Interplay of Rice Stripe Virus and Rice Black Streaked Dwarf Virus during Their Acquisition and Accumulation in Insect Vector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect, Plant Virus, Virus Acquisition
2.2. Total RNA Extraction and RT-PCR
2.3. Immunofluorescence Microscopy
2.4. RT-qPCR to Quantify Relative mRNA Transcript Levels
3. Results
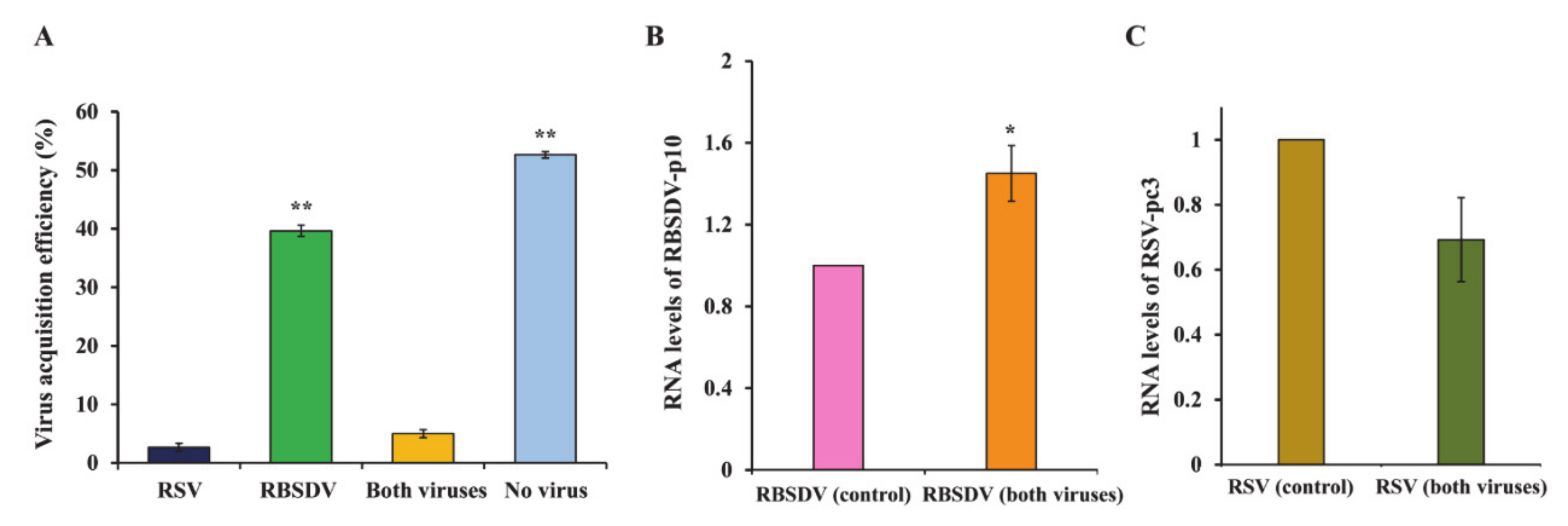
3.1. Acquisition Efficiency of RSV and RBSDV by SBPHs after Successively Feeding on Plants Infected with a Different Virus
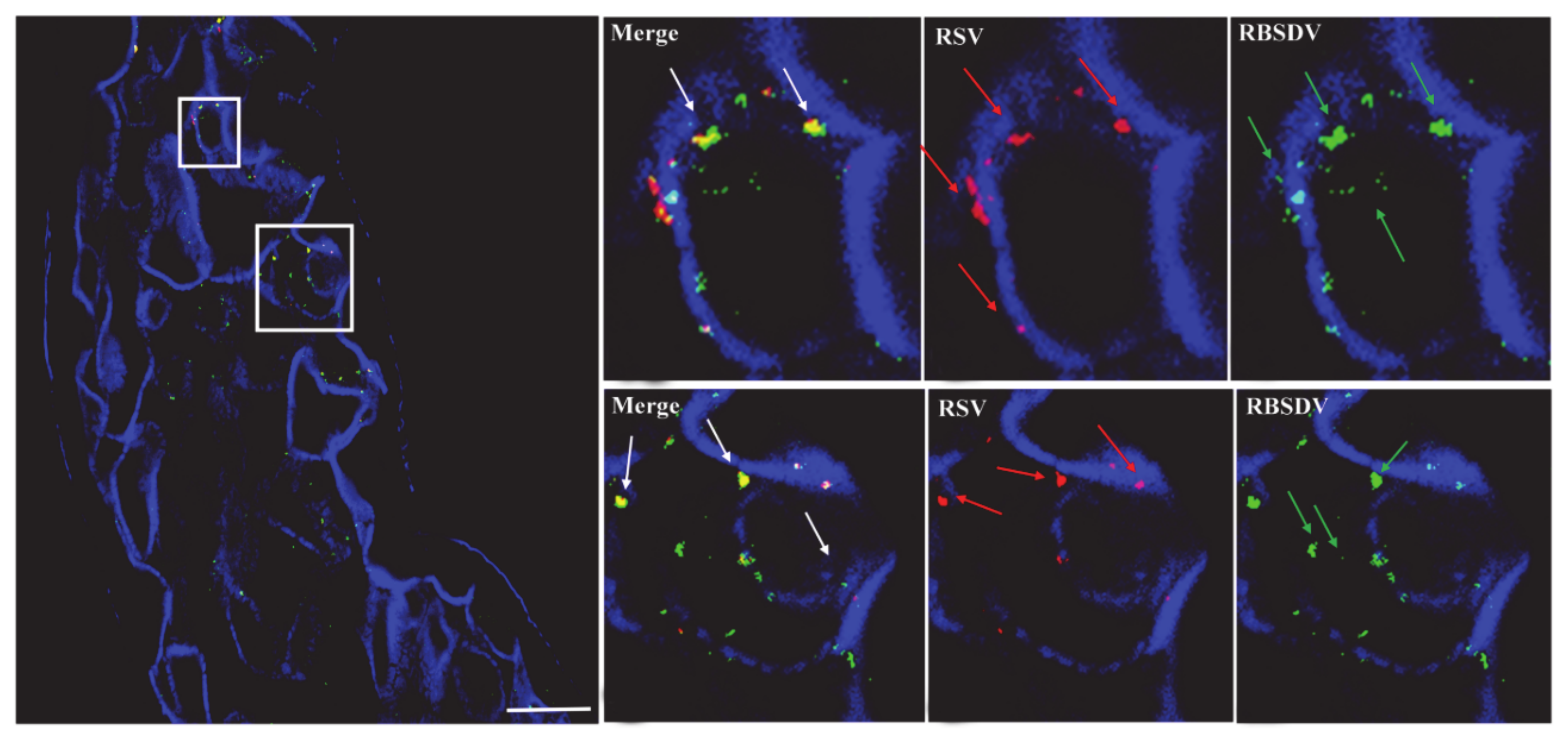
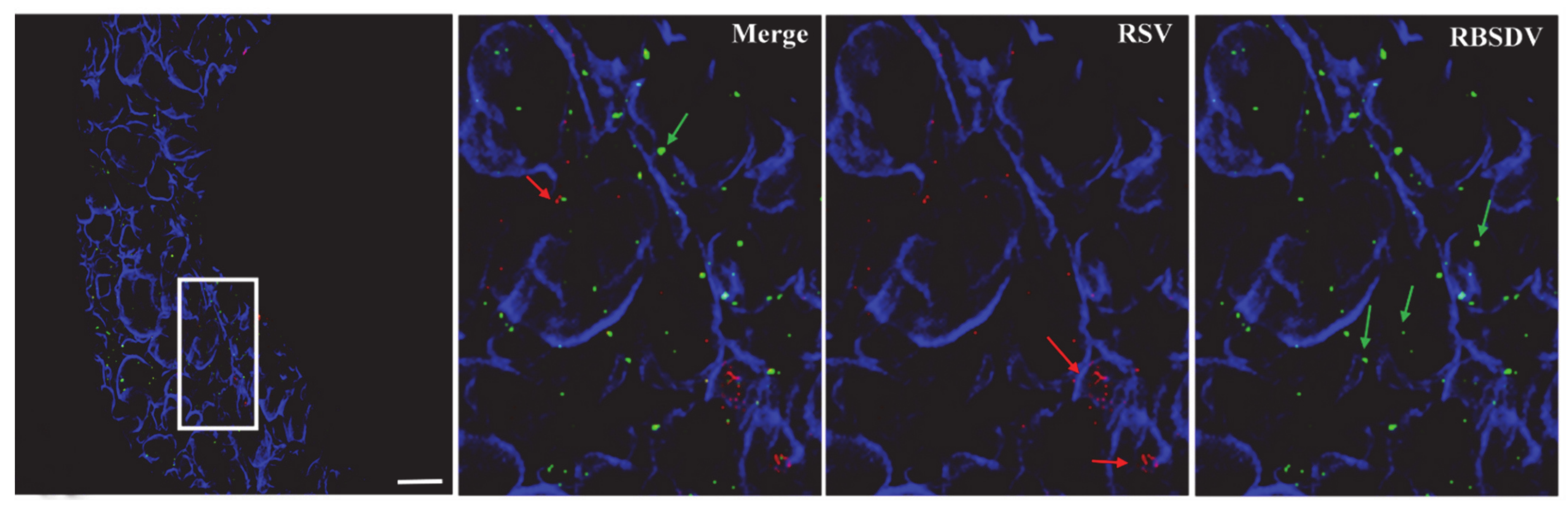
3.2. Localization of RSV and RBSDV in Midgut Epithelial Cells from Insects Successively Feeding on Plants Infected with a Different Virus
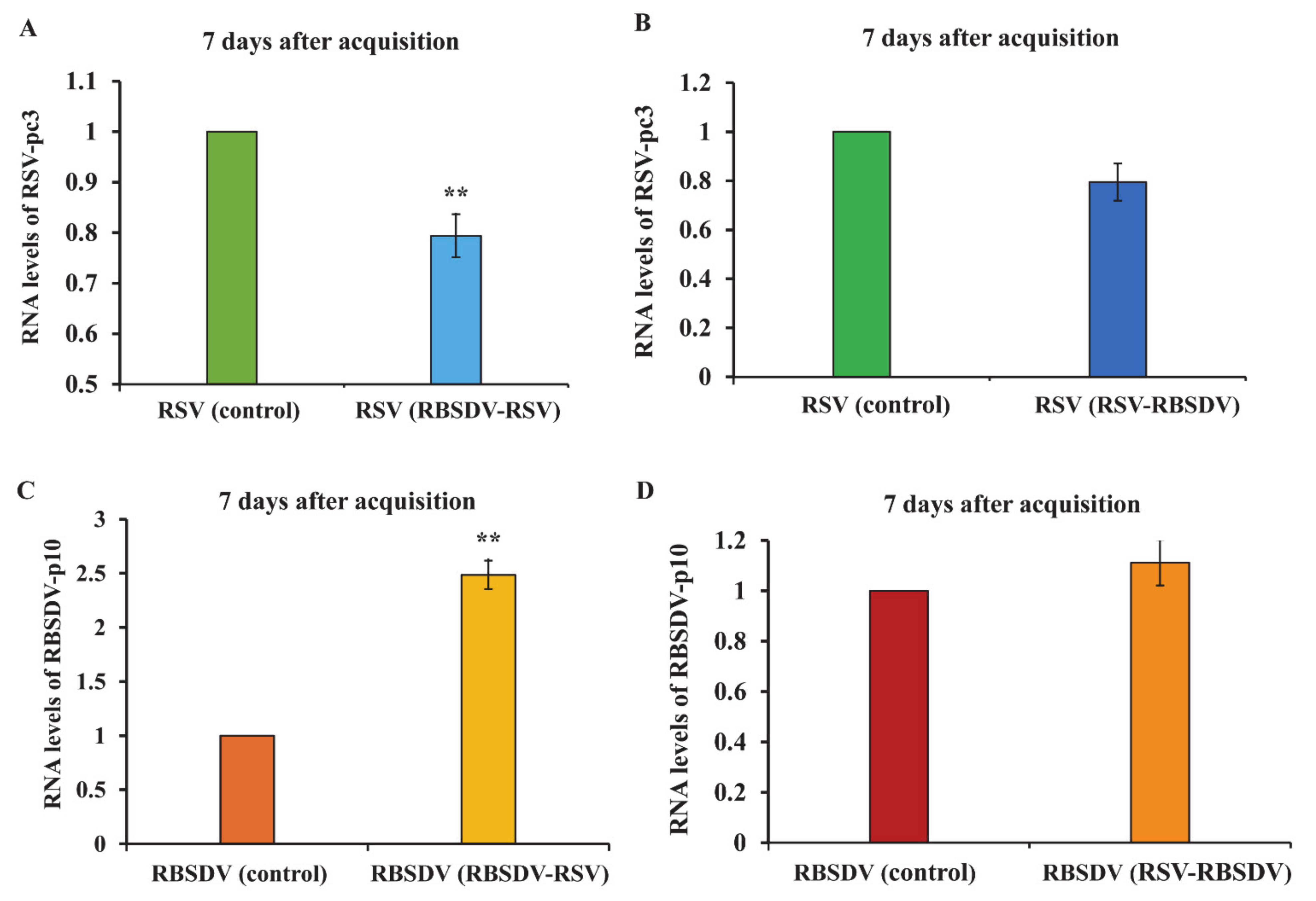
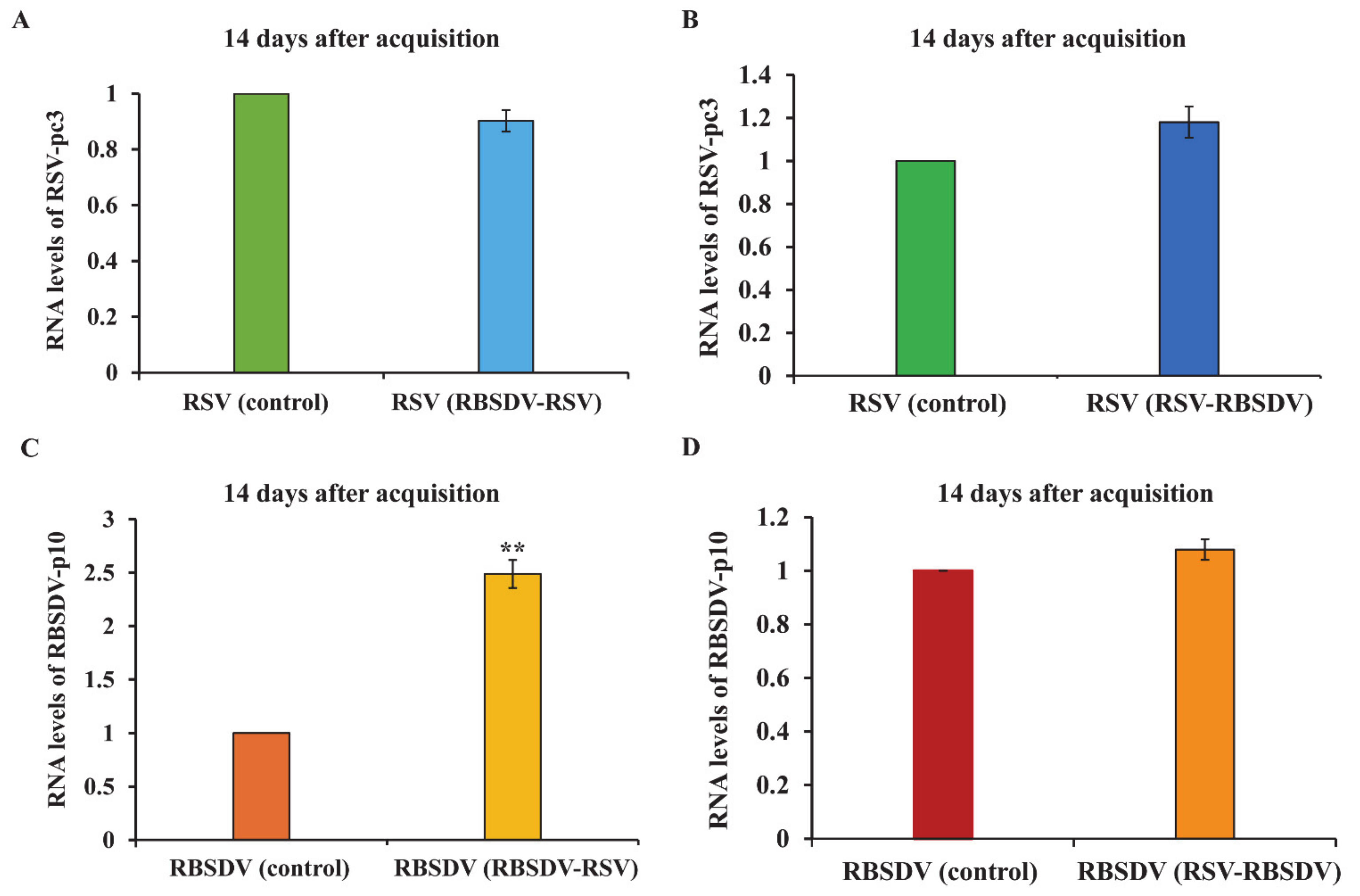
3.3. Viral Titer of RSV and RBSDV in Insects Successively Feeding on Plants Infected by Different Viruses
3.4. Acquisition and Accumulation of RSV and RBSDV in SBPHs That Fed on Plants Coinfected with Both Viruses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Li, L.; Wang, X.; Zhou, G. Transmission of Rice stripe virus acquired from frozen infected leaves by the small brown planthopper (Laodelphax striatellus Fallen). J. Virol. Method. 2007, 146, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Wu, J.; Zhou, Y.; Zhou, X. Identification of a movement protein of the tenuivirus rice stripe virus. J. Virol. 2008, 82, 12304–12311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, T.Y.; Yang, J.G.; Liao, F.L.; Gao, F.L.; Lu, L.M.; Zhang, X.T.; Li, F.; Wu, Z.