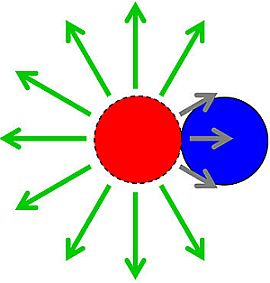
Spatial Vulnerability: Bacterial Arrangements, Microcolonies, and Biofilms as Responses to Low Rather than High Phage Densities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phage Adsorption to Free Bacteria
2.1.1. Phage Movement towards Bacterial Targets

2.1.2. Basic Adsorption Calculations


2.2. Phage Interaction with Bacterial Arrangements
2.2.1. Increased Target Size
 to describe reductions in phage adsorption rates to bacteria that stem from shading, such that
to describe reductions in phage adsorption rates to bacteria that stem from shading, such that  < k. Note that the umlaut’s intention is to imply a description of properties associated with bacterial arrangements, with the double dots literally suggestive of a diplococcus.
< k. Note that the umlaut’s intention is to imply a description of properties associated with bacterial arrangements, with the double dots literally suggestive of a diplococcus.  P, where n is the number of bacteria making up an individual arrangement. That is, the target size of an arrangement increases by a factor of n relative to free bacteria while at the same time decreases by a factor of
P, where n is the number of bacteria making up an individual arrangement. That is, the target size of an arrangement increases by a factor of n relative to free bacteria while at the same time decreases by a factor of  /k. The increase due to n, however, likely is greater than the decrease described by
/k. The increase due to n, however, likely is greater than the decrease described by  /k, at least so long as arrangements are not sequestered within phage-excluding volumes such as (perhaps) defects in the glass walls of chemostats [29]. Larger arrangements, in other words, almost inevitably will tend to serve as larger targets for phage encounter than will either individual bacteria or smaller arrangements.
/k, at least so long as arrangements are not sequestered within phage-excluding volumes such as (perhaps) defects in the glass walls of chemostats [29]. Larger arrangements, in other words, almost inevitably will tend to serve as larger targets for phage encounter than will either individual bacteria or smaller arrangements. ), which is the increased diameter of a two-fold larger volume, and approximately two (n) times larger than the target size of individual cocci. These values in other words range from where shading is substantial (1.26 times) to where shading instead is minimal (~2 times; for illustration, see Figure 2). Given diversity in arrangement shape it is clear that using arrangement diameter as a proxy for target size is a simplification, though one which I retain both for the sake of mathematical convenience and because assuming that targets are spherical may be the most reasonable of default assumptions. Clearly though, and as indicated in the above calculation (Figure 2), surface area (as equivalent to the “~2 times” calculation) provides a more intuitive perspective on target size and particularly so given non-spherical as well as relatively immobile targets. The larger and more important point, however, is that in terms of target size, arrangements should be inherently more vulnerable to phage encounter than individual bacteria.
), which is the increased diameter of a two-fold larger volume, and approximately two (n) times larger than the target size of individual cocci. These values in other words range from where shading is substantial (1.26 times) to where shading instead is minimal (~2 times; for illustration, see Figure 2). Given diversity in arrangement shape it is clear that using arrangement diameter as a proxy for target size is a simplification, though one which I retain both for the sake of mathematical convenience and because assuming that targets are spherical may be the most reasonable of default assumptions. Clearly though, and as indicated in the above calculation (Figure 2), surface area (as equivalent to the “~2 times” calculation) provides a more intuitive perspective on target size and particularly so given non-spherical as well as relatively immobile targets. The larger and more important point, however, is that in terms of target size, arrangements should be inherently more vulnerable to phage encounter than individual bacteria. . Twice its volume (V2) therefore is
. Twice its volume (V2) therefore is  , which as a sphere is equal to
, which as a sphere is equal to  . For
. For  , then r2 = 21/3r1. With such shading, then, diameter increases by only 21/3 = 1.26 fold.
, then r2 = 21/3r1. With such shading, then, diameter increases by only 21/3 = 1.26 fold.
 . Twice its volume (V2) therefore is
. Twice its volume (V2) therefore is  , which as a sphere is equal to
, which as a sphere is equal to  . For
. For  , then r2 = 21/3r1. With such shading, then, diameter increases by only 21/3 = 1.26 fold.
, then r2 = 21/3r1. With such shading, then, diameter increases by only 21/3 = 1.26 fold.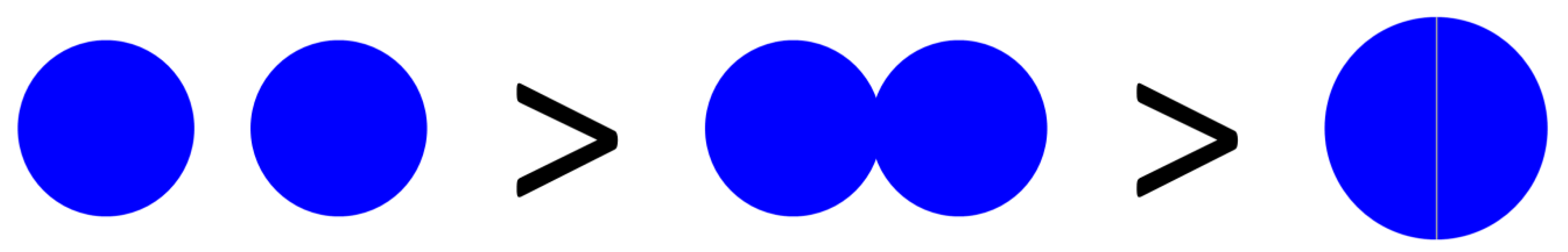
2.2.2. Increased Multiplicity of Adsorption

 > k holds, then Nt/N0 > At/A0. That is, fewer arrangements will remain fractionally unadsorbed (At/A0) than would individual, free bacteria (Nt/N0 ), holding bacterial size and adsorption susceptibility otherwise constant. Here n
> k holds, then Nt/N0 > At/A0. That is, fewer arrangements will remain fractionally unadsorbed (At/A0) than would individual, free bacteria (Nt/N0 ), holding bacterial size and adsorption susceptibility otherwise constant. Here n  Pt is equivalent to MOIactual for arrangements. Note though that it is my preference to instead use the term multiplicity of adsorption, i.e., MOA, rather than multiplicity of infection because while an arrangement can be wholly adsorbed by a phage, subsequent infection of the whole arrangement is a more complicated process versus the infection of individual phage-adsorbed bacteria.
