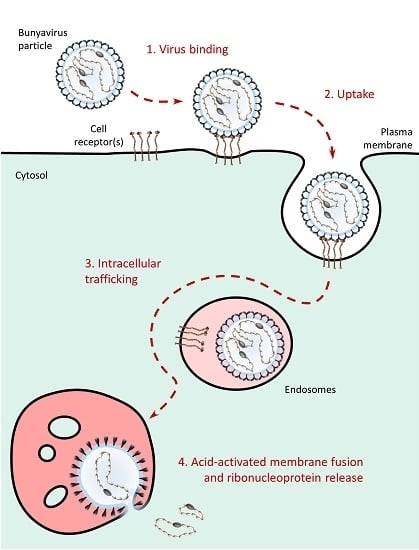
Early Bunyavirus-Host Cell Interactions
Abstract
:1. Introduction
2. Bunyavirus Genome Organization and Virion Structure
3. Receptors for Arbo-Bunyaviruses in Mammalian Hosts
4. Receptors for Plant-Specific Bunyaviruses
5. Receptors for Aerosol-Transmitted Bunyaviruses: How Hantaviruses Target Cells
6. Bunyavirus Uptake
7. Bunyavirus Intracellular Trafficking
8. Bunyavirus-Cell Membrane Fusion
9. Concluding Remarks and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ANDV | Andes virus |
| BCCV | Black Creek Canal virus |
| BMP | phospholipid bis(monoacylglycerol) |
| CCHFV | Crimean-Congo hemorrhagic fever virus |
| CME | clathrin-mediated endocytosis |
| DAF | decay-accelerated factor |
| DC | dendritic cell |
| DC-SIGN | dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin |
| DN | dominant negative |
| EE | early endosome |
| ER | endoplasmic reticulum |
| GAG | glycosaminoglycan |
| HTNV | Hantaan virus |
| HUVEC | human umbilical vein endothelial cell |
| LACV | La Crosse virus |
| LE | late endosome |
| LL | di-leucine |
| L-SIGN | liver/lymph node-specific intercellular adhesion molecule 3-grabbing non-integrin |
| MVB | multivesicular body |
| NMMHC-IIA | non-muscle myosin heavy chain IIA |
| NY-1V | New York-1 virus |
| OROV | Oropouche virus |
| PHV | Prospect Hill virus |
| PSI | plexin-semaphorin-integrin |
| PUUV | Puumula virus |
| RNP | ribonucleoprotein |
| RVFV | Rift Valley fever virus |
| SEOV | Seoul virus |
| SFTSV | severe fever with thrombocytopenia syndrome virus |
| siRNA | small interfering RNA |
| SNV | Sin Nombre virus |
| TOSV | Toscana virus |
| TSWV | tomato spotted wilt virus |
| UUKV | Uukuniemi virus |
References
- Schmaljohn, C.; Elliott, R.M. Bunyaviridae. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: PA, USA, 2014; Volume 1, pp. 1244–1282. [Google Scholar]
- Junglen, S.; Drosten, C. Virus discovery and recent insights into virus diversity in arthropods. Curr. Opin. Microbiol. 2013, 16, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Vaheri, A.; Strandin, T.; Hepojoki, J.; Sironen, T.; Henttonen, H.; Makela, S.; Mustonen, J. Uncovering the mysteries of hantavirus infections. Nat. Rev. Microbiol. 2013, 11, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M.; Brennan, B. Emerging phleboviruses. Curr. Opin. Virol. 2014, 5, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M. Orthobunyaviruses: recent genetic and structural insights. Nat. Rev. Microbiol. 2014, 12, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Lasecka, L.; Baron, M.D. The molecular biology of nairoviruses, an emerging group of tick-borne arboviruses. Arch. Virol. 2014, 159, 1249–1265. [Google Scholar] [CrossRef] [PubMed]
- Rotenberg, D.; Jacobson, A.L.; Schneweis, D.J.; Whitfield, A.E. Thrips transmission of tospoviruses. Curr. Opin. Virol. 2015, 15, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Leger, P.; Lozach, P.Y. Bunyaviruses: from transmission by arthropods to virus entry into the mammalian host first-target cells. Future Virol. 2015, 10, 859–881. [Google Scholar] [CrossRef]
- Horne, K.M.; Vanlandingham, D.L. Bunyavirus-vector interactions. Viruses 2014, 6, 4373–4397. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C.; Zhang, Y.Z. The evolution and emergence of hantaviruses. Curr. Opin. Virol. 2015, 10, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, R.; Melin, L. Synthesis, assembly and intracellular transport of Bunyaviridae. membrane proteins. In The Bunyaviridae; Elliott, R.M., Ed.; Plenum Press: New York, NY, USA, 1996; pp. 159–188. [Google Scholar]
- Ferron, F.; Li, Z.; Danek, E.I.; Luo, D.; Wong, Y.; Coutard, B.; Lantez, V.; Charrel, R.; Canard, B.; Walz, T.; Lescar, J. The hexamer structure of Rift Valley fever virus nucleoprotein suggests a mechanism for its assembly into ribonucleoprotein complexes. PLoS Pathog. 2011, 7, e1002030. [Google Scholar] [CrossRef] [PubMed]
- Raymond, D.D.; Piper, M.E.; Gerrard, S.R.; Skiniotis, G.; Smith, J.L. Phleboviruses encapsidate their genomes by sequestering RNA bases. Proc. Natl. Acad. Sci. USA 2012, 109, 19208–19213. [Google Scholar] [CrossRef] [PubMed]
- Reguera, J.; Cusack, S.; Kolakofsky, D. Segmented negative strand RNA virus nucleoprotein structure. Curr. Opin. Virol. 2014, 5, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dutta, S.; Karlberg, H.; Devignot, S.; Weber, F.; Hao, Q.; Tan, Y.J.; Mirazimi, A.; Kotaka, M. Structure of Crimean-Congo hemorrhagic fever virus nucleoprotein: superhelical homo-oligomers and the role of caspase-3 cleavage. J. Virol. 2012, 86, 12294–12303. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.D.; Surtees, R.; Walter, C.T.; Ariza, A.; Bergeron, E.; Nichol, S.T.; Hiscox, J.A.; Edwards, T.A.; Barr, J.N. Structure, function, and evolution of the Crimean-Congo hemorrhagic fever virus nucleocapsid protein. J. Virol. 2012, 86, 10914–10923. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, W.; Ji, W.; Deng, M.; Sun, Y.; Zhou, H.; Yang, C.; Deng, F.; Wang, H.; Hu, Z.; Lou, Z.; Rao, Z. Crimean-Congo hemorrhagic fever virus nucleoprotein reveals endonuclease activity in bunyaviruses. Proc. Natl. Acad. Sci. USA 2012, 109, 5046–5051. [Google Scholar] [CrossRef] [PubMed]
