Spatiotemporal Dynamics of Highly Pathogenic Avian Influenza Subtype H5N8 in Poultry Farms, South Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection and Management
2.2. Spatiotemporal Analysis
3. Results
3.1. Descriptive Analysis
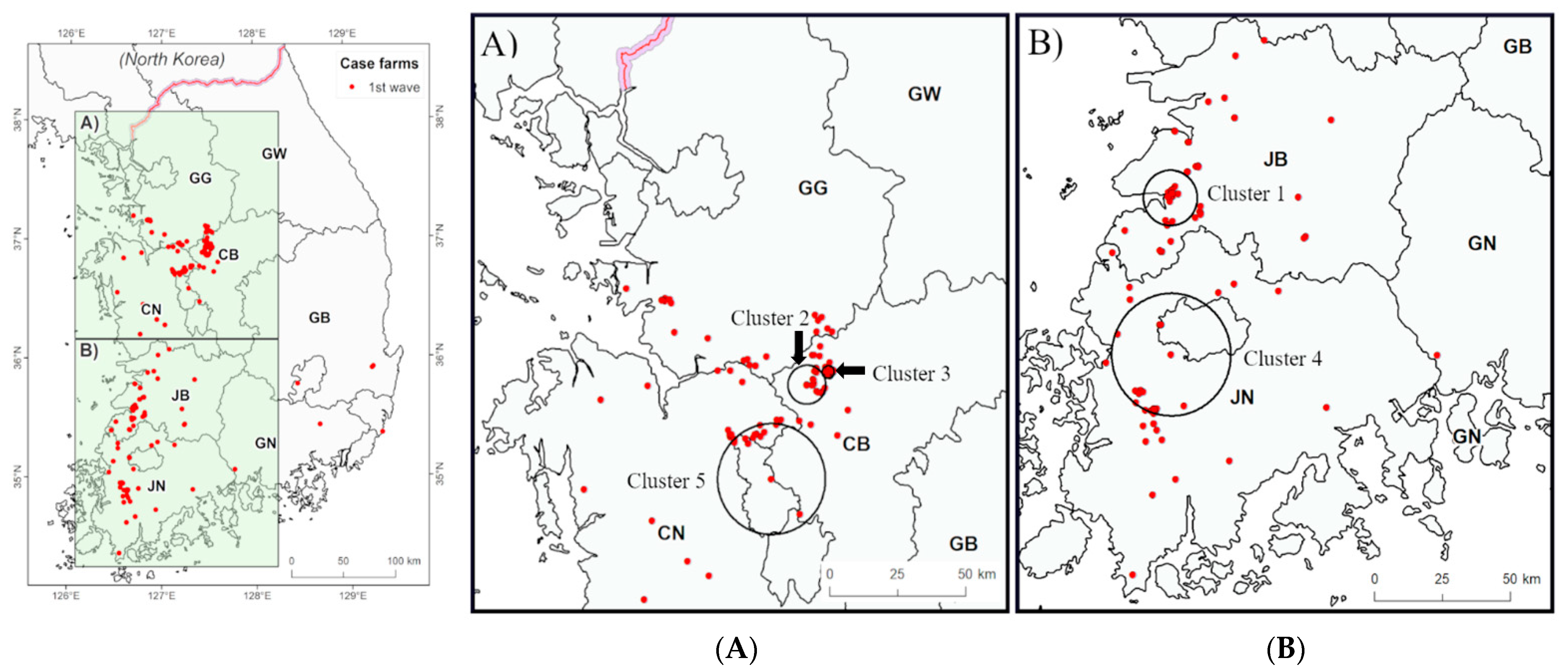
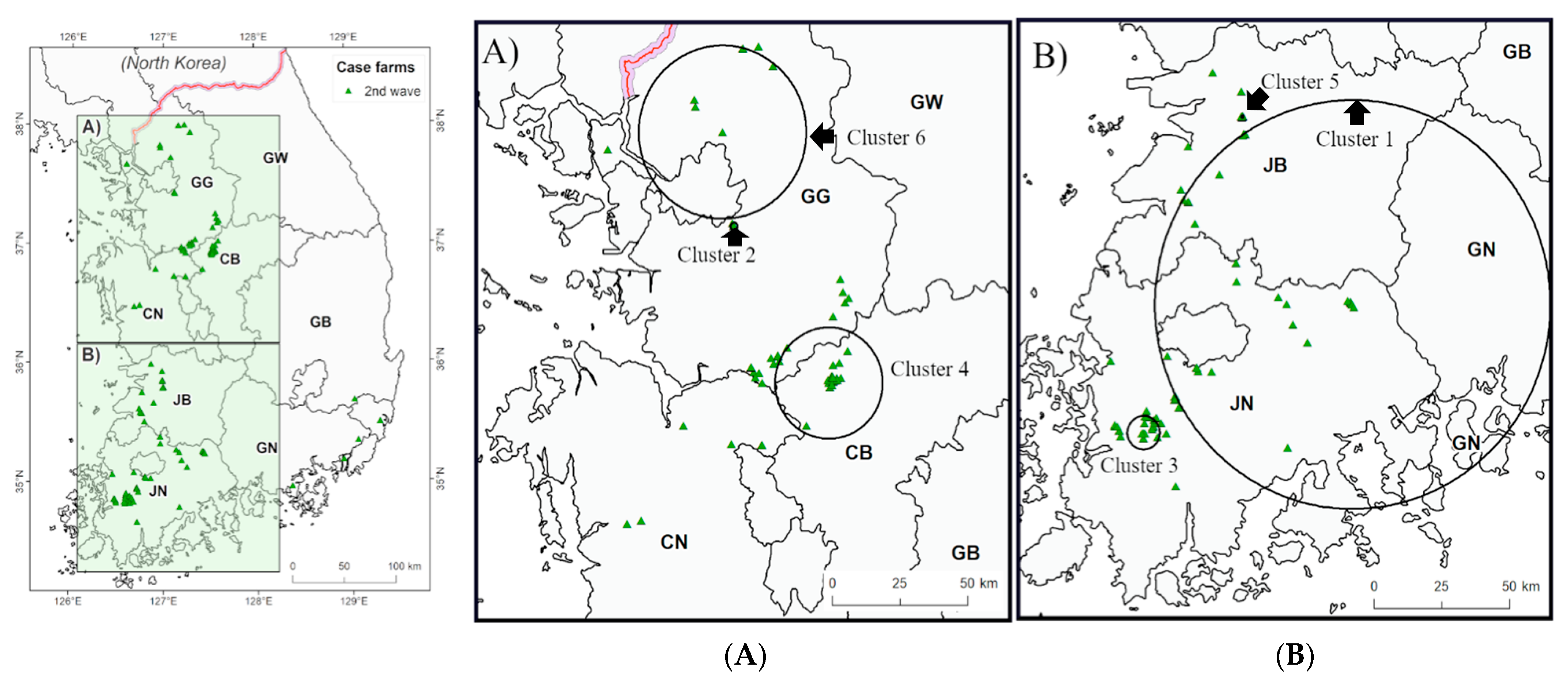
3.2. Spatiotemporal Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexander, D.J. An overview of the epidemiology of avian influenza. Vaccine 2007, 25, 5637–5644. [Google Scholar] [CrossRef]
- Short, K.R.; Richard, M.; Verhagen, J.H.; van Riel, D.; Schrauwen, E.J.; van den Brand, J.M.; Manz, B.; Bodewes, R.; Herfst, S. One health, multiple challenges: The inter-species transmission of influenza a virus. One Health 2015, 1, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y. Novel Reassortant Influenza A (H5N8) Viruses, South Korea, 2014. Emerg. Infect. Dis. 2014, 20, 1087. [Google Scholar] [CrossRef]
- Jeong, J.; Kang, H.-M.; Lee, E.-K.; Song, B.-M.; Kwon, Y.-K.; Kim, H.-R.; Choi, K.-S.; Kim, J.-Y.; Lee, H.-J.; Moon, O.-K. Highly pathogenic avian influenza virus (H5N8) in domestic poultry and its relationship with migratory birds in South Korea during 2014. Vet. Microbiol. 2014, 173, 249–257. [Google Scholar] [CrossRef]
- Verhagen, J.H.; Herfst, S.; Fouchier, R.A. How a virus travels the world. Science 2015, 347, 616–617. [Google Scholar] [CrossRef] [PubMed]
- Pandit, P.S.; Bunn, D.A.; Pande, S.A.; Aly, S.S. Modeling highly pathogenic avian influenza transmission in wild birds and poultry in West Bengal, India. Sci. Rep. 2013, 3, 2175. [Google Scholar] [CrossRef] [Green Version]