-J.; Lin, Q.Y.; Xie, L.H.; et al. Genetic diversity and population structure of rice stripe virus in China. J. Gen. Virol. 2009, 90, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.D.; Chen, J.P.; Zhang, H.M.; Sun, X.L.; Zhu, J.L.; Wang, A.G.; Sheng, W.X.; Adams, M.J. Recent rice stripe virus epidemics in zhejiang province, China, and experiments on sowing date, disease yield loss relationships and seedling susceptibility. Plant Dis. 2008, 92, 1190–1196. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.D.; Chen, J.P.; Wang, A.G.; Jiang, X.H.; Adams, M.J. Studies on the epidemiology and yield losses from rice black-streaked dwarf disease in a recent epidemic in zhejiang province, China. Plant Pathol. 2009, 58, 815–825. [Google Scholar] [CrossRef]
- Li, L.; Li, H.W.; Dong, H.B.; Wang, X.F.; Zhou, G.H. Transmission by Laodelphax striatellus Fallen of rice black streaked dwarf virus from frozen infected rice leaves to healthy plants of rice and maize. J. Phytopathol. 2011, 159, 1–5. [Google Scholar] [CrossRef]
- Lu, L.; Wang, Q.; Huang, D.; Xu, Q.; Zhou, X.; Wu, J. Rice black-streaked dwarf virus P10 suppresses protein kinase C in insect vector through changing the subcellular localization of LsRACK1. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 1767. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.K.; Lian, S.; Kim, S.M.; Park, S.H.; Kim, K.H. Current insights into research on rice stripe virus. Plant Pathol. J. 2013, 29, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Choi, J.Y.; Park, D.H.; Park, M.G.; Kim, J.Y.; Wang, M.; Kim, H.J.; Kim, W.J.; Je, Y.H. Suppression of rice stripe virus replication in Laodelphax striatellus using vector insect-derived double-stranded RNAs. Plant Pathol. 2020, 36, 280–288. [Google Scholar] [CrossRef]
