Inactivation of RNA Viruses by Gamma Irradiation: A Study on Mitigating Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus Stocks
2.2. Virus Titration
2.3. Statistics and D10 Value Quantification
2.4. Sample Preparation
2.5. Radiation Procedure
3. Results
3.1. Dose Curves for the Inactivation of Surrogate Viruses
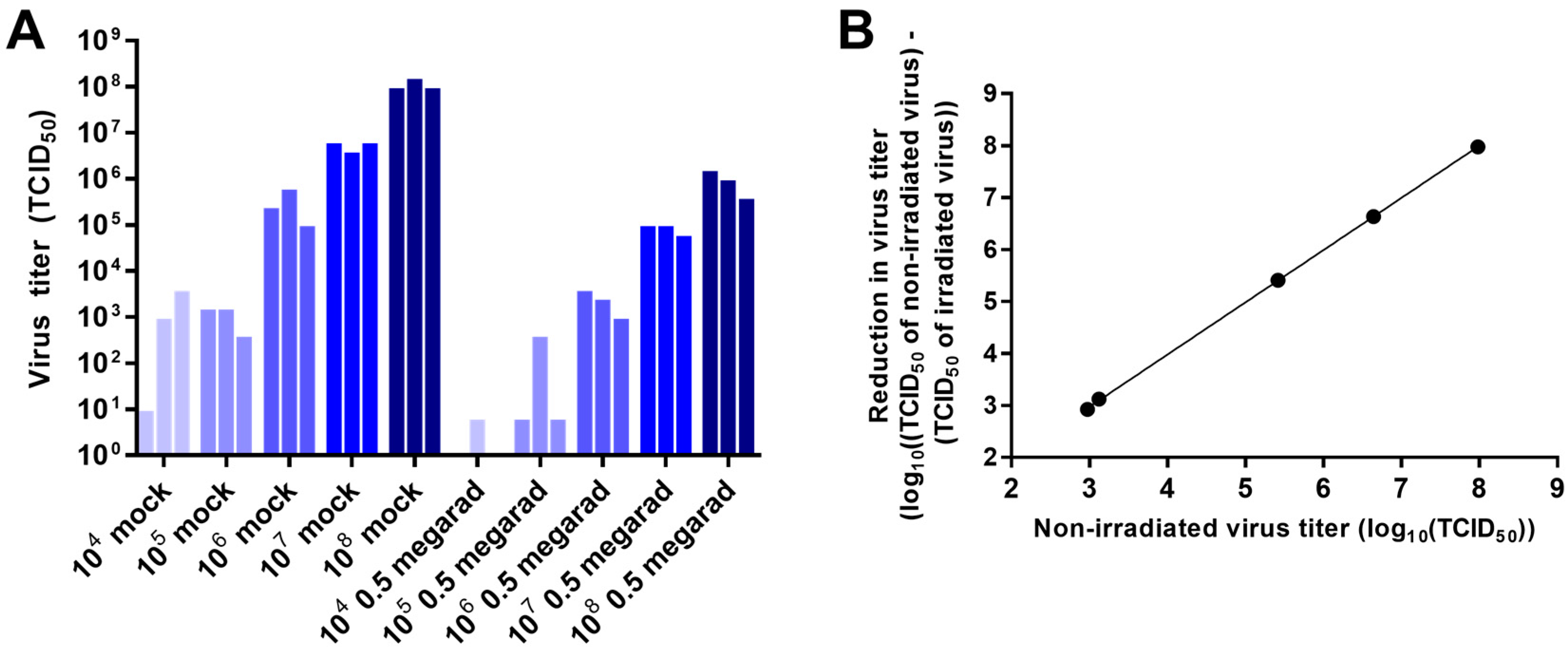
3.2. Effect of Virus Concentration on Inactivation by Gamma Irradiation