- Ariza, A.; Tanner, S.J.; Walter, C.T.; Dent, K.C.; Shepherd, D.A.; Wu, W.; Matthews, S.V.; Hiscox, J.A.; Green, T.J.; Luo, M.; et al. Nucleocapsid protein structures from orthobunyaviruses reveal insight into ribonucleoprotein architecture and RNA polymerization. Nucleic Acids Res. 2013, 41, 5912–5926. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Li, P.; Elliott, R.M.; Dong, C. Structure of Schmallenberg orthobunyavirus nucleoprotein suggests a novel mechanism of genome encapsidation. J. Virol. 2013, 87, 5593–5601. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Ouyang, S.; Liang, M.; Niu, F.; Shaw, N.; Wu, W.; Ding, W.; Jin, C.; Peng, Y.; Zhu, Y.; et al. Structure of severe fever with thrombocytopenia syndrome virus nucleocapsid protein in complex with suramin reveals therapeutic potential. J. Virol. 2013, 87, 6829–6839. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Q.; Pan, X.; Fernandez de Castro, I.; Sun, Y.; Guo, Y.; Tao, X.; Risco, C.; Sui, S.F.; Lou, Z. Bunyamwera virus possesses a distinct nucleocapsid protein to facilitate genome encapsidation. Proc. Natl. Acad. Sci. USA 2013, 110, 9048–9053. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Shaw, N.; Wang, Y.E.; Jiao, L.; Ding, W.; Li, X.; Zhu, P.; Upur, H.; Ouyang, S.; Cheng, G.; et al. Structure of the Leanyer orthobunyavirus nucleoprotein-RNA complex reveals unique architecture for RNA encapsidation. Proc. Natl. Acad. Sci. USA 2013, 110, 9054–9059. [Google Scholar] [CrossRef] [PubMed]
- Olal, D.; Dick, A.; Woods, V.L., Jr.; Liu, T.; Li, S.; Devignot, S.; Weber, F.; Saphire, E.O.; Daumke, O. Structural insights into RNA encapsidation and helical assembly of the Toscana virus nucleoprotein. Nucleic Acids Res. 2014, 42, 6025–6037. [Google Scholar] [CrossRef] [PubMed]
- Raymond, D.D.; Piper, M.E.; Gerrard, S.R.; Smith, J.L. Structure of the Rift Valley fever virus nucleocapsid protein reveals another architecture for RNA encapsidation. Proc. Natl. Acad. Sci. USA 2010, 107, 11769–11774. [Google Scholar] [CrossRef] [PubMed]
- Reguera, J.; Malet, H.; Weber, F.; Cusack, S. Structural basis for encapsidation of genomic RNA by La Crosse orthobunyavirus nucleoprotein. Proc. Natl. Acad. Sci. USA 2013, 110, 7246–7251. [Google Scholar] [CrossRef] [PubMed]
- Olal, D.; Daumke, O. Structure of the Hantavirus nucleoprotein provides insights into the mechanism of RNA encapsidation. Cell Rep. 2016, 14, 2092–2099. [Google Scholar] [CrossRef] [PubMed]
- Surtees, R.; Ariza, A.; Punch, E.K.; Trinh, C.H.; Dowall, S.D.; Hewson, R.; Hiscox, J.A.; Barr, J.N.; Edwards, T.A. The crystal structure of the Hazara virus nucleocapsid protein. BMC Struct. Biol. 2015, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Eifan, S.; Schnettler, E.; Dietrich, I.; Kohl, A.; Blomstrom, A.L. Non-structural proteins of arthropod-borne bunyaviruses: roles and functions. Viruses 2013, 5, 2447–2468. [Google Scholar] [CrossRef] [PubMed]
- Vera-Otarola, J.; Solis, L.; Soto-Rifo, R.; Ricci, E.P.; Pino, K.; Tischler, N.D.; Ohlmann, T.; Darlix, J.L.; Lopez-Lastra, M. The Andes hantavirus NSs protein is expressed from the viral small mRNA by a leaky scanning mechanism. J. Virol. 2012, 86, 2176–2187. [Google Scholar] [CrossRef] [PubMed]
- Plyusnin, A. Genetics of hantaviruses: implications to taxonomy. Arch. Virol. 2002, 147, 665–682. [Google Scholar] [CrossRef] [PubMed]
- Jaaskelainen, K.M.; Plyusnina, A.; Lundkvist, A.; Vaheri, A.; Plyusnin, A. Tula hantavirus isolate with the full-length ORF for nonstructural protein NSs survives for more consequent passages in interferon-competent cells than the isolate having truncated NSs ORF. Virol J. 2008, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Plyusnin, A.; Beaty, B.J.; Elliott, R.M.; Goldbach, R.; Kormelink, R.; Lundkvist, Å.; Schmaljohn, C.S.; Tesh, R.B. The Bunyaviridae. In virus taxonomy: ninth report of the international committee on taxonomy of viruses. 2012 International committee on taxonomy of viruses. Elsevier Inc. 2012. [Google Scholar]
- Sanchez, A.J.; Vincent, M.J.; Nichol, S.T. Characterization of the glycoproteins of Crimean-Congo hemorrhagic fever virus. J. Virol. 2002, 76, 7263–7275. [Google Scholar] [CrossRef] [PubMed]
- Dessau, M.; Modis, Y. Crystal structure of glycoprotein C from Rift Valley fever virus. Proc. Natl. Acad. Sci. USA 2013, 110, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Garry, C.E.; Garry, R.F. Proteomics computational analyses suggest that the carboxyl terminal glycoproteins of Bunyaviruses are class II viral fusion protein (beta-penetrenes). Theor. Biol. Med. Model. 2004, 1, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tischler, N.D.; Gonzalez, A.; Perez-Acle, T.; Rosemblatt, M.; Valenzuela, P.D. Hantavirus Gc glycoprotein: evidence for a class II fusion protein. J. Gen. Virol. 2005, 86, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Plassmeyer, M.L.; Soldan, S.S.; Stachelek, K.M.; Martin-Garcia, J.; Gonzalez-Scarano, F. California serogroup Gc (G1) glycoprotein is the principal determinant of pH-dependent cell fusion and entry. Virology 2005, 338, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Munoz, N.; Salazar-Quiroz, N.; Tischler, N.D. Hantavirus Gn and Gc envelope glycoproteins: key structural units for virus cell entry and virus assembly. Viruses 2014, 6, 1801–1822. [Google Scholar] [CrossRef] [PubMed]