- Dent, J.E.; Kiss, I.Z.; Kao, R.R.; Arnold, M. The potential spread of highly pathogenic avian influenza virus via dynamic contacts between poultry premises in Great Britain. BMC Vet. Res. 2011, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Richardson, B.; Takle, E.; Chai, L.; Schmitt, D.; Xin, H. Airborne transmission may have played a role in the spread of 2015 highly pathogenic avian influenza outbreaks in the United States. Sci. Rep. 2019, 9, 11755. [Google Scholar] [CrossRef] [Green Version]
- Diggle, P.J.; Chetwynd, A.G.; Häggkvist, R.; Morris, S.E. Second-order analysis of space-time clustering. Stat. Methods Med. Res. 1995, 4, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.D. Identifying space–time disease clusters. Acta Trop. 2004, 91, 291–299. [Google Scholar] [CrossRef]
- Picado, A.; Guitian, F.; Pfeiffer, D. Space–time interaction as an indicator of local spread during the 2001 FMD outbreak in the UK. Prev. Vet. Med. 2007, 79, 3–19. [Google Scholar] [CrossRef]
- Wilesmith, J.; Stevenson, M.; King, C.; Morris, R. Spatio-temporal epidemiology of foot-and-mouth disease in two counties of Great Britain in 2001. Prev. Vet. Med. 2003, 61, 157–170. [Google Scholar] [CrossRef]
- Picado, A.; Speybroeck, N.; Kivaria, F.; Mosha, R.M.; Sumaye, R.D.; Casal, J.; Berkvens, D. Foot-and-mouth disease in Tanzania from 2001 to 2006. Transbound. Emerg. Dis. 2011, 58, 44–52. [Google Scholar] [CrossRef]
- Porphyre, T.; McKenzie, J.; Stevenson, M. A descriptive spatial analysis of bovine tuberculosis in intensively controlled cattle farms in New Zealand. Vet. Res. 2007, 38, 465–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metras, R.; Porphyre, T.; Pfeiffer, D.U.; Kemp, A.; Thompson, P.N.; Collins, L.M.; White, R.G. Exploratory space-time analyses of Rift Valley fever in South Africa in 2008–2011. PLoS Negl. Trop. Dis. 2012, 6, e1808. [Google Scholar] [CrossRef] [PubMed]