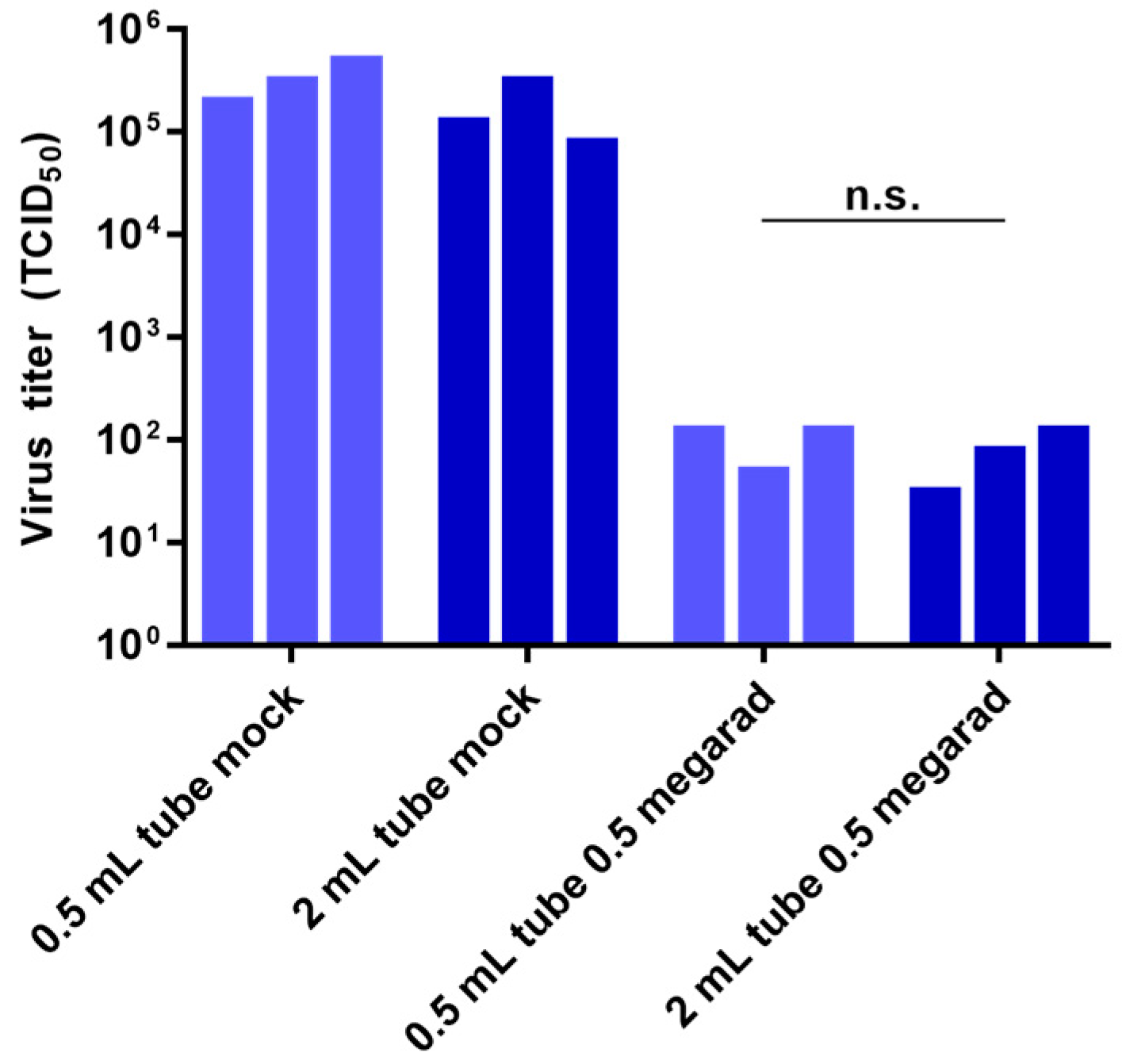
3.3. Effect of Sample Volume on Inactivation by Gamma Irradiation
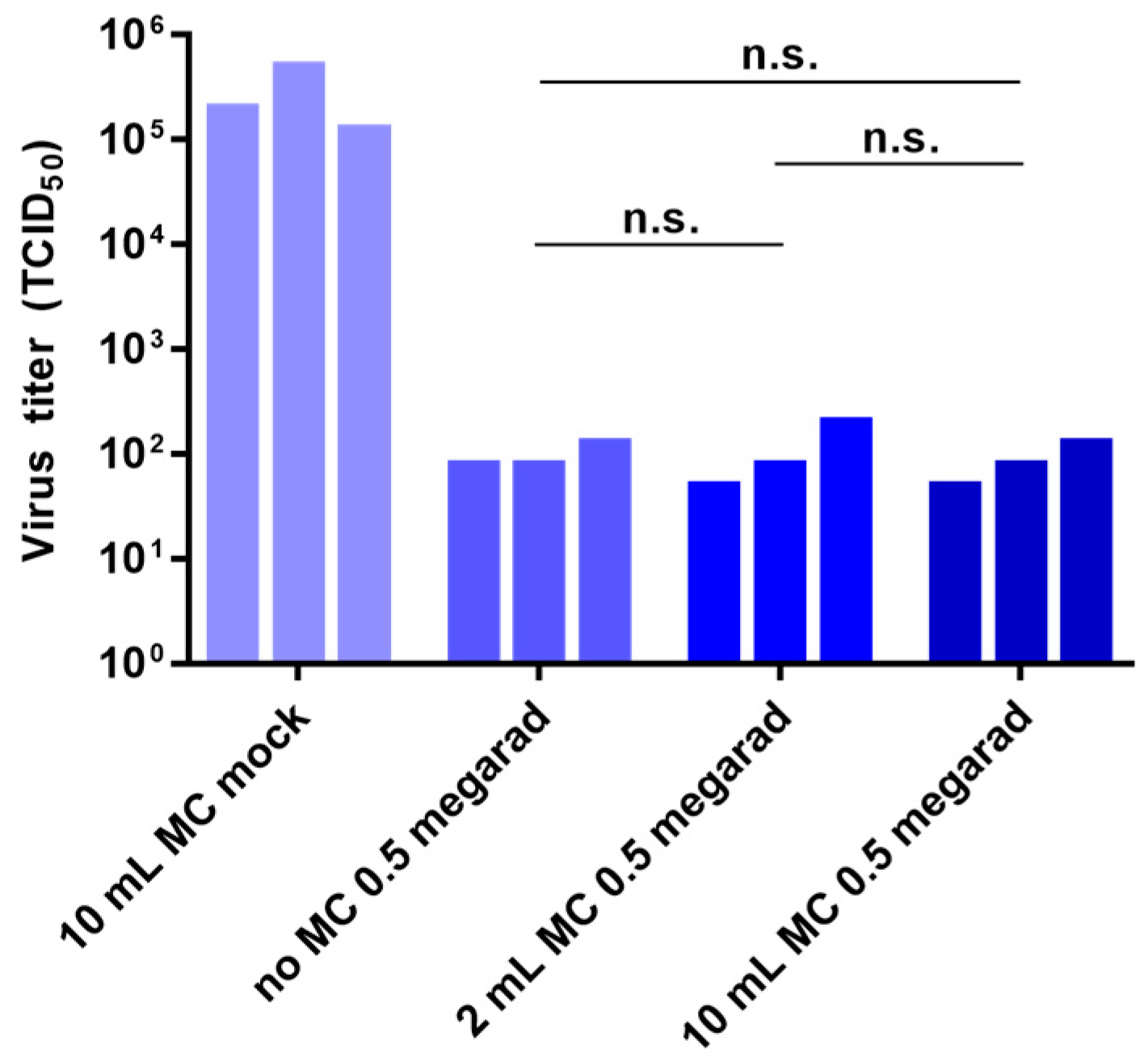
3.4. Effect of Air Volume on Inactivation by Gamma Irradiation
3.5. Effect of Media Composition on Inactivation by Gamma Irradiation
3.6. Effect of External Disinfectants on Inactivation
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Centers for Disease Control and Prevention Division of Select Agents and Toxins and Animal and Plant Health Inspection Service Agriculture Select Agent Services Federal Select Agent Program—Non-Viable Select Agents and Nonfunctional Select Toxins and Rendering Samples free of Select Agents and Toxins. Available online: http://www.selectagents.gov/guidance-nonviable.html (accessed on 1 January 2016).