- Overby, A.K.; Pettersson, R.F.; Grunewald, K.; Huiskonen, J.T. Insights into bunyavirus architecture from electron cryotomography of Uukuniemi virus. Proc. Natl Acad. Sci. U S A 2008, 105, 2375–2379. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, A.N.; Sherman, M.B.; Morais, M.C.; Holbrook, M.R.; Watowich, S.J. Three-dimensional organization of Rift Valley fever virus revealed by cryoelectron tomography. J. Virol. 2008, 82, 10341–10348. [Google Scholar] [CrossRef] [PubMed]
- Huiskonen, J.T.; Overby, A.K.; Weber, F.; Grunewald, K. Electron cryo-microscopy and single-particle averaging of Rift Valley fever virus: evidence for Gn-Gc glycoprotein heterodimers. J. Virol. 2009, 83, 3762–3769. [Google Scholar] [CrossRef] [PubMed]
- Huiskonen, J.T.; Hepojoki, J.; Laurinmaki, P.; Vaheri, A.; Lankinen, H.; Butcher, S.J.; Grunewald, K. Electron cryotomography of Tula hantavirus suggests a unique assembly paradigm for enveloped viruses. J. Virol. 2010, 84, 4889–4897. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.B.; Freiberg, A.N.; Holbrook, M.R.; Watowich, S.J. Single-particle cryo-electron microscopy of Rift Valley fever virus. Virology 2009, 387, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Bowden, T.A.; Bitto, D.; McLees, A.; Yeromonahos, C.; Elliott, R.M.; Huiskonen, J.T. Orthobunyavirus ultrastructure and the curious tripodal glycoprotein spike. PLoS Pathog. 2013, 9, e1003374. [Google Scholar] [CrossRef] [PubMed]
- Battisti, A.J.; Chu, Y.K.; Chipman, P.R.; Kaufmann, B.; Jonsson, C.B.; Rossmann, M.G. Structural studies of Hantaan virus. J. Virol. 2011, 85, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Boulant, S.; Stanifer, M.; Lozach, P.Y. Dynamics of virus-receptor interactions in virus binding, signaling, and endocytosis. Viruses 2015, 7, 2794–2815. [Google Scholar] [CrossRef] [PubMed]
- Helenius, A. Virus Entry and Uncoating. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014. [Google Scholar]
- Jolly, C.L.; Sattentau, Q.J. Attachment factors. Adv. Exp. Med. Biol. 2013, 790, 1–23. [Google Scholar] [PubMed]
- Grove, J.; Marsh, M. The cell biology of receptor-mediated virus entry. J. Cell. Biol. 2011, 195, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- De Boer, S.M.; Kortekaas, J.; de Haan, C.A.; Rottier, P.J.; Moormann, R.J.; Bosch, B.J. Heparan sulfate facilitates Rift Valley fever virus entry into the cell. J. Virol. 2012, 86, 13767–13771. [Google Scholar] [CrossRef] [PubMed]
- Pietrantoni, A.; Fortuna, C.; Remoli, M.E.; Ciufolini, M.G.; Superti, F. Bovine lactoferrin inhibits Toscana virus infection by binding to heparan sulphate. Viruses 2015, 7, 480–495. [Google Scholar] [CrossRef] [PubMed]
- Riblett, A.M.; Blomen, V.A.; Jae, L.T.; Altamura, L.A.; Doms, R.W.; Brummelkamp, T.R.; Wojcechowskyj, J.A. A Haploid genetic screen identifies heparan sulfate proteoglycans supporting Rift Valley fever virus infection. J. Virol. 2015, 90, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Lozach, P.Y.; Kuhbacher, A.; Meier, R.; Mancini, R.; Bitto, D.; Bouloy, M.; Helenius, A. DC-SIGN as a receptor for phleboviruses. Cell Host Microbe 2011, 10, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, H.; Li, X.; Zhang, X.; Liu, W.; Kuhl, A.; Kaup, F.; Soldan, S.S.; Gonzalez-Scarano, F.; Weber, F.; He, Y.; et al. Severe fever with thrombocytopenia virus glycoproteins are targeted by neutralizing antibodies and can use DC-SIGN as a receptor for pH-dependent entry into human and animal cell lines. J. Virol. 2013, 87, 4384–4394. [Google Scholar] [CrossRef] [PubMed]
- Suda, Y.; Fukushi, S.; Tani, H.; Murakami, S.; Saijo, M.; Horimoto, T.; Shimojima, M. Analysis of the entry mechanism of Crimean-Congo hemorrhagic fever virus, using a vesicular stomatitis virus pseudotyping system. Arch. Virol. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Connolly-Andersen, A.M.; Douagi, I.; Kraus, A.A.; Mirazimi, A. Crimean-Congo hemorrhagic fever virus infects human monocyte-derived dendritic cells. Virology 2009, 390, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Connolly-Andersen, A.M.; Moll, G.; Andersson, C.; Akerstrom, S.; Karlberg, H.; Douagi, I.; Mirazimi, A. Crimean-Congo hemorrhagic fever virus activates endothelial cells. J. Virol. 2011, 85, 7766–7774. [Google Scholar] [CrossRef] [PubMed]
- Peyrefitte, C.N.; Perret, M.; Garcia, S.; Rodrigues, R.; Bagnaud, A.; Lacote, S.; Crance, J.M.; Vernet, G.; Garin, D.; Bouloy, M.; et al. Differential activation profiles of Crimean-Congo hemorrhagic fever virus- and Dugbe virus-infected antigen-presenting cells. J. Gen. Virol. 2010, 91, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Svajger, U.; Anderluh, M.; Jeras, M.; Obermajer, N. C-type lectin DC-SIGN: an adhesion, signalling and antigen-uptake molecule that guides dendritic cells in immunity. Cell. Signal. 2010, 22, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, L.; Roosendahl, P.; Ng, W.C.; Brooks, A.G.; Reading, P.C.; Londrigan, S.L. Endocytic function is critical for influenza A virus infection via DC-SIGN and L-SIGN. Sci. Rep. 2016, 6, 19428. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qi, Y.; Liu, C.; Gao, W.; Chen, P.; Fu, L.; Peng, B.; Wang, H.; Jing, Z.; Zhong, G.; et al. Nonmuscle myosin heavy chain IIA is a critical factor contributing to the efficiency of early infection of severe fever with thrombocytopenia syndrome virus. J. Virol. 