. 2020; 12(9):1031. https://0-doi-org.brum.beds.ac.uk/10.3390/v12091031
Chicago/Turabian StyleTang, Ling, Wangjun Tang, Xiaofang Li, Chuanxia Hu, Di Wu, Tianhou Wang, and Guimei He. 2020. "Avian Influenza Virus Prevalence and Subtype Diversity in Wild Birds in Shanghai, China, 2016–2018" Viruses 12, no. 9: 1031. https://0-doi-org.brum.beds.ac.uk/10.3390/v12091031