Pt is equivalent to MOIactual for arrangements. Note though that it is my preference to instead use the term multiplicity of adsorption, i.e., MOA, rather than multiplicity of infection because while an arrangement can be wholly adsorbed by a phage, subsequent infection of the whole arrangement is a more complicated process versus the infection of individual phage-adsorbed bacteria. Pt versus MN=kPt. With M defined in this manner, then the fraction of phage targets expected to remain unadsorbed is equal to
Pt versus MN=kPt. With M defined in this manner, then the fraction of phage targets expected to remain unadsorbed is equal to  (= At/A0) and e-kPt (= Nt/N0), respectively, which are restatements of Equations (3) and (1), respectively. Note that
(= At/A0) and e-kPt (= Nt/N0), respectively, which are restatements of Equations (3) and (1), respectively. Note that  < e-kPt if as expected n
< e-kPt if as expected n  Pt > kPt, implying that At/A0 < Nt/N0.
Pt > kPt, implying that At/A0 < Nt/N0. , also may decline as n increases. It may be harder, that is, for environmental phages on average to encounter individual bacteria that are found within larger arrangements (such as a large microcolony) versus individual bacteria that are found in smaller arrangements (such as a diplococcus). Arrangement vulnerability to adsorption, given this tendency, therefore might increase less rapidly as a function of the number of bacteria that they contain.
, also may decline as n increases. It may be harder, that is, for environmental phages on average to encounter individual bacteria that are found within larger arrangements (such as a large microcolony) versus individual bacteria that are found in smaller arrangements (such as a diplococcus). Arrangement vulnerability to adsorption, given this tendency, therefore might increase less rapidly as a function of the number of bacteria that they contain.2.2.3. Phage Propagation within Arrangements
 Pt should adequately describe the likelihood of arrangement encounter with a phage. Further, n
Pt should adequately describe the likelihood of arrangement encounter with a phage. Further, n  Pt defines the actual multiplicity, a.k.a., multiplicity of adsorption, of a bacterial arrangement with the value
Pt defines the actual multiplicity, a.k.a., multiplicity of adsorption, of a bacterial arrangement with the value  specifying inefficiencies in this phage adsorption process in comparison to free bacteria. In addition, phage adsorption to arrangements is not identical to phage adsorption to free bacteria because only a fraction of the number of bacteria making up an arrangement become initially phage adsorbed (i.e., 1/n) rather than all of the bacteria making up a free bacterium (1/1). Furthermore, with bacterial arrangements an initial (“primary”) phage adsorption could give rise to a variety of subsequent outcomes including infection of only the adsorbed bacterium, subsequent infection of a fraction of the bacteria found within the arrangement, or indeed subsequent infection of all of the bacteria making up an arrangement. The latter result, of course, is the more costly of outcomes to the affected bacteria, just as it is in terms of prey aggregation more generally [36]. The result is that an additional assumption must be made to argue that the vulnerability of bacterial arrangements to phages can be greater than that seen for individual bacteria. This assumption, in particular, is that the efficiency of phage propagation among bacteria found within arrangements must be greater than that which can be sustained among free bacteria.
specifying inefficiencies in this phage adsorption process in comparison to free bacteria. In addition, phage adsorption to arrangements is not identical to phage adsorption to free bacteria because only a fraction of the number of bacteria making up an arrangement become initially phage adsorbed (i.e., 1/n) rather than all of the bacteria making up a free bacterium (1/1). Furthermore, with bacterial arrangements an initial (“primary”) phage adsorption could give rise to a variety of subsequent outcomes including infection of only the adsorbed bacterium, subsequent infection of a fraction of the bacteria found within the arrangement, or indeed subsequent infection of all of the bacteria making up an arrangement. The latter result, of course, is the more costly of outcomes to the affected bacteria, just as it is in terms of prey aggregation more generally [36]. The result is that an additional assumption must be made to argue that the vulnerability of bacterial arrangements to phages can be greater than that seen for individual bacteria. This assumption, in particular, is that the efficiency of phage propagation among bacteria found within arrangements must be greater than that which can be sustained among free bacteria.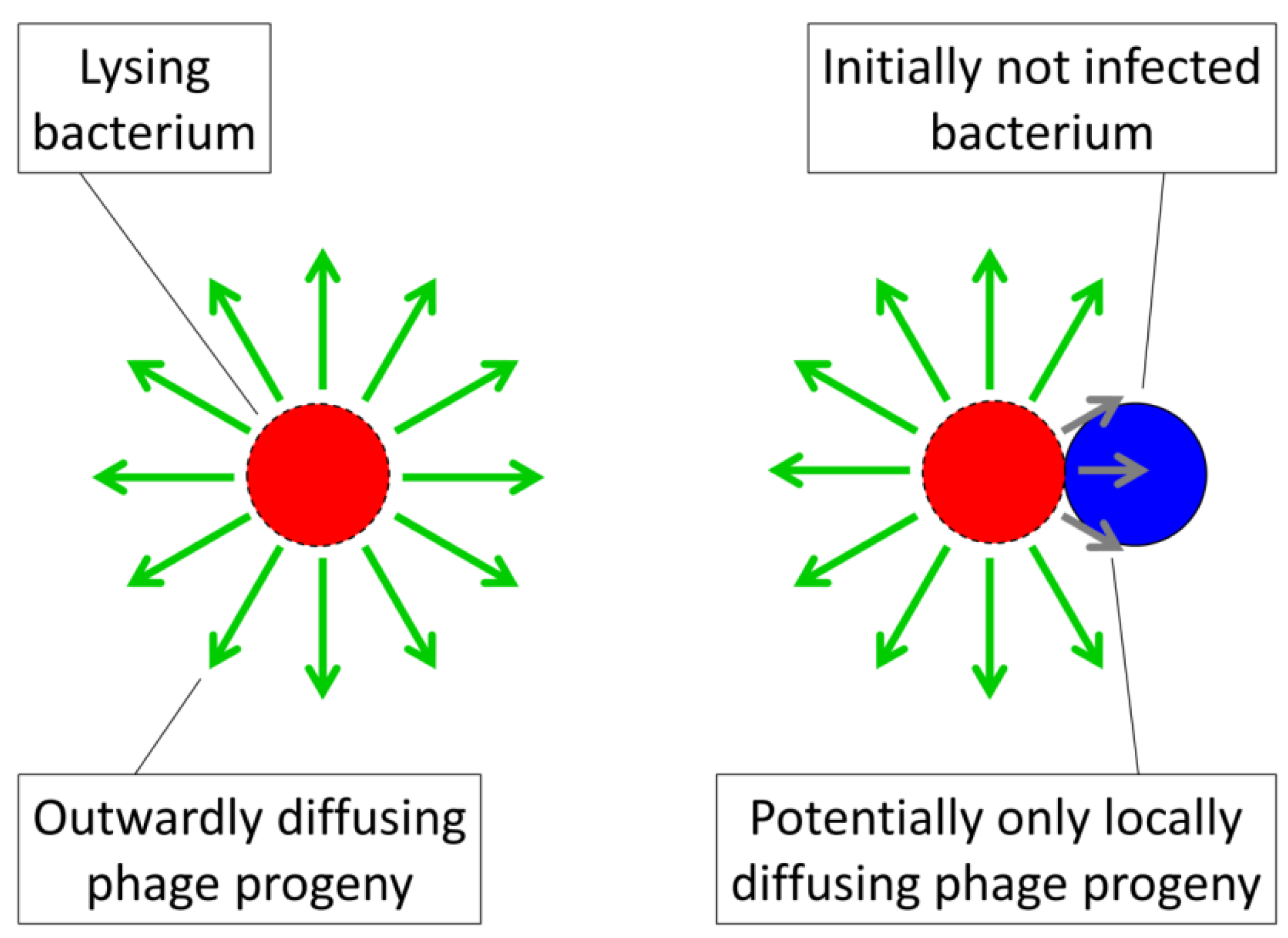