2014, 88, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Leger, P.; Tetard, M.; Youness, B.; Cordes, N.; Rouxel, R.N.; Flamand, M.; Lozach, P.Y. Differential use of the C-type lectins L-SIGN and DC-SIGN for phlebovirus endocytosis. Traffic 2016, 17, 639–656. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Feng, Y.; Zhu, Z.; Dimitrov, D.S. Identification of a putative Crimean-Congo hemorrhagic fever virus entry factor. Biochem. Biophys. Res. Commun. 2011, 411, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.B.; Ullman, D.E.; Sherwood, J.L.; German, T.L. Immunoprecipitation of a 50-kDa protein: a candidate receptor component for tomato spotted wilt tospovirus (Bunyaviridae) in its main vector, Frankliniella occidentalis. Virus Res. 2000, 67, 109–118. [Google Scholar] [CrossRef]
- Bandla, M.D.; Campbell, L.R.; Ullman, D.E.; Sherwood, J.L. Interaction of tomato spotted wilt tospovirus (TSWV) glycoproteins with a thrips midgut protein, a potential cellular receptor for TSWV. Phytopathology 1998, 88, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Krautkramer, E.; Zeier, M. Hantavirus causing hemorrhagic fever with renal syndrome enters from the apical surface and requires decay-accelerating factor (DAF/CD55). J. Virol. 2008, 82, 4257–4264. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovskaya, I.N.; Shepley, M.; Shaw, R.; Ginsberg, M.H.; Mackow, E.R. Beta3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl Acad. Sci. U S A 1998, 95, 7074–7079. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovskaya, I.N.; Brown, E.J.; Ginsberg, M.H.; Mackow, E.R. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by beta3 integrins. J. Virol. 1999, 73, 3951–3959. [Google Scholar] [PubMed]
- Matthys, V.S.; Gorbunova, E.E.; Gavrilovskaya, I.N.; Mackow, E.R. Andes virus recognition of human and Syrian hamster beta3 integrins is determined by an L33P substitution in the PSI domain. J. Virol. 2010, 84, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kwon, Y.C.; Kim, S.I.; Park, J.M.; Lee, K.H.; Ahn, B.Y. A hantavirus causing hemorrhagic fever with renal syndrome requires gC1qR/p32 for efficient cell binding and infection. Virology 2008, 381, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Mou, D.L.; Wang, Y.P.; Huang, C.X.; Li, G.Y.; Pan, L.; Yang, W.S.; Bai, X.F. Cellular entry of Hantaan virus A9 strain: specific interactions with beta3 integrins and a novel 70kDa protein. Biochem. Biophys. Res. Commun. 2006, 339, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Raftery, M.J.; Lalwani, P.; Krautkrmer, E.; Peters, T.; Scharffetter-Kochanek, K.; Kruger, R.; Hofmann, J.; Seeger, K.; Kruger, D.H.; Schonrich, G. Beta2 integrin mediates hantavirus-induced release of neutrophil extracellular traps. J. Exp. Med. 2014, 211, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Pokidysheva, E.; Zhang, Y.; Battisti, A.J.; Bator-Kelly, C.M.; Chipman, P.R.; Xiao, C.; Gregorio, G.G.; Hendrickson, W.A.; Kuhn, R.J.; Rossmann, M.G. Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Cell 2006, 124, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Silvas, J.A.; Popov, V.L.; Paulucci-Holthauzen, A.; Aguilar, P.V. Extracellular vesicles mediate receptor-independent transmission of novel tick-borne bunyavirus. J. Virol. 2015, 90, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, R.L.; Batuman, O.; Webster, C.G.; Adkins, S. Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Annu. Rev. Virol. 2015, 2, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Tentchev, D.; Verdin, E.; Marchal, C.; Jacquet, M.; Aguilar, J.M.; Moury, B. Evolution and structure of tomato spotted wilt virus populations: evidence of extensive reassortment and insights into emergence processes. J. Gen. Virol. 2011, 92, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Nagata, T.; Inoue-Nagata, A.K.; Prins, M.; Goldbach, R.; Peters, D. Impeded thrips transmission of defective tomato spotted wilt virus isolates. Phytopathology 2000, 90, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Resende Rde, O.; de Haan, P.; de Avila, A.C.; Kitajima, E.W.; Kormelink, R.; Goldbach, R.; Peters, D. Generation of envelope and defective interfering RNA mutants of tomato spotted wilt virus by mechanical passage. J. Gen. Virol. 1991, 72, 2375–2383. [Google Scholar] [CrossRef] [PubMed]
- Ullman, D.E.; Westcot, D.M.; Chenault, K.D.; Sherwood, J.L.; German, T.L.; Bandla, M.D.; Cantone, F.A.; Duer, H.L. Compartmentalization, intracellular transport, and autophagy of tomato spotted wilt tospovirus proteins in infected thrips cells. Phytopathology 1996, 85, 644–654. [Google Scholar] [CrossRef]
- Sakimura, K. The present status of thrips-borne viruses. In Biological Tranmission of Disease Agents; Maramorosch, K., Ed.; Academic Press: New York, NY, USA, 1962; pp. 33–40. [Google Scholar]
- Ullman, D.E.; Cho, J.J.; Mau, R.F.L.; Westcot, D.M.; Cantone, D.M. A midgut barrier to TSWV acquisition by adult western flower thrips. Phytopathology 1992, 82, 1333–1342. [Google Scholar] [CrossRef]