- Hibino, H. Biology and epidemiology of rice viruses. Ann. Rev. Phytopathol. 1996, 34, 249–274. [Google Scholar] [CrossRef]
- Matsukura, K.; Sanada-Morimura, S.; Fujii, T.; Matsumura, M. Potential risks of poaceous plants as infectious sources of rice black streaked dwarf virus transmitted by the small brown planthopper, Laodelphax striatellus. Plant Dis. 2019, 103, 1244–1248. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, N.; Ren, Y.; Wang, X. Insights into insect vector transmission and epidemiology of plant infections. Front. Microbiol. 2021, 12, 628262. [Google Scholar] [CrossRef]
- Fang, S.; Yu, J.; Feng, J.; Han, C.; Li, D.; Liu, Y. Identification of rice black-streaked dwarf fijivirus in maize with rough dwarf disease in China. Arch. Virol. 2001, 146, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Andika, I.B.; Shen, J.; Lv, Y.; Ji, Y.; Sun, L.; Chen, J. Characterization of rice black streaked dwarf virus and rice stripe virus derived siRNAs in singly and doubly infected insect vector Laodelphax striatellus. PLoS ONE 2013, 8, e66007. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.Y.; Xu, L.Z.; Wu, Y.; Wang, P.; Shi, J.J.; Zhai, B.P. Geographic variation of diapause and sensitive stages of photoperiodic response in Laodelphax striatellus Fallen (Hemiptera: Delphacidae). J. Insect. Sci. 2016, 16, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Su, Q.; Shi, X.; Pan, H.; Jiao, X.; Zhang, Y. Persistently transmitted viruses restrict the transmission of other viruses by affecting their vectors. Front. Physiol. 2018, 9, 1261. [Google Scholar] [CrossRef]