2.2.4. Inefficiencies in Phage Propagation
 . This represents the number of bacteria within an arrangement that will be lost, on average, as a consequence of phage adsorption of a single bacterium within that arrangement. This number,
. This represents the number of bacteria within an arrangement that will be lost, on average, as a consequence of phage adsorption of a single bacterium within that arrangement. This number,  , and can range up to the total number of bacteria making up an arrangement (n). Another way of viewing
, and can range up to the total number of bacteria making up an arrangement (n). Another way of viewing  , however, is that
, however, is that  > 1 implies a phage reproductive number within a bacterial arrangement that is greater than 0 such that some degree of phage propagation within an arrangement occurs along with consequent bacterial death. If insufficient phage release from infected bacteria and/or insufficient subsequent bacterial infection occurs within an arrangement, or subsequent infections don’t happen fast enough, then complete eradication of an arrangement by an infecting phage may not happen, such that
> 1 implies a phage reproductive number within a bacterial arrangement that is greater than 0 such that some degree of phage propagation within an arrangement occurs along with consequent bacterial death. If insufficient phage release from infected bacteria and/or insufficient subsequent bacterial infection occurs within an arrangement, or subsequent infections don’t happen fast enough, then complete eradication of an arrangement by an infecting phage may not happen, such that  < n.
< n.
 ) is the fraction of arrangements that become phage adsorbed over some interval, t, and
) is the fraction of arrangements that become phage adsorbed over some interval, t, and  is the number of bacteria per arrangement that are lost to this adsorption (assuming, for simplicity, that
is the number of bacteria per arrangement that are lost to this adsorption (assuming, for simplicity, that  is independent of the actual multiplicity of phage adsorption to a given arrangement). Were
is independent of the actual multiplicity of phage adsorption to a given arrangement). Were  = 1, then though arrangements are more likely to be adsorbed than free cells, nevertheless no more bacteria would be lost per arrangement adsorption. Indeed, to the extent that the initial bacterial infection is less likely, that is, given
= 1, then though arrangements are more likely to be adsorbed than free cells, nevertheless no more bacteria would be lost per arrangement adsorption. Indeed, to the extent that the initial bacterial infection is less likely, that is, given  < k along with
< k along with  = 1, then overall existence as an arrangement could result in less vulnerability to phages rather than more. Such a situation would occur, for example, were phage infections abortive or perhaps could result instead were infections substantially reduced in burst size or extended in latent period such that phage propagation through an arrangement were substantially impaired. Alternatively, the equality
= 1, then overall existence as an arrangement could result in less vulnerability to phages rather than more. Such a situation would occur, for example, were phage infections abortive or perhaps could result instead were infections substantially reduced in burst size or extended in latent period such that phage propagation through an arrangement were substantially impaired. Alternatively, the equality  = n would imply complete arrangement loss following each phage adsorption of an arrangement. The parameter,
= n would imply complete arrangement loss following each phage adsorption of an arrangement. The parameter,  , is thus a description of arrangement vulnerability to phages post-adsorption, ranging from minimal (
, is thus a description of arrangement vulnerability to phages post-adsorption, ranging from minimal (  = 1, or even
= 1, or even  = 0) to maximal (
= 0) to maximal (  = n ). A visual summary of the models represented by Equations (1) and (4) is presented in Figure 4.
= n ). A visual summary of the models represented by Equations (1) and (4) is presented in Figure 4.2.2.5. An Important Special Case
 < n, but Equation (4) does not reflect that if one adsorbing phage fails to clear an arrangement then perhaps more than one adsorbing phage will, with greater likelihood, succeed in doing so. One way to address this concern is simply by setting
< n, but Equation (4) does not reflect that if one adsorbing phage fails to clear an arrangement then perhaps more than one adsorbing phage will, with greater likelihood, succeed in doing so. One way to address this concern is simply by setting  = n, that is, an assumption of complete arrangement loss per primary phage adsorption.
= n, that is, an assumption of complete arrangement loss per primary phage adsorption.