- Ritzenthaler, C. Parallels and distinctions in the direct cell-to-cell spread of the plant and animal viruses. Curr. Opin. Virol. 2011, 1, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Storms, M.M.; Kormelink, R.; Peters, D.; Van Lent, J.W.; Goldbach, R.W. The nonstructural NSm protein of tomato spotted wilt virus induces tubular structures in plant and insect cells. Virology 1995, 214, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Kormelink, R.; Storms, M.; Van Lent, J.; Peters, D.; Goldbach, R. Expression and subcellular location of the NSm protein of tomato spotted wilt virus (TSWV), a putative viral movement protein. Virology 1994, 200, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Paape, M.; Solovyev, A.G.; Erokhina, T.N.; Minina, E.A.; Schepetilnikov, M.V.; Lesemann, D.E.; Schiemann, J.; Morozov, S.Y.; Kellmann, J.W. At-4/1, an interactor of the tomato spotted wilt virus movement protein, belongs to a new family of plant proteins capable of directed intra- and intercellular trafficking. Mol. Plant. Microbe Interact. 2006, 19, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Amari, K.; Boutant, E.; Hofmann, C.; Schmitt-Keichinger, C.; Fernandez-Calvino, L.; Didier, P.; Lerich, A.; Mutterer, J.; Thomas, C.L.; Heinlein, M.; et al. A family of plasmodesmal proteins with receptor-like properties for plant viral movement proteins. PLoS Pathog. 2010, 6, e1001119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, J.F. 2010: the year of the lung. Int. J. Tuberc. Lung Dis. 2010, 14, 1–4. [Google Scholar] [PubMed]
- Dalrymple, N.A.; Mackow, E.R. Virus interactions with endothelial cell receptors: implications for viral pathogenesis. Curr. Opin. Virol. 2014, 7, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovskaya, I.; Gorbunova, E.; Matthys, V.; Dalrymple, N.; Mackow, E. The role of the endothelium in HPS pathogenesis and potential therapeutic approaches. Adv. Virol. 2012, 2012, 467059. [Google Scholar] [CrossRef] [PubMed]
- Hepojoki, J.; Vaheri, A.; Strandin, T. The fundamental role of endothelial cells in hantavirus pathogenesis. Front. Microbiol. 2014, 5, 727. [Google Scholar] [CrossRef] [PubMed]
- Macneil, A.; Nichol, S.T.; Spiropoulou, C.F. Hantavirus pulmonary syndrome. Virus Res. 2011, 162, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Rasmuson, J.; Andersson, C.; Norrman, E.; Haney, M.; Evander, M.; Ahlm, C. Time to revise the paradigm of hantavirus syndromes? Hantavirus pulmonary syndrome caused by European hantavirus. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Schmaljohn, C.; Hjelle, B. Hantaviruses: a global disease problem. Emerg. Infect. Dis. 1997, 3, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.S.G.; Wennerberg, K.; Armulik, A.; Lohikangas, L. Fibronectin-integrin interactions. Front. Biosci. 1995, 2, 126–146. [Google Scholar] [CrossRef]
- Raymond, T.; Gorbunova, E.; Gavrilovskaya, I.N.; Mackow, E.R. Pathogenic hantaviruses bind plexin-semaphorin-integrin domains present at the apex of inactive, bent alphavbeta3 integrin conformers. Proc. Natl. Acad. Sci. U S A 2005, 102, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Askari, J.A.; Buckley, P.A.; Mould, A.P.; Humphries, M.J. Linking integrin conformation to function. J. Cell Sci. 2009, 122, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Tucker, S.P.; Compans, R.W. Virus infection of polarized epithelial cells. Adv. Virus Res. 1993, 42, 187–247. [Google Scholar] [PubMed]
- Bomsel, M.; Alfsen, A. Entry of viruses through the epithelial barrier: pathogenic trickery. Nat. Rev. Mol. Cell. Biol. 2003, 4, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, N.F.; Howard, C.; McKeating, J.A. Over the fence or through the gate: how viruses infect polarized cells. Immunotherapy 2012, 4, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Schoenenberger, C.A.; Zuk, A.; Zinkl, G.M.; Kendall, D.; Matlin, K.S. Integrin expression and localization in normal MDCK cells and transformed MDCK cells lacking apical polarity. J. Cell Sci. 1994, 107, 527–541. [Google Scholar] [PubMed]
- Manninen, A. Epithelial polarity--generating and integrating signals from the ECM with integrins. Exp. Cell. Res. 2015, 334, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Conforti, G.; Dominguez-Jimenez, C.; Zanetti, A.; Gimbrone, M.A., Jr.; Cremona, O.; Marchisio, P.C.; Dejana, E. Human endothelial cells express integrin receptors on the luminal aspect of their membrane. Blood 1992, 80, 437–446. [Google Scholar] [PubMed]
- Aplin, J.D.; Spanswick, C.; Behzad, F.; Kimber, S.J.; Vicovac, L. Integrins beta 5, beta 3 and alpha v are apically distributed in endometrial epithelium. Mol. Hum. Reprod. 1996, 2, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Gawaz, M.; Neumann, F.J.; Dickfeld, T.; Reininger, A.; Adelsberger, H.; Gebhardt, A.; Schomig, A. Vitronectin receptor (alpha(v)beta3) mediates platelet adhesion to the luminal aspect of endothelial cells: implications for reperfusion in acute myocardial infarction. Circulation 1997, 96, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Byzova, T.V.; Plow, E.F. Activation of alphaVbeta3 on vascular cells controls recognition of prothrombin. J. Cell. Biol. 1998, 143, 2081–2092. [Google Scholar] [CrossRef] [PubMed]
- Raftery, M.J.; Kraus, A.A.; Ulrich, R.; Kruger, D.H.; Schonrich, G. Hantavirus infection of dendritic cells. J. Virol. 2002, 76, 10724–10733. [Google Scholar] [CrossRef] [PubMed]