- Liu, X.D.; Zhai, B.P.; Liu, C.M. Outbreak reasons of Laodelphax striatellus population. Chin. J. Appl. Entomol. 2006, 43, 141–146, (in Chinese with English summary). [Google Scholar]
- Wu, N.; Zhang, L.; Ren, Y.; Wang, X. Rice black-streaked dwarf virus: From multiparty interactions among plant–virus–vector to intermittent epidemics. Mol. Plant Pathol. 2020, 21, 1007–1019. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; el Ammar, D.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Ann. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef] [Green Version]
- Dietzgen, R.G.; Mann, K.S.; Johnson, K.N. Plant virus–insect vector interactions: Current and potential future research directions. Viruses 2016, 8, 303. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, Z.; Zhang, X.; Kang, L.; Liu, R.; Ciu, F. Genomic variations in the 3′-termini of rice stripe virus in the rotation between vector insect and host plant. New Phytol. 2018, 219, 1085–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, F.; Liu, W.; Wu, N.; Zhang, L.; Zhou, X.; Wang, X. Invasion of midgut epithelial cells by a persistently transmitted virus is mediated by sugar transporter 6 in its insect vector. PLoS Pathogen. 2018, 14, e1007201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Chen, D.; Hu, J.; Zhang, K.; Kang, L.; Chen, Y.; Huang, L.; Zhang, L.; Xiang, Y.; Song, Q.; et al. The α-tubulin of Laodelphax striatellus mediates the passage of rice stripe virus (RSV) and enhances horizontal transmission. PLoS Pathogen. 2020, 16, e1008710. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, A. Studies on insect transmission of rice virus diseases in Japan. Bull. Nat. Inst. Agr. Sci. C 1962, 14, 1–112. [Google Scholar] [CrossRef]