- Vergne, T.; Gogin, A.; Pfeiffer, D.U. Statistical Exploration of Local Transmission Routes for African Swine Fever in Pigs in the Russian Federation, 2007-2014. Transbound. Emerg. Dis. 2017, 64, 504–512. [Google Scholar] [CrossRef] [Green Version]
- Guinat, C.; Nicolas, G.; Vergne, T.; Bronner, A.; Durand, B.; Courcoul, A.; Gilbert, M.; Guerin, J.L.; Paul, M.C. Spatio-temporal patterns of highly pathogenic avian influenza virus subtype H5N8 spread, France, 2016 to 2017. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2018, 23, 1700791. [Google Scholar] [CrossRef]
- Loth, L.; Pham, L.T.; Stevenson, M.A. Spatio-temporal distribution of outbreaks of highly pathogenic avian influenza virus subtype H5N1 in Vietnam, 2015–2018. Transbound. Emerg. Dis. 2019. [Google Scholar] [CrossRef]
- Animal & Plant Quarantine Agency. 2014–2016 Epidemiologic Reports of Highly Pathogenic Avian Influenza. 2016. Available online: https://ebook.qia.go.kr/home/view.php?host=main&site=20180306_101633&listPageNow=0&list2PageNow=0&code=0&code2=0&code3=0&optionlisttype=&searchcode=0&searchcode2=0&searchdate=0&searchkey=site&searchval=2014+%B0%ED%BA%B4%BF%F8%BC%BA&searchandor=&dummy=&&orders=A (accessed on 9 September 2020). (In Korea).
- Mafra, M.O.A. Food and Rural Affairs. Act on the Prevention of Contagious Animal Diseaes. Available online: https://elaw.klri.re.kr/eng_service/lawView.do?hseq=4939&lang=ENG (accessed on 23 October 2020).
- Oh, S.-M. Self-declaration of the recovery of freedom from Highly Pathogenic Avian Influenza in poultry by Republic of Korea. Available online: http://www.oie.int (accessed on 27 May 2020).