- Ravkov, E.V.; Nichol, S.T.; Compans, R.W. Polarized entry and release in epithelial cells of Black Creek Canal virus, a New World hantavirus. J. Virol. 1997, 71, 1147–1154. [Google Scholar] [PubMed]
- Rowe, R.K.; Pekosz, A. Bidirectional virus secretion and nonciliated cell tropism following Andes virus infection of primary airway epithelial cell cultures. J. Virol. 2006, 80, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Buranda, T.; Swanson, S.; Bondu, V.; Schaefer, L.; Maclean, J.; Mo, Z.; Wycoff, K.; Belle, A.; Hjelle, B. Equilibrium and kinetics of Sin Nombre hantavirus binding at DAF/CD55 functionalized bead surfaces. Viruses 2014, 6, 1091–1111. [Google Scholar] [CrossRef] [PubMed]
- Coyne, C.B.; Bergelson, J.M. Virus-induced ABL and FYN kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 2006, 124, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Sobo, K.; Rubbia-Brandt, L.; Brown, T.D.; Stuart, A.D.; McKee, T.A. Decay-accelerating factor binding determines the entry route of echovirus 11 in polarized epithelial cells. J. Virol. 2011, 85, 12376–12386. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.; Drake, M.J.; Bruce, E.A.; Riblett, A.M.; Didigu, C.A.; Wilen, C.B.; Malani, N.; Male, F.; Lee, F.H.; Bushman, F.D.; et al. The major cellular sterol regulatory pathway is required for Andes virus infection. PLoS Pathog. 2014, 10, e1003911. [Google Scholar] [CrossRef] [PubMed]
- Kleinfelter, L.M.; Jangra, R.K.; Jae, L.T.; Herbert, A.S.; Mittler, E.; Stiles, K.M.; Wirchnianski, A.S.; Kielian, M.; Brummelkamp, T.R.; Dye, J.M.; et al. Haploid genetic screen reveals a profound and direct dependence on cholesterol for hantavirus membrane fusion. MBio 2015, 6, e00801. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Munoz, N.; Darlix, J.L.; Tischler, N.D. Development of a lentiviral vector system to study the role of the Andes virus glycoproteins. Virus Res. 2010, 153, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Bangphoomi, N.; Takenaka-Uema, A.; Sugi, T.; Kato, K.; Akashi, H.; Horimoto, T. Akabane virus utilizes alternative endocytic pathways to entry into mammalian cell lines. J. Vet. Med. Sci. 2014, 76, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.I.; Rodrigues, A.H.; Silva, M.L.; Mortara, R.A.; Rossi, M.A.; Jamur, M.C.; Oliver, C.; Arruda, E. Oropouche virus entry into HeLa cells involves clathrin and requires endosomal acidification. Virus Res. 2008, 138, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Shtanko, O.; Nikitina, R.A.; Altuntas, C.Z.; Chepurnov, A.A.; Davey, R.A. Crimean-Congo hemorrhagic fever virus entry into host cells occurs through the multivesicular body and requires ESCRT regulators. PLoS Pathog. 2014, 10, e1004390. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Johansson, C.; Mirazimi, A. Crimean-Congo hemorrhagic fever virus entry and replication is clathrin-, pH- and cholesterol-dependent. J. Gen. Virol. 2009, 90, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Lozach, P.Y.; Burleigh, L.; Staropoli, I.; Navarro-Sanchez, E.; Harriague, J.; Virelizier, J.L.; Rey, F.A.; Despres, P.; Arenzana-Seisdedos, F.; Amara, A. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J. Biol. Chem. 2005, 280, 23698–23708. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Torrelles, J.B.; Schlesinger, L.S. Mutation in the DC-SIGN cytoplasmic triacidic cluster motif markedly attenuates receptor activity for phagocytosis and endocytosis of mannose-containing ligands by human myeloid cells. J. Leukoc. Biol. 2008, 84, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.R.; Moraz, M.L.; Pasquato, A.; Helenius, A.; Lozach, P.Y.; Kunz, S. Role of DC-SIGN in Lassa virus entry into human dendritic cells. J. Virol. 2013, 87, 11504–11515. [Google Scholar] [CrossRef] [PubMed]
- Hollidge, B.S.; Nedelsky, N.B.; Salzano, M.V.; Fraser, J.W.; Gonzalez-Scarano, F.; Soldan, S.S. Orthobunyavirus entry into neurons and other mammalian cells occurs via clathrin-mediated endocytosis and requires trafficking into early endosomes. J. Virol. 2012, 86, 7988–8001. [Google Scholar] [CrossRef] [PubMed]
- Garrison, A.R.; Radoshitzky, S.R.; Kota, K.P.; Pegoraro, G.; Ruthel, G.; Kuhn, J.H.; Altamura, L.A.; Kwilas, S.A.; Bavari, S.; Haucke, V.; et al. Crimean-Congo hemorrhagic fever virus utilizes a clathrin- and early endosome-dependent entry pathway. Virology 2013, 444, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Lozach, P.Y.; Mancini, R.; Bitto, D.; Meier, R.; Oestereich, L.; Overby, A.K.; Pettersson, R.F.; Helenius, A. Entry of bunyaviruses into mammalian cells. Cell. Host Microbe 2010, 7, 488–499. [Google Scholar] [CrossRef] [PubMed]
- De Boer, S.M.; Kortekaas, J.; Spel, L.; Rottier, P.J.; Moormann, R.J.; Bosch, B.J. Acid-activated structural reorganization of the Rift Valley fever virus Gc fusion protein. J. Virol. 2012, 86, 13642–13652. [Google Scholar] [CrossRef] [PubMed]
- Harmon, B.; Schudel, B.R.; Maar, D.; Kozina, C.; Ikegami, T.; Tseng, C.T.; Negrete, O.A. Rift Valley fever virus strain MP-12 enters mammalian host cells via caveola-mediated endocytosis. J. Virol. 2012, 86, 12954–12970. [Google Scholar] [CrossRef] [PubMed]