- Salazar, M.I.; Richardson, J.H.; Sánchez-Vargas, I.; Olson, K.E.; Beaty, B.J. Dengue virus type 2: Replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Dumón, A.D.; Caro, A.E.B.; Mattio, M.F.; Alemandri, V.; del Vas, M.; Truol, G. Co-infection with a wheat rhabdovirus causes a reduction in Mal de Río Cuarto virus titer in its planthopper vector. Bull. Entomol. Res. 2017, 108, 232–240. [Google Scholar] [CrossRef]
- Öhlund, P.; Lundén, H.; Blomström, A.L. Insect specific virus evolution and potential effects on vector competence. Virus Genes 2019, 55, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Huo, Y.; Liu, W.; Zhang, F.; Chen, X.; Li, L.; Liu, Q.; Zhou, Y.; Wei, T.; Fang, R.; Wang, X. Transovarial transmission of a plant virus is mediated by vitellogenin of its insect vector. PLoS Pathog. 2014, 10, e1003949. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Zhou, G. A one-step real time RT-PCR assay for quantifying rice stripe virus in rice and in the small brown planthopper (Laodelphax striatellus Fallen). J. Virol. Methods. 2008, 151, 181–187. [Google Scholar] [CrossRef]
- Zhang, P.; Mar, T.T.; Liu, W.; Li, L.; Wang, X. Simultaneous detection and differentiation of rice black streaked dwarf virus (RBSDV) and southern rice black streaked dwarf virus (SRBSDV) by duplex real time RT-PCR. Virol. J. 2013, 10, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Falk, B.W.; Tsai, J.H. Biology and molecular biology of viruses in the genus Tenuivirus. Ann. Rev. Phytopathol. 1998, 36, 139–163. [Google Scholar] [CrossRef]