 > k/
> k/  . That is, when this inequality holds then bacteria found within bacterial arrangements are more vulnerable to phages than are phage-susceptible bacteria that are “free”. In words: Bacteria found within arrangements are more susceptible to phage attack if losses due to existence within a phage‑adsorbed arrangement,
. That is, when this inequality holds then bacteria found within bacterial arrangements are more vulnerable to phages than are phage-susceptible bacteria that are “free”. In words: Bacteria found within arrangements are more susceptible to phage attack if losses due to existence within a phage‑adsorbed arrangement,  , are greater than increases in individual bacterial vulnerability to “primary” adsorptions that come from not existing within an arrangement (that is, k/
, are greater than increases in individual bacterial vulnerability to “primary” adsorptions that come from not existing within an arrangement (that is, k/  where k >
where k >  ).
). (phage adsorption constant considering reductions due to shading of bacteria by bacteria found within bacterial arrangements), n (number of bacteria found per arrangement), N (bacterial density of overall environment), L (phage latent period, which is the duration of a phage infection), and
(phage adsorption constant considering reductions due to shading of bacteria by bacteria found within bacterial arrangements), n (number of bacteria found per arrangement), N (bacterial density of overall environment), L (phage latent period, which is the duration of a phage infection), and  (number of bacteria per arrangement lost subsequent to phage infection of one cell in the arrangement). Likelihood of phage adsorption of bacterial arrangements is n
(number of bacteria per arrangement lost subsequent to phage infection of one cell in the arrangement). Likelihood of phage adsorption of bacterial arrangements is n  and density of arrangements within environments is equal to N/n = N0/n (or indeed n0
and density of arrangements within environments is equal to N/n = N0/n (or indeed n0  and N0/n0, respectively, to reflect that n changes as a function of time in the figure). The inequality t ≥ 2L indicates how phage acquisition of bacteria within a bacterial arrangement, according to this model, involves at least two sequential rounds of phage infection. The absence of cells in the lower right is intentional as too is the reduction in cell number to nt in the lower left. Both of these reductions in cell number, going from middle to bottom, indicate phage-induced bacterial lysis.
and N0/n0, respectively, to reflect that n changes as a function of time in the figure). The inequality t ≥ 2L indicates how phage acquisition of bacteria within a bacterial arrangement, according to this model, involves at least two sequential rounds of phage infection. The absence of cells in the lower right is intentional as too is the reduction in cell number to nt in the lower left. Both of these reductions in cell number, going from middle to bottom, indicate phage-induced bacterial lysis.
 (phage adsorption constant considering reductions due to shading of bacteria by bacteria found within bacterial arrangements), n (number of bacteria found per arrangement), N (bacterial density of overall environment), L (phage latent period, which is the duration of a phage infection), and
(phage adsorption constant considering reductions due to shading of bacteria by bacteria found within bacterial arrangements), n (number of bacteria found per arrangement), N (bacterial density of overall environment), L (phage latent period, which is the duration of a phage infection), and  (number of bacteria per arrangement lost subsequent to phage infection of one cell in the arrangement). Likelihood of phage adsorption of bacterial arrangements is n
(number of bacteria per arrangement lost subsequent to phage infection of one cell in the arrangement). Likelihood of phage adsorption of bacterial arrangements is n  and density of arrangements within environments is equal to N/n = N0/n (or indeed n0
and density of arrangements within environments is equal to N/n = N0/n (or indeed n0  and N0/n0, respectively, to reflect that n changes as a function of time in the figure). The inequality t ≥ 2L indicates how phage acquisition of bacteria within a bacterial arrangement, according to this model, involves at least two sequential rounds of phage infection. The absence of cells in the lower right is intentional as too is the reduction in cell number to nt in the lower left. Both of these reductions in cell number, going from middle to bottom, indicate phage-induced bacterial lysis.
and N0/n0, respectively, to reflect that n changes as a function of time in the figure). The inequality t ≥ 2L indicates how phage acquisition of bacteria within a bacterial arrangement, according to this model, involves at least two sequential rounds of phage infection. The absence of cells in the lower right is intentional as too is the reduction in cell number to nt in the lower left. Both of these reductions in cell number, going from middle to bottom, indicate phage-induced bacterial lysis.
 /k = 0.5 such that k/
/k = 0.5 such that k/  = 2) but each primary adsorption results in the loss of ten bacteria (
= 2) but each primary adsorption results in the loss of ten bacteria (  = 10), then the resulting 10 > 2 would imply that arrangements are more vulnerable to phages than are free bacteria. Indeed, in this example bacteria found within arrangements would be five times more vulnerable. Note further that if arrangements can reduce
= 10), then the resulting 10 > 2 would imply that arrangements are more vulnerable to phages than are free bacteria. Indeed, in this example bacteria found within arrangements would be five times more vulnerable. Note further that if arrangements can reduce  to 1, then arrangements will be expected to display lower vulnerability to phages than free bacteria so long as individual bacteria found within arrangements are less vulnerable to primary phage adsorptions than free bacteria (i.e., again, such that k >
to 1, then arrangements will be expected to display lower vulnerability to phages than free bacteria so long as individual bacteria found within arrangements are less vulnerable to primary phage adsorptions than free bacteria (i.e., again, such that k >  ). This is an observation that could very well explain the utility of abortive infection systems to bacteria, i.e., phage-resistance mechanisms in which both adsorbed bacteria and adsorbing phages die [30]. Alternatively, for
). This is an observation that could very well explain the utility of abortive infection systems to bacteria, i.e., phage-resistance mechanisms in which both adsorbed bacteria and adsorbing phages die [30]. Alternatively, for  = 0, which is the case given successful bacterial display of, for example, antibacterial restriction-modification systems, then biologically the inequality no longer holds, i.e., 0 > k/
= 0, which is the case given successful bacterial display of, for example, antibacterial restriction-modification systems, then biologically the inequality no longer holds, i.e., 0 > k/  . Logically, though, in this case arrangements should be no more or less vulnerable to phages than free bacteria since, in fact, neither would be vulnerable. See Table 1, under “Higher” phage densities for summary. Note, though, that calculations relevant to the “Lower” phage densities portion of the Table, i.e., Equation (7), are not discussed until Sections 2.3.3 and 2.3.4.