- QGIS Development Team. QGIS Geographic Infromation System; Open Source Geospatial Foundation: Chicago, IL, USA, 2020. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Rowlingson, B.S.; Diggle, P.J. Splancs: Spatial point pattern analysis code in S-Plus. Comput. Geosci. 1993, 19, 627–655. [Google Scholar] [CrossRef]
- Kulldorff, M. A spatial scan statistic. Commun. Stat. Theory Methods 2007, 26, 1481–1496. [Google Scholar] [CrossRef]
- Kulldorff, M.; Heffernan, R.; Hartman, J.; Assuncao, R.; Mostashari, F. A space-time permutation scan statistic for disease outbreak detection. PLoS Med. 2005, 2, e59. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.C.; Lee, Y.-J.; Song, B.-M.; Kang, H.-M.; Lee, E.-K.; Hanna, A.; Gilbert, M.; Brown, I.H.; Pybus, O.G. Wild waterfowl migration and domestic duck density shape the epidemiology of highly pathogenic H5N8 influenza in the Republic of Korea. Infect. Genet. Evol. 2015, 34, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, D.; Robinson, T.P.; Stevenson, M.; Stevens, K.B.; Rogers, D.J.; Clements, A.C. Spatial Analysis in Epidemiology; Oxford University Press: Oxford, UK, 2008; Volume 142. [Google Scholar]
- Ahmed, S.; Ersbøll, A.K.; Biswas, P.; Christensen, J.P. The space–time clustering of highly pathogenic avian influenza (HPAI) H5N1 outbreaks in Bangladesh. Epidemiol. Infect. 2010, 138, 843–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, Y.J.; Choi, W.S.; Kim, Y.I.; Lee, I.W.; Kwon, H.I.; Park, S.J.; Kim, E.H.; Kim, S.M.; Kwon, J.J.; Song, M.S.; et al. Genetic characteristics of highly pathogenic H5N8 avian influenza viruses isolated from migratory wild birds in South Korea during 2014–2015. Arch. Virol. 2016, 161, 2749–2764. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Bahl, J.; Swayne, D.E.; Lee, Y.N.; Lee, Y.J.; Song, C.S.; Lee, D.H. Domestic ducks play a major role in the maintenance and spread of H5N8 highly pathogenic avian influenza viruses in South Korea. Transbound. Emerg. Dis. 2020, 67, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-T.; Pak, S.-I. The contribution of farm vehicle movements for a highly pathogenic avian influenza epidemic in 2014 in the Republic of Korea. J. Prev. Vet. Med. 2019, 43, 182–188. [Google Scholar] [CrossRef]
- Ameji, O.; Abdu, P.; Sa’idu, L.; Kabir, J.; Assam, A. Awareness, knowledge, readiness to report outbreak and biosecurity practices towards highly pathogenic avian influenza in Kogi State, Nigeria. Int. J. Poult. Sci. 2012, 11, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Animal & Plant Quarantine Agency. High Pathogenic Avian Influenza; the Blue Book; Yong-Sang, K., Ed.; Imun Company: Noida, IN, Canada, 2015. [Google Scholar]
- Song, B.-M.; Lee, E.-K.; Lee, Y.-N.; Heo, G.-B.; Lee, H.-S.; Lee, Y.-J. Phylogeographical characterization of H5N8 viruses isolated from poultry and wild birds during 2014–2016 in South Korea. J. Vet. Sci. 2017, 18, 89–94. [Google Scholar] [CrossRef] [Green Version]
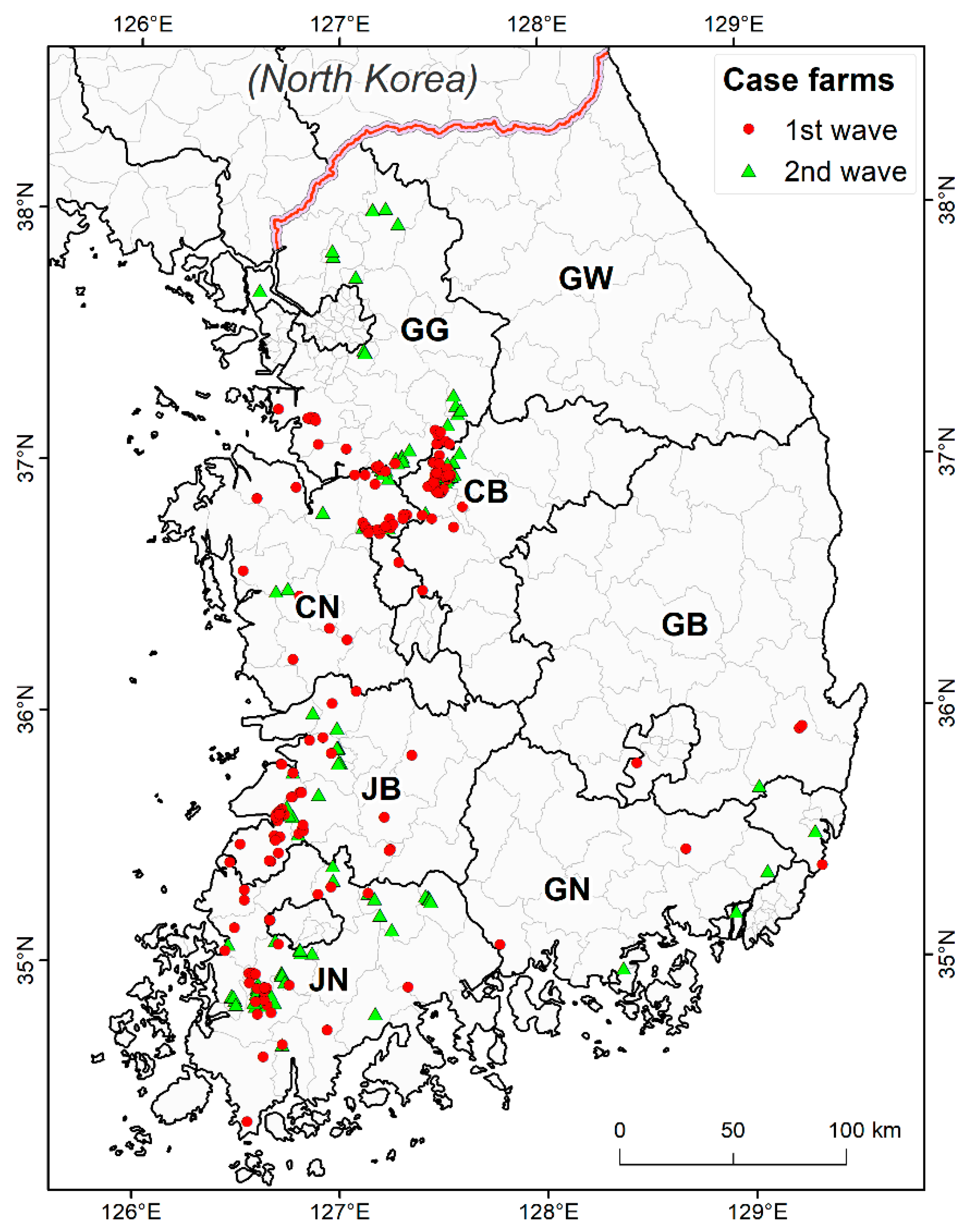
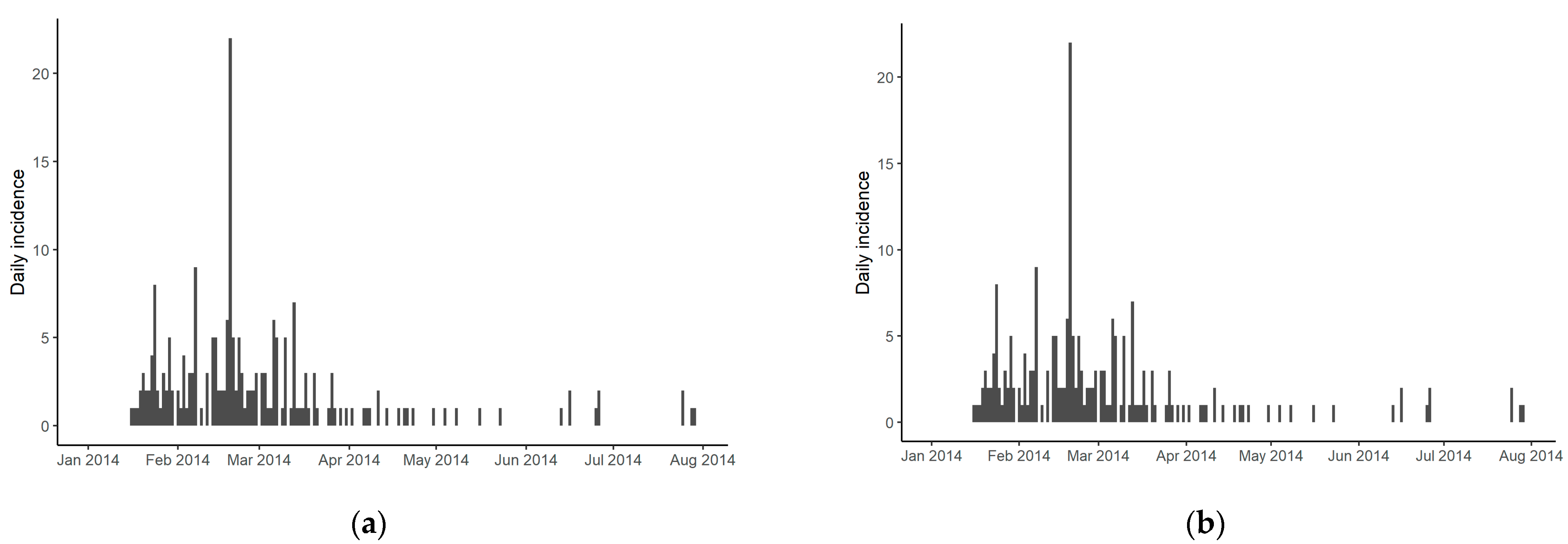

| Wave | Duck (%) | Chicken (%) | Others (%) | Total Number of Cases | Starts | End | Number of Days |
|---|---|---|---|---|---|---|---|
| First | 166 (78.3) | 39 (18.4) | 7 (3.3) | 212 | 15 January 2014 | 29 July 2014 | 196 |
| Second | 117 (72.2) | 39 (24.1) | 6 (3.7) | 162 | 24 September 2014 | 10 June 2015 | 260 |
| Third | 14 (82.4) | 0 (0.0) | 3 (17.6) | 17 | 14 September 2015 | 15 November 2015 | 63 |
| Fourth | 2 (100.0) | 0 (0.0) | 0 (0.0) | 2 | 23 March 2016 | 05 April 2016 | 14 |
| Total | 299 (76.1) | 78 (19.8) | 16 (4.1) | 393 | 533 |