- Link, P.A.; Fuchs, M. Transmission specificity of plant viruses by vectors. J. Plant Pathol. 2005, 87, 153–165. [Google Scholar]
- He, D.C.; Zhan, J.; Cheng, Z.B.; Xie, L.H. Viruliferous rate of small brown planthopper is a good indicator of rice stripe disease epidemics. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Zhang, A.H.; Yan, C.; Meng, F.S.; Huo, L.Z.; Di, D.P.; Miao, H.Q.; Wang, X. Comparison RBSDV bearing rates transmission rates among different generations of artificial reared Laodelphax striatellus Fallén. Acta Phytopathol. Sin. 2017, 47, 551–557. [Google Scholar] [CrossRef]
- Jia, D.; Chen, Q.; Mao, Q.; Zhang, X.; Wu, W.; Chen, H.; Yu, X.; Wang, Z.; Wei, T. Vector mediated transmission of persistently transmitted plant viruses. Curr. Opin. Virol. 2018, 28, 127–132. [Google Scholar] [CrossRef]
- Lu, G.; Li, S.H.; Zhou, C.H.; Qian, X.; Xiang, Q.; Yang, T.; Wu, J.; Zhou, Y.; Ding, X.S.; Tao, X. Tenuivirus utilizes its glycoprotein as a helper component to overcome insect midgut barriers for its circulative and propagative transmission. PLoS Pathogen. 2019, 15, e1007655. [Google Scholar] [CrossRef] [Green Version]
- Linak, J.A.; Jacobson, A.L.; Sit, T.L.; Kennedy, G.G. Relationships of virus titers and transmission rates among sympatric and allopatric virus isolates and thrips vectors support local adaptation. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Mukhopadyay, S. Interactions of insect vectors with plants in relation to transmission of plant viruses. Proc. Indian Acad. Sci. 1984, 93, 349–357. [Google Scholar] [CrossRef]