- Jordan, R.T.; Kempe, L.L. Inactivation of some animal viruses with gamma radiation from cobalt-60. Proc. Soc. Exp. Biol. Med. 1956, 91, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Shaffer, H.L. Sterilization and preservation by radiation sterilization. In Disinfection, 353 Sterilization, and Preservation; Block, S.S., Ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2001; pp. 729–746. [Google Scholar]
- Anderson, A.W.; Nordan, H.C.; Cain, R.F.; Parrish, G.; Duggan, D. Studies on a radioresistant Micrococcus. I. Isolation, morphology, cultural characteristics, and resistance to gamma radiation. Food Technol. 1956, 10, 575–577. [Google Scholar]
- Davies, R.; Sinskey, A.J. Radiation-resistant mutants of Salmonella typhimurium LT2: Development and characterization. J. Bacteriol. 1973, 113, 133–144. [Google Scholar] [PubMed]
- Sullivan, R.; Fassolitis, A.C.; Larkin, E.P.; Read, R.B.; Peeler, J.T. Inactivation of thirty viruses by gamma radiation. Appl. Microbiol. 1971, 22, 61–65. [Google Scholar] [PubMed]
- International Organization for Standardization, (ISO). Sterilization of Healthcare Products—Requirements for Validation and Routine Control—Radiation Sterilization; ISO 11137; International Organization for Standardization: Geneva, Switzerland, 1995. [Google Scholar]
- Kenny, M.T.; Albright, K.L.; Emery, J.B.; Bittle, J.L. Inactivation of rubella virus by gamma radiation. J. Virol. 1969, 4, 807–810. [Google Scholar] [PubMed]
- Rohwer, R.G. Scrapie infectious agent is virus-like in size and susceptibility to inactivation. Nature 1984, 308, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Sommer, R.; Pribil, W.; Appelt, S.; Gehringer, P.; Eschweiler, H.; Leth, H.; Cabaj, A.; Haider, T. Inactivation of bacteriophages in water by means of non-ionizing (UV-253.7 nm) and ionizing (gamma) radiation: A comparative approach. Water Res. 2001, 35, 3109–3116. [Google Scholar] [CrossRef]
- House, C.; House, J.A.; Yedloutschnig, R.J. Inactivation of viral agents in bovine serum by gamma irradiation. Can. J. Microbiol. 1990, 36, 737–740. [Google Scholar] [CrossRef] [PubMed]
- De Roda Husman, A.M.; Bijkerk, P.; Lodder, W.; Van Den Berg, H.; Pribil, W.; Cabaj, A.; Gehringer, P.; Sommer, R.; Duizer, E. Calicivirus inactivation by nonionizing (253.7-nanometer-wavelength [UV]) and ionizing (gamma) radiation. Appl. Environ. Microbiol. 2004, 70, 5089–5093. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, Z.; Su, W. Ion Irradiation and Biomolecular Radiation Damage II. Indirect Effect. 2010; arXiv:1004.4394 [physics.bio-ph]. [Google Scholar]
- Lomax, M.E.; Folkes, L.K.; O’Neill, P. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 2013, 25, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, H.; Iida, Y.; Matsuda, A.; Kuwabara, M. Damage induced by hydroxyl radicals generated in the hydration layer of gamma-irradiated frozen aqueous solution of DNA. J. Radiat. Res. 1996, 37, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Summers, W.C.; Szybalski, W. Gamma-irradiation of deoxyribonucleic acid in dilute solutions. II. Molecular mechanisms responsible for inactivation of phage, its transfecting DNA, and of bacterial transforming activity. J. Mol. Biol. 1967, 26, 227–235. [Google Scholar] [CrossRef]