- Filone, C.M.; Hanna, S.L.; Caino, M.C.; Bambina, S.; Doms, R.W.; Cherry, S. Rift valley fever virus infection of human cells and insect hosts is promoted by protein kinase C epsilon. PLoS ONE 2010, 5, e15483. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Park, J.; Lee, S.; Park, B.; Shin, J.; Song, K.J.; Ahn, T.I.; Hwang, S.Y.; Ahn, B.Y.; Ahn, K. Hantaan virus enters cells by clathrin-dependent receptor-mediated endocytosis. Virology 2002, 294, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Buranda, T.; BasuRay, S.; Swanson, S.; Agola, J.; Bondu, V.; Wandinger-Ness, A. Rapid parallel flow cytometry assays of active GTPases using effector beads. Anal. Biochem. 2013, 442, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, H.N.; Jonsson, C.B. New and Old World hantaviruses differentially utilize host cytoskeletal components during their life cycles. Virology 2008, 374, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.C.; Vacca, F.; Gruenberg, J. Endosome maturation, transport and functions. Semin. Cell. Dev. Biol. 2014, 31, 2–10. [Google Scholar] [CrossRef] [PubMed]
- White, J.M.; Whittaker, G.R. Fusion of Enveloped Viruses in Endosomes. Traffic 2016, 17, 593–614. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.C.; Gruenberg, J. Ion flux and the function of endosomes and lysosomes: pH is just the start: the flux of ions across endosomal membranes influences endosome function not only through regulation of the luminal pH. Bioessays. 2011, 33, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Lozach, P.Y.; Huotari, J.; Helenius, A. Late-penetrating viruses. Curr. Opin. Virol. 2011, 1, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Muñoz, N.; Barriga, G.P.; Valenzuela, P.D.T.; Tischler, N.D. Aromatic and polar residues spanning the candidate fusion peptide of Andes virus are essential for membrane fusion and infection. J. Gen. Virol. 2011, 92, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, J.; Takashima, I.; Hashimoto, N. Cell fusion by haemorrhagic fever with renal syndrome (HFRS) viruses and its application for titration of virus infectivity and neutralizing antibody. Arch. Virol. 1985, 86, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Johansson, C.; Lundkvist, A.; Mirazimi, A. Microtubule-dependent and microtubule-independent steps in Crimean-Congo hemorrhagic fever virus replication cycle. Virology 2009, 385, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Celma, C.C.; Roy, P. Rift Valley fever virus structural proteins: expression, characterization and assembly of recombinant proteins. Virol J. 2008, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Bitto, D.; Halldorsson, S.; Caputo, A.; Huiskonen, J.T. Low pH and anionic lipid dependent fusion of Uukuniemi phlebovirus to liposomes. J. Biol. Chem. 2016, 291, 6412–6422. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ming, Z.; Xiaochun, W.; Hong, W. Rab7: role of its protein interaction cascades in endo-lysosomal traffic. Cell. Signal. 2011, 23, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Acuna, R.; Bignon, E.; Mancini, R.; Lozach, P.Y.; Tischler, N.D. Acidification triggers Andes hantavirus membrane fusion and rearrangement of Gc into a stable post-fusion homotrimer. J. Gen. Virol. 2015, 96, 3192–3197. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.C. Viral membrane fusion. Virology 2015, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Kielian, M. Mechanisms of Virus Membrane Fusion Proteins. Annu. Rev. Virol. 2014, 1, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, A.E.; Ullman, D.E.; German, T.L. tomato spotted wilt virus glycoprotein G(c) is cleaved at acidic pH. Virus Res. 2005, 110, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Goli, J.; Clark, G.; Brauburger, K.; Elliott, R.M. Functional analysis of the Bunyamwera orthobunyavirus Gc glycoprotein. J. Gen. Virol. 2009, 90, 2483–2492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plassmeyer, M.L.; Soldan, S.S.; Stachelek, K.M.; Roth, S.M.; Martin-Garcia, J.; Gonzalez-Scarano, F. Mutagenesis of the La Crosse Virus glycoprotein supports a role for Gc (1066–1087) as the fusion peptide. Virology 2007, 358, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Soldan, S.S.; Hollidge, B.S.; Wagner, V.; Weber, F.; Gonzalez-Scarano, F. La Crosse virus (LACV) Gc fusion peptide mutants have impaired growth and fusion phenotypes, but remain neurotoxic. Virology 2010, 404, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, D.R.; Cooke, C.; Prabakaran, I.; Boland, J.; Nathanson, N.; Gonzalez-Scarano, F. Expression of the La Crosse M segment proteins in a recombinant vaccinia expression system mediates pH-dependent cellular fusion. Virology 1993, 193, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Kielian, M. Class II virus membrane fusion proteins. Virology 2006, 344, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Vaney, M.C.; Rey, F.A. Class II enveloped viruses. Cell. Microbiol. 2011, 13, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Modis, Y. Class II fusion proteins. Adv. Exp. Med. Biol. 2013, 790, 150–166. [Google Scholar] [PubMed]
- Liao, M.; Kielian, M. Domain III from class II fusion proteins functions as a dominant-negative inhibitor of virus membrane fusion. J. Cell. Biol. 2005, 171, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.G.; Yang, P.L.; Harrison, S.C. Peptide inhibitors of dengue-virus entry target a late-stage fusion intermediate. PLoS Pathog. 2010, 6, e1000851. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.W.; Smith, J.M.; Ripoll, D.R.; Spik, K.W.; Taylor, S.L.; Badger, C.V.; Grant, R.J.; Ogg, M.M.; Wallqvist, A.; Guttieri, M.C.; et al. A fusion-inhibiting peptide against Rift Valley fever virus inhibits multiple, diverse viruses. PLoS Negl. Trop. Dis. 2013, 7, e2430. [Google Scholar] [CrossRef] [PubMed]