. Logically, though, in this case arrangements should be no more or less vulnerable to phages than free bacteria since, in fact, neither would be vulnerable. See Table 1, under “Higher” phage densities for summary. Note, though, that calculations relevant to the “Lower” phage densities portion of the Table, i.e., Equation (7), are not discussed until Sections 2.3.3 and 2.3.4. ≥ 1. Calculations relevant to lower phage densities, as found in the bottom portion of the table, are not discussed until Section 2.3.3 and especially section 2.3.4.
≥ 1. Calculations relevant to lower phage densities, as found in the bottom portion of the table, are not discussed until Section 2.3.3 and especially section 2.3.4.
| Environmental Phage Density (P) | Phage Propagation Ability Through Arrangements (  ) ) | |||
|---|---|---|---|---|
| Higher | Lower | |||
| Higher (bacterial losses dominate dynamics) | For  [lesser or no impediments to phage propagation within arrangements] | For  [impediments less than absolute] | For  [e.g., abortive infections] | For  [e.g., phage restriction] |
| Lower (bacterial gains dominate dynamics) | For  [which, as P → 0, is more likely] |  | [assuming phage-independent advantages to arrangement formation, i.e., μA - μN > 0, and that μA - μN > P(   -k)holds, which is likely given both P → 0 and -k)holds, which is likely given both P → 0 and  → 0] → 0] | |
2.3. Utility of Group Living in Light of Phages
 ) than for free bacteria (k) but nonetheless that arrangements can be more vulnerable to exploitation by phages. If we assume that arrangements nevertheless provide bacteria with selective benefits, then it should be possible to consider how large these benefits must be to offset costs stemming from this presumed increased susceptibility of arrangements to phage infection. Before moving on to that issue, however, I first address two underlying considerations, (1) how existence within clonal associations in fact might benefit bacteria and (2) experimental evidence that phages can propagate through bacterial arrangements and/or microcolonies.
) than for free bacteria (k) but nonetheless that arrangements can be more vulnerable to exploitation by phages. If we assume that arrangements nevertheless provide bacteria with selective benefits, then it should be possible to consider how large these benefits must be to offset costs stemming from this presumed increased susceptibility of arrangements to phage infection. Before moving on to that issue, however, I first address two underlying considerations, (1) how existence within clonal associations in fact might benefit bacteria and (2) experimental evidence that phages can propagate through bacterial arrangements and/or microcolonies.2.3.1. Selective Benefits of Living in Arrangements
2.3.2. Susceptibility of Bacterial Arrangements and Microcolonies to Phage Exploitation
2.3.3. Phage-Mediated Costs of Existing as Arrangements


 P
P  N/n in Equation (7), or simply kPN in Equation (8), describes those bacteria that have been lost from the unadsorbed bacterial pool, N, as a consequence of phage adsorption either of themselves (Equation (7) and Equation (8)) or of the arrangement in which those bacteria are located (Equation (7)). As noted above (i.e., A0 = N0/n in Section 2.2.2), the expression N / n as found in Equation (7) is a description of the density of arrangements consisting of n bacteria that are found in the environment in question. Also as above, the parameter
N/n in Equation (7), or simply kPN in Equation (8), describes those bacteria that have been lost from the unadsorbed bacterial pool, N, as a consequence of phage adsorption either of themselves (Equation (7) and Equation (8)) or of the arrangement in which those bacteria are located (Equation (7)). As noted above (i.e., A0 = N0/n in Section 2.2.2), the expression N / n as found in Equation (7) is a description of the density of arrangements consisting of n bacteria that are found in the environment in question. Also as above, the parameter  describes the number of bacteria that will be lost to phage infection given phage adsorption of an arrangement. Lastly, n
describes the number of bacteria that will be lost to phage infection given phage adsorption of an arrangement. Lastly, n  P is a description of the per-arrangement rate of bacterial adsorption by phages given an environmental phage density of P.
P is a description of the per-arrangement rate of bacterial adsorption by phages given an environmental phage density of P. P and each of those adsorptions results in a loss of
P and each of those adsorptions results in a loss of  bacteria. This compares with the rate of loss of individual bacteria as described by kP in Equation (8). The expression, μN with either subscript, by contrast, describes in both equations the gains in bacterial density that occur as a consequence of bacterial replication. Thus, If
bacteria. This compares with the rate of loss of individual bacteria as described by kP in Equation (8). The expression, μN with either subscript, by contrast, describes in both equations the gains in bacterial density that occur as a consequence of bacterial replication. Thus, If  P
P  > µA , then the bacterial population will experience a net decline in number whereas
> µA , then the bacterial population will experience a net decline in number whereas  P
P  < µA indicates net gains and
< µA indicates net gains and  P
P  = µA defines a steady state. For Equation (8) the equivalent expressions instead are respectively kP > μN , kP < μN , and kP = μN, where P in the latter can be described as an inundation threshold or even phage minimum inhibitory concentration, that is, of bacteria [25]. In the absence of phages, the bacterial population will simply grow at rates specified by μA or μN.
= µA defines a steady state. For Equation (8) the equivalent expressions instead are respectively kP > μN , kP < μN , and kP = μN, where P in the latter can be described as an inundation threshold or even phage minimum inhibitory concentration, that is, of bacteria [25]. In the absence of phages, the bacterial population will simply grow at rates specified by μA or μN.
 ). Specifically, the larger
). Specifically, the larger  P
P  then the more bacteria found within arrangements that are lost to phage infection. For example, twice as many bacteria will be lost per unit time for bacterial arrangements (
then the more bacteria found within arrangements that are lost to phage infection. For example, twice as many bacteria will be lost per unit time for bacterial arrangements (  P
P  from Equation (7)) versus free bacteria (kP from Equation (8)) if
from Equation (7)) versus free bacteria (kP from Equation (8)) if  P
P  /k = 2.