- Salvaudón, L.; De Moraes, C.M.; Mescher, M.C. Outcomes of co-infection by two potyviruses: Implications for the evolution of manipulative strategies. Proc. Biol. Sci. 2013, 280, 20122959. [Google Scholar] [CrossRef] [Green Version]
- Bourdin, D.; Lecoq, H. Evidence that heteroencapsidation between two potyviruses is involved in aphid transmission of a non-aphid-transmissible isolate from mixed infections. Phytopathology 1991, 81, 1459–1464. [Google Scholar] [CrossRef]
- Brault, V.; Uzest, M.; Monsion, B.; Jacquot, E.; Blanc, S. Aphids as transport devices for plant viruses. C. R. Biol. 2010, 333, 524–538. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moya Fernández, M.B.; Liu, W.; Zhang, L.; Hajano, J.-U.-D.; Wang, X. Interplay of Rice Stripe Virus and Rice Black Streaked Dwarf Virus during Their Acquisition and Accumulation in Insect Vector. Viruses 2021, 13, 1121. https://0-doi-org.brum.beds.ac.uk/10.3390/v13061121
Moya Fernández MB, Liu W, Zhang L, Hajano J-U-D, Wang X. Interplay of Rice Stripe Virus and Rice Black Streaked Dwarf Virus during Their Acquisition and Accumulation in Insect Vector. Viruses. 2021; 13(6):1121. https://0-doi-org.brum.beds.ac.uk/10.3390/v13061121
Chicago/Turabian StyleMoya Fernández, Marcia Beatriz, Wenwen Liu, Lu Zhang, Jamal-U-Ddin Hajano, and Xifeng Wang. 2021. "Interplay of Rice Stripe Virus and Rice Black Streaked Dwarf Virus during Their Acquisition and Accumulation in Insect Vector" Viruses 13, no. 6: 1121. https://0-doi-org.brum.beds.ac.uk/10.3390/v13061121