| Wave | Cluster | Radius (km) | Start | End | Number of Days | Number of Outbreaks | Expected Outbreaks | Observed to Expected Ratio | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| First | 1 | 9.86 | 15 January 2014 | 24 January 2014 | 10 | 20 | 2.3 | 8.8 | 0.001 |
| 2 | 6.91 | 01 February 2014 | 07 February 2014 | 7 | 15 | 1.8 | 8.5 | 0.001 | |
| 3 | 2.21 | 17 February 2014 | 19 February 2014 | 3 | 28 | 4.1 | 6.8 | 0.001 | |
| 4 | 24.84 | 10 March 2014 | 06 April 2014 | 28 | 17 | 3.9 | 4.4 | 0.002 | |
| 5 | 19.74 | 10 March 2014 | 08 April 2014 | 30 | 11 | 2.2 | 5.1 | 0.030 | |
| Second | 1 | 5.95 | 24 September 2014 | 12 October 2014 | 19 | 14 | 1.9 | 7.2 | 0.001 |
| 2 | 72.59 | 17 October 2014 | 19 November 2014 | 34 | 11 | 2.3 | 4.8 | 0.025 | |
| 3 | 1.30 | 22 December 2014 | 26 December 2014 | 5 | 5 | 0.2 | 32.5 | 0.001 | |
| 4 | 19.76 | 22 February 2015 | 20 March 2015 | 27 | 36 | 10.2 | 3.5 | 0.001 | |
| 5 | 0.46 | 26 March 2015 | 12 April 2015 | 18 | 11 | 0.8 | 13.5 | 0.001 | |
| 6 | 30.74 | 16 April 2015 | 21 May 2015 | 36 | 6 | 0.4 | 16.2 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.-H.; Bae, S.H.; Cho, S. Spatiotemporal Dynamics of Highly Pathogenic Avian Influenza Subtype H5N8 in Poultry Farms, South Korea. Viruses 2021, 13, 274. https://0-doi-org.brum.beds.ac.uk/10.3390/v13020274
Kim W-H, Bae SH, Cho S. Spatiotemporal Dynamics of Highly Pathogenic Avian Influenza Subtype H5N8 in Poultry Farms, South Korea. Viruses. 2021; 13(2):274. https://0-doi-org.brum.beds.ac.uk/10.3390/v13020274
Chicago/Turabian StyleKim, Woo-Hyun, Sun Hak Bae, and Seongbeom Cho. 2021. "Spatiotemporal Dynamics of Highly Pathogenic Avian Influenza Subtype H5N8 in Poultry Farms, South Korea" Viruses 13, no. 2: 274. https://0-doi-org.brum.beds.ac.uk/10.3390/v13020274