/k = 2.  P
P  /k-fold more arrangement-associated bacteria will be lost versus free bacteria for any given phage density, P. Forming into an arrangement, and thereby incurring costs of additional vulnerability to phages, therefore should be worthwhile to bacteria only to the extent that μA, or some other measure of bacterial fitness, increases as a consequence of group living to a larger extent than bacterial fitness decreases as a result of incurring a greater spatial vulnerability to phages, i.e., as described by
/k-fold more arrangement-associated bacteria will be lost versus free bacteria for any given phage density, P. Forming into an arrangement, and thereby incurring costs of additional vulnerability to phages, therefore should be worthwhile to bacteria only to the extent that μA, or some other measure of bacterial fitness, increases as a consequence of group living to a larger extent than bacterial fitness decreases as a result of incurring a greater spatial vulnerability to phages, i.e., as described by  P
P  /k.
/k.2.3.4. Importance of Reduced Vulnerability to Phages
 = 1 but nonetheless where it is possible for
= 1 but nonetheless where it is possible for  .tif (Equation (7) versus Equation (8)). At a minimum, however, μA =
.tif (Equation (7) versus Equation (8)). At a minimum, however, μA =  P
P  is necessary for bacterial fitness, as measured here in terms of increases in bacterial growth rates, to offset costs due to phage adsorption, and this compares with μN = kP for free bacteria; see Abedon and Thomas-Abedon [19] along with references cited, or Abedon [25], for derivation of the latter.
is necessary for bacterial fitness, as measured here in terms of increases in bacterial growth rates, to offset costs due to phage adsorption, and this compares with μN = kP for free bacteria; see Abedon and Thomas-Abedon [19] along with references cited, or Abedon [25], for derivation of the latter.  P
P  > kP, then the growth rate or other measure of the fitness of bacterial arrangements must be greater than that of free bacteria by that amount, e.g., μA-μN >
> kP, then the growth rate or other measure of the fitness of bacterial arrangements must be greater than that of free bacteria by that amount, e.g., μA-μN >  P
P  - kP =P(
- kP =P( 
 - k), to offset increased phage-associated costs that are borne by bacterial arrangements. This fitness improvement, however, need not be substantial unless phage densities (P) are also substantial. Thus, as
- k), to offset increased phage-associated costs that are borne by bacterial arrangements. This fitness improvement, however, need not be substantial unless phage densities (P) are also substantial. Thus, as  or
or  increase so too does the potential for phages to block the evolution of bacterial arrangements, but at the same time such increases do not serve as absolute blocks on this evolution. The alternative perspective is that given sufficiently high phage densities—but not too high, as indicated in the previous paragraph—then evolution could tend to favor reductions in
increase so too does the potential for phages to block the evolution of bacterial arrangements, but at the same time such increases do not serve as absolute blocks on this evolution. The alternative perspective is that given sufficiently high phage densities—but not too high, as indicated in the previous paragraph—then evolution could tend to favor reductions in  even if bacteria otherwise experience benefits from forming into arrangements, that is, reduced formation of arrangements could serve as a bacterial anti-phage strategy. In simple terms, a coccus might encounter a phage approximately half as often as a diplococcus.
even if bacteria otherwise experience benefits from forming into arrangements, that is, reduced formation of arrangements could serve as a bacterial anti-phage strategy. In simple terms, a coccus might encounter a phage approximately half as often as a diplococcus. 2.3.5. Reduced Bacterial Densities as Phage-Resistance Strategy
3. Experimental Section
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Ann. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar]
- Kjelleberg, S.; Givskov, M. The Biofilm Mode of Life: Mechanisms and Adaptations; Horizon Biosciences: Norfolk, UK, 2007. [Google Scholar]
- Ramage, G.; Culshaw, S.; Jones, B.; Williams, C. Are we any closer to beating the biofilm: Novel methods of biofilm control. Curr. Opin. Infect. Dis. 2010, 23, 560–566. [Google Scholar]
- Gino, E.; Starosvetsky, J.; Kurzbaum, E.; Armon, R. Combined chemical-biological treatment for prevention/rehabilitation of clogged wells by an iron-oxidizing bacterium. Environ. Sci. Technol. 2010, 44, 3123–3129. [Google Scholar]
- Cos, P.; Tote, K.; Horemans, T.; Maes, L. Biofilms: An extra hurdle for effective antimicrobial therapy. Curr. Pharm. Des. 2010, 16, 2279–2295. [Google Scholar]
- Abedon, S.T. Kinetics of phage-mediated biocontrol of bacteria. Foodborne Pathog. Dis. 2009, 6, 807–815. [Google Scholar]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar]
- Abedon, S.T. Bacteriophages and biofilms. In Biofilms: Formation, Development and Properties; Bailey, W.C., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2010; pp. 1–58, Chapter 1. [Google Scholar]
- Abedon, S.T. Bacteriophages and Biofilms: Ecology, Phage Therapy, Plaques; Nova Science Publishers: Hauppauge, NY, USA, 2011. [Google Scholar]
- Abedon, S.T.; Thomas-Abedon, C.; Thomas, A.; Mazure, H. Bacteriophage prehistory: Is or is not Hankin, 1896, a phage reference? Bacteriophage 2011, 1, 174–178. [Google Scholar] [CrossRef]