- Ward, R.L. Mechanisms of poliovirus inactivation by the direct and indirect effects of ionizing radiation. Radiat. Res. 1980, 83, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Divers, E.; Ma, Y.; Li, J. Inactivation of a human norovirus surrogate, human norovirus virus-like particles, and vesicular stomatitis virus by gamma irradiation. Appl. Environ. Microbiol. 2011, 77, 3507–3517. [Google Scholar] [CrossRef] [PubMed]
- Elliott, L.H.; McCormick, J.B.; Johnson, K.M. Inactivation of Lassa, Marburg, and Ebola viruses by gamma irradiation. J. Clin. Microbiol. 1982, 16, 704–708. [Google Scholar] [PubMed]
- Korystov, Y. Contributions of the direct and indirect effects of ionizing radiation to reproductive cell death. Radiat. Res. 1992, 129, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Thayer, D.W.; Boyd, G. Effect of irradiation temperature on inactivation of Escherichia coli O157:H7 and Staphylococcus aureus. J. Food Prot. 2001, 64, 1624–1626. [Google Scholar] [PubMed]
- Sullivan, R.; Scarpino, P.V.; Fassolitis, A.C.; Larkin, E.P.; Peeler, J.T. Gamma radiation inactivation of coxsackievirus B-2. Appl. Microbiol. 1973, 26, 14–17. [Google Scholar] [PubMed]
- Da Silva Aquino, K.A. Sterilization by Gamma Irradiation. In Gamma Radiation; Adrovic, F., Ed.; InTech: Vienna, Austria, 2012; pp. 171–206. [Google Scholar]
- Crucq, A.S.; Slegers, C.; Deridder, V.; Tilquin, B. Radiosensitivity study of cefazolin sodium. Talanta 2000, 52, 873–877. [Google Scholar] [CrossRef]
- Marzi, A.; Ebihara, H.; Callison, J.; Groseth, A.; Williams, K.J.; Geisbert, T.W.; Feldmann, H. Vesicular stomatitis virus-based Ebola vaccines with improved cross-protective efficacy. J. Infect. Dis. 2011, 204 (Suppl. 3), S1066–S1074. [Google Scholar] [CrossRef] [PubMed]
- Lemon, K.; de Vries, R.D.; Mesman, A.W.; McQuaid, S.; van Amerongen, G.; Yüksel, S.; Ludlow, M.; Rennick, L.J.; Kuiken, T.; Rima, B.K.; et al. Early target cells of measles virus after aerosol infection of non-human primates. PLoS Pathog. 2011, 7, e1001263. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Wang, M.K.; Rennick, L.J.; Full, F.; Gableske, S.; Mesman, A.W.; Gringhuis, S.I.; Geijtenbeek, T.B.H.; Duprex, W.P.; Gack, M.U. Antagonism of the phosphatase PP1 by the measles virus V protein is required for innate immune escape of MDA5. Cell Host Microbe 2014, 16, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Enterlein, S.; Warfield, K.L.; Swenson, D.L.; Stein, D.A.; Smith, J.L.; Gamble, C.S.; Kroeker, A.D.; Iversen, P.L.; Bavari, S.; Mühlberger, E. VP35 knockdown inhibits Ebola virus amplification and protects against lethal infection in mice. Antimicrob. Agents Chemother. 2006, 50, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.; Tatsuo, H.; Hidaka, Y.; Aoki, T.; Minagawa, H.; Yanagi, Y. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J. Virol. 2001, 75, 4399–4401. [Google Scholar] [CrossRef] [PubMed]
- Hierholzer, J.C.; Killington, R.A. Virus isolation and quantification. In Virology Methods; Mahy, B., Kangro, H.O., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 25–46. [Google Scholar]
- Kertesz, Z.I.; Parsons, G.F. Ozone Formation in Air Exposed to Cobalt-60 Gamma Radiation. Science 1963, 142, 1289–1290. [Google Scholar] [CrossRef] [PubMed]
- Meryman, H.T. Mechanics of freezing in living cells and tissues. Science 1956, 124, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Department of Agriculture. Gamma Irradiation as a Treatment to Address Pathogens of Animal Biosecurity Concern, Final Policy Review; Department of Agriculture: Canberra, Australia, 2014. [Google Scholar]
- Klaponski, N.; Cutts, T.; Gordon, D.; Theriault, S. A