- Barriga, G.P.; Villalón-Letelier, F.; Márquez, C.L.; Bignon, E.A.; Acuña, R.; Ross, B.H.; Monasterio, O.; Mardones, G.A.; Vidal, S.E.; Tischler, N.D. Inhibition of the hantavirus fusion process by predicted domain III and stem peptides from glycoprotein Gc. PLoS Negl. Trop. Dis. 2016. under review. [Google Scholar]
- Qin, Z.L.; Zheng, Y.; Kielian, M. Role of conserved histidine residues in the low-pH dependence of the Semliki Forest virus fusion protein. J. Virol. 2009, 83, 4670–4677. [Google Scholar] [CrossRef] [PubMed]
- Delos, S.E.; La, B.; Gilmartin, A.; White, J.M. Studies of the “chain reversal regions” of the avian sarcoma/leukosis virus (ASLV) and Ebolavirus fusion proteins: analogous residues are important, and a His residue unique to EnvA affects the pH dependence of ASLV entry. J. Virol. 2010, 84, 5687–5694. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, F.A.; Stauffer, F.; Lima, C.S.; Juliano, M.A.; Juliano, L.; Da Poian, A.T. Membrane fusion induced by vesicular stomatitis virus depends on histidine protonation. J. Biol. Chem. 2003, 278, 13789–13794. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, T.; Mueller, D.S.; Mark, A.E.; Young, P.R.; Kobe, B. The Role of histidine residues in low-pH-mediated viral membrane fusion. Structure 2006, 14, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Edgcomb, S.P.; Murphy, K.P. Variability in the pKa of histidine side-chains correlates with burial within proteins. Proteins 2002, 49, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hacker, J.K.; Hardy, J.L. Adsorptive endocytosis of California encephalitis virus into mosquito and mammalian cells: a role for G1. Virology 1997, 23540–23547. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Franceschini, A.; Horvath, P.; Tetard, M.; Mancini, R.; von Mering, C.; Helenius, A.; Lozach, P.Y. Genome-wide small interfering RNA screens reveal VAMP3 as a novel host factor required for Uukuniemi virus late penetration. J. Virol. 2014, 88, 8565–8578. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, K.C.; McLane, L.M.; Maqbool, T.; Panda, D.; Gordesky-Gold, B.; Cherry, S. A genome-wide RNAi screen reveals that mRNA decapping restricts bunyaviral replication by limiting the pools of DCP2-accessible targets for cap-snatching. Genes Dev. 2013, 27, 1511–1525. [Google Scholar] [CrossRef] [PubMed]
- Eckerle, I.; Lenk, M.; Ulrich, R.G. More novel hantaviruses and diversifying reservoir hosts--time for development of reservoir-derived cell culture models? Viruses 2014, 6, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, K.B.; Nguyen Hoang, A.T.; Gupta, S.; Ahlm, C.; Svensson, M.; Klingstrom, J. Andes hantavirus-infection of a 3D human lung tissue model reveals a late peak in progeny virus production followed by increased levels of proinflammatory cytokines and VEGF-A. PLoS ONE 2016, 11, e0149354. [Google Scholar] [CrossRef] [PubMed]
- Bell-Sakyi, L.; Kohl, A.; Bente, D.A.; Fazakerley, J.K. Tick cell lines for study of Crimean-Congo hemorrhagic fever virus and other arboviruses. Vector Borne Zoonotic Dis. 2012, 12, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Mazelier, M.R.; R.N.; Zumstein, M.; Mancini, R.; Bell-Sakyi, L.; Lozach, P.Y. Uukuniemi virus as a tick-borne virus model. J. Virol. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Modis, Y. A novel membrane fusion protein family in Flaviviridae? Trends Microbiol. 2014, 22, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.; Bonneau, R.; Holbrook, M.R.; Watowich, S.J.; Birmanns, S.; Wriggers, W.; Freiberg, A.N. An assembly model of rift valley Fever virus. Front. Microbiol. 2012, 3, 254. [Google Scholar] [CrossRef] [PubMed]





| Transmission | Receptor/Cofactor | Bunyavirus | Genus | Ref. |
|---|---|---|---|---|
| Arthropod bite | DC-SIGN | SFTSV, UUKV, RVFV | Phlebovirus | [50,51,52,53,54,55,61,62,63,64,65] |
| LACV | Orthobunyavirus | |||
| CCHFV | Nairovirus | |||
| L-SIGN | RVFV, SFTSV, TOSV, UUKV | Phlebovirus | ||
| NMMHC-IIA | SFTSV | |||
| Heparan Sulfate | RVFV, TOSV | |||
| Nucleolin | CCHFV | Nairovirus | ||
| 50-kDa protein | TSWV | Tospovirus | ||
| Aerosol inhalation | β3 integrin | ANDV, SNV, HTNV, PUUV, SEOV, NY-1V | Hantavirus | [66,67,68,69,70,71,72] |
| β1 integrin | PHV, TULV | |||
| β2 integrin | HTNV | |||
| gC1qR | HTNV | |||
| DAF | HTNV, PUUV | |||
| 70-kDa protein | HTNV |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albornoz, A.; Hoffmann, A.B.; Lozach, P.-Y.; Tischler, N.D. Early Bunyavirus-Host Cell Interactions. Viruses 2016, 8, 143. https://0-doi-org.brum.beds.ac.uk/10.3390/v8050143
Albornoz A, Hoffmann AB, Lozach P-Y, Tischler ND. Early Bunyavirus-Host Cell Interactions. Viruses. 2016; 8(5):143. https://0-doi-org.brum.beds.ac.uk/10.3390/v8050143
Chicago/Turabian StyleAlbornoz, Amelina, Anja B. Hoffmann, Pierre-Yves Lozach, and Nicole D. Tischler. 2016. "Early Bunyavirus-Host Cell Interactions" Viruses 8, no. 5: 143. https://0-doi-org.brum.beds.ac.uk/10.3390/v8050143