- Twort, F.W. An investigation on the nature of ultra-microscopic viruses. Lancet 1915, ii, 1241–1243. [Google Scholar]
- Twort, F.W. An investigation on the nature of ultra-microscopic viruses. Bacteriophage 2011, 1, 127–129. [Google Scholar]
- Abedon, S.T.; Yin, J. Impact of spatial structure on phage population growth. In Bacteriophage Ecology; Abedon, S.T., Ed.; Cambridge University Press: Cambridge, UK, 2008; Volume 15, pp. 94–113, Chapter 4. [Google Scholar]
- Krone, S.M.; Abedon, S.T. Modeling phage plaque growth. In Bacteriophage Ecology; Abedon, S.T., Ed.; Cambridge University Press: Cambridge, UK, 2008; Volume 15, pp. 415–438, Chapter 16. [Google Scholar]
- Abedon, S.T.; Yin, J. Bacteriophage plaques: Theory and analysis. Meth. Mol. Biol. 2009, 501, 161–174. [Google Scholar]
- Gallet, R.; Shao, Y.; Wang, I.N. High adsorption rate is detrimental to bacteriophage fitness in a biofilm-like environment. BMC Evol. Biol. 2009, 9, 241. [Google Scholar]
- Gallet, R.; Kannoly, S.; Wang, I.N. Effects of bacteriophage traits on plaque formation. BMC Microbiol. 2011, 11, 181. [Google Scholar]
- Abedon, S.T. Communication among phages, bacteria, and soil environments. In Biocommunication of Soil Microorganisms; Witzany, G., Ed.; Springer: New York, NY, USA, 2011; Volume 23, pp. 37–65, Chapter 2. [Google Scholar]
- Abedon, S.T.; Thomas-Abedon, C. Phage therapy pharmacology. Curr. Pharm. Biotechnol. 2010, 11, 28–47. [Google Scholar]
- Gill, J.J.; Hyman, P. Phage choice, isolation and preparation for phage therapy. Curr. Pharm. Biotechnol. 2010, 11, 2–14. [Google Scholar]
- Lu, T.K.; Collins, J.J. Dispersing biofilms with engineered enzymatic bacteriophage. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 11197–11202. [Google Scholar]
- Goodridge, L.D. Designing phage therapeutics. Curr. Pharm. Biotechnol. 2010, 11, 15–27. [Google Scholar]
- Mondes, R.; O'Toole, G.A. The developmental model of microbial biofilms: Ten years of a paradigm up for review. Trends Microbiol. 2009, 17, 73–87. [Google Scholar]
- Murray, A.G.; Jackson, G.A. Viral dynamics: A model of the effects of size, shape, motion, and abundance of single-celled planktonic organisms and other particles. Mar. Ecol. Prog. Ser. 1992, 89, 103–116. [Google Scholar] [CrossRef]
- Abedon, S. Phage therapy pharmacology: Calculating phage dosing. Adv. Appl. Microbiol. 2011, 77, 1–40. [Google Scholar]
- Thingstad, T.F. Elements of a theory for the mechanisms controlling abundance, diversity, and biogeochemical role of lytic bacterial viruses in aquatic systems. Limnol. Oceanogr. 2000, 45, 1320–1328. [Google Scholar] [CrossRef]
- Thingstad, T.F.; Bratbak, G.; Heldal, M. Aquatic phage ecology. In Bacteriophage Ecology; Abedon, S.T., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 251–280, Chapter 10. [Google Scholar]
- Chao, L.; Levin, B.R.; Stewart, F.M. A complex community in a simple habitat: An experimental study with bacteria and phage. Ecology 1977, 58, 369–378. [Google Scholar]
- Schrag, S.; Mittler, J.E. Host-parasite persistence: The role of spatial refuges in stabilizing bacteria-phage interactions. Am. Nat. 1996, 148, 348–377. [Google Scholar]
- Hyman, P.; Abedon, S.T. Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 2010, 70, 217–248. [Google Scholar]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar]
- Abedon, S.T. Bacterial 'immunity' against bacteriophages. Bacteriophage. 2012, 2. Available online: http://www.landesbioscience.com/journals/bacteriophage/article/18609/ (accessed on 13 April 2012).
- Abedon, S.T. Lysis of lysis inhibited bacteriophage T4-infected cells. J. Bacteriol. 1992, 174, 8073–8080. [Google Scholar]
- Abedon, S.T. Envisaging bacteria as phage targets. Bacteriophage 2011, 1, 228–230. [Google Scholar]
- Stent, G.S. Molecular Biology of Bacterial Viruses; WH Freeman and Co.: San Francisco, CA, USA, 1963. [Google Scholar]
- Ioannou, C.C.; Bartumeus, F.; Krause, J.; Ruxton, G.D. Unified effects of aggregation reveal larger prey groups take longer to find. Proc. Biol. Sci 2011, 278, 2985–2990. [Google Scholar] [Green Version]
- Abedon, S.T. Phage population growth: Constraints, games, adaptation. In Bacteriophage Ecology; Abedon, S.T., Ed.; Cambridge University Press: Cambridge, UK, 2008; Volume 15, pp. 64–93, Chapter 3. [Google Scholar]
- Kasman, L.M.; Kasman, A.; Westwater, C.; Dolan, J.; Schmidt, M.G.; Norris, J.S. Overcoming the phage replication threshold: A mathematical model with implications for phage therapy. J. Virol. 2002, 76, 5557–5564. [Google Scholar]
- Abedon, S.T. Bacteriophage T4 resistance to lysis-inhibition collapse. Genet. Res. 1999, 74, 1–11. [Google Scholar]
- Young, K.D. The selective value of bacterial shape. Microbiol. Mol. Biol. Rev. 2006, 70, 660–703. [Google Scholar]
- Young, K.D. Bacterial morphology: Why have different shapes? Curr. Opin. Microbiol. 2007, 10, 596–600. [Google Scholar]
- Azeredo, J.; Sutherland, I.W. The use of phages for the removal of infectious biofilms. Curr. Pharm. Biotechnol. 2008, 9, 261–266. [Google Scholar]
- Turner, G.F.; Pitcher, T.J. Attack abatement: A model for group protection by combined avoidance and dilution. Am. Nat. 1986, 128, 228–240. [Google Scholar]
- Babic, A.; Berkmen, M.B.; Lee, C.A.; Grossman, A.D. Efficient gene transfer in bacterial cell chains. MBio 2011, 2, e00027-11. [Google Scholar]
- Abedon, S.T. Phages, ecology, evolution. In Bacteriophage Ecology; Abedon, S.T., Ed.; Cambridge University Press: Cambridge, UK, 2008; Volume 15, pp. 1–28, Chapter 1. [Google Scholar]
- Hagens, S.; Loessner, M.J. Bacteriophage for biocontrol of foodborne pathogens: Calculations and considerations. Curr. Pharm. Biotechnol. 2010, 11, 58–68. [Google Scholar]
- Goodridge, L.D. Phages, bacteria, and food. In Bacteriophage Ecology; Abedon, S.T., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 302–331, Chapter 12. [Google Scholar]
- Xavier, J.B.; Foster, K.R. Cooperation and conflict in microbial biofilms. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 876–881. [Google Scholar]
- Wimpenny, J. Ecological determinants of biofilm formation. Biofouling 1996, 10, 43–63. [Google Scholar]
- Nadell, C.D.; Xavier, J.B.; Levin, S.A.; Foster, K.R. The evolution of quorum sensing in bacterial biofilms. PLoS Biol. 2008, 6, e14. [Google Scholar]
- Crombach, A.; Hogeweg, P. Evolution of resource cycling in ecosystems and individuals. BMC Evol. Biol. 2009, 9, 122. [Google Scholar]
- Hoffmeister, M.; Martin, W. Interspecific evolution: Microbial symbiosis, endosymbiosis and gene transfer. Environ. Microbiol. 2003, 5, 641–649. [Google Scholar]
- Searcy, D.G. Metabolic integration during the evolutionary origin of mitochondria. Cell Res. 2003, 13, 229–238. [Google Scholar]
- Kreft, J.U. Biofilms promote altruism. Microbiology 2004, 150, 2751–2760. [Google Scholar]
- Nadell, C.D.; Bassler, B.L. A fitness trade-off between local competition and dispersal in Vibrio cholerae biofilms. Proc. Natl. Acad. Sci U. S. A. 2011, 108, 14181–14185. [Google Scholar]
- Takahashi, N.; Nyvad, B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar]
- Kolter, R. Biofilms in lab and nature: A molecular geneticist's voyage to microbial ecology. Int. Microbiol. 2010, 13, 1–7. [Google Scholar]
- Whiteley, M.; Ott, J.R.; Weaver, E.A.; McLean, R.J.C. Effects of community composition and growth rate on aquifer biofilm bacteria and their susceptibility to betadine disinfection. Environ. Microbiol. 2001, 3, 43–52. [Google Scholar]
- Matz, C. Biofilms as refuge against predation. In The Biofilm Mode of Life: Mechanisms and Adaptations; Kjelleberg, S., Givskov, M., Eds.; Horizon Bioscience: Norfolk, UK, 2007; pp. 195–213, Chapter11. [Google Scholar]
- Thurlow, L.R.; Hanke, M.L.; Fritz, T.; Angle, A.; Aldrich, A.; Williams, S.H.; Engebretsen, I.L.; Bayles, K.W.; Horswill, A.R.; Kielian, T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 2011, 186, 6585–6596. [Google Scholar]
- Arciola, C.R. Host defense against implant infection: The ambivalent role of phagocytosis. Int. J. Artif. Organs 2010, 33, 565–567. [Google Scholar]
- Jensen, P.O.; Givskov, M.; Bjarnsholt, T.; Moser, C. The immune system vs. Pseudomonas aeruginosa biofilms. FEMS Immunol. Med. Microbiol. 2010, 59, 292–305. [Google Scholar]
- Costerton, J.W.; Cheng, J.-J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial biofilms in nature and disease. Ann. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar]
- Barron, B.A.; Fischetti, V.A.; Zabriskie, J.B. Studies of the bacteriophage kinetics of multicellular systems: A statistical model for the estimation of burst size per cell in streptococci. J. Appl. Bacteriol. 1970, 33, 436–442. [Google Scholar]
- Friend, P.L.; Slade, A.D. Characteristics of group A streptococcal bacteriophages. J. Bacteriol. 1966, 92, 148–154. [Google Scholar]
- Fischetti, V.A.; Barron, B.; Zabriskie, J.B. Studies on streptococcal bacteriophages. I. burst size and intracellular growth of group A and group C streptococcal bacteriophages. J. Exp. Med. 1968, 127, 475–488. [Google Scholar] [CrossRef]
- Kaplan, D.A.; Naumovski, L.; Rothschild, B.; Collier, R.J. Appendix: A model of plaque formation. Gene 1981, 13, 221–225. [Google Scholar]
- Doolittle, M.M.; Cooney, J.J.; Caldwell, D.E. Tracing the interaction of bacteriophage with bacterial biofilms using fluorescent and chromogenic probes. J. Indust. Microbiol. 1996, 16, 331–341. [Google Scholar]
- Abedon, S.T. Bacteriophage intraspecific cooperation and defection. In Contemporary Trends in Bacteriophage Research; Adams, H.T., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2009; pp. 191–215, Chapter 7. [Google Scholar]
- Bohannan, B.J.M.; Lenski, R.E. Effect of prey heterogeneity on the response of a food chain to resource enrichment. Am. Nat. 1999, 153, 73–82. [Google Scholar]
- Bohannan, B.J.M.; Lenski, R.E. Linking genetic change to community evolution: Insights from studies of bacteria and bacteriophage. Ecol. Lett. 2000, 3, 362–377. [Google Scholar]
- Terborgh, J.; Lopez, L.; Nunez, V.; Rao, M.; Shahabuddin, G.; Orihuela, G.; Riveros, M.; Ascanio, R.; Adler, G.H.; Lambert, T.D.; Balbas, L. Ecological meltdown in predator-free forest fragments. Science 2001, 294, 1923–1926. [Google Scholar]
- Abedon, S.T. Phage evolution and ecology. Adv. Appl. Microbiol. 2009, 67, 1–45. [Google Scholar]
- Kutter, E.; De Vos, D.; Gvasalia, G.; Alavidze, Z.; Gogokhia, L.; Kuhl, S.; Abedon, S.T. Phage therapy in clinical practice: Treatment of human infections. Curr. Pharm. Biotechnol. 2010, 11, 69–86. [Google Scholar]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abedon, S.T. Spatial Vulnerability: Bacterial Arrangements, Microcolonies, and Biofilms as Responses to Low Rather than High Phage Densities. Viruses 2012, 4, 663-687. https://0-doi-org.brum.beds.ac.uk/10.3390/v4050663
Abedon ST. Spatial Vulnerability: Bacterial Arrangements, Microcolonies, and Biofilms as Responses to Low Rather than High Phage Densities. Viruses. 2012; 4(5):663-687. https://0-doi-org.brum.beds.ac.uk/10.3390/v4050663
Chicago/Turabian StyleAbedon, Stephen T. 2012. "Spatial Vulnerability: Bacterial Arrangements, Microcolonies, and Biofilms as Responses to Low Rather than High Phage Densities" Viruses 4, no. 5: 663-687. https://0-doi-org.brum.beds.ac.uk/10.3390/v4050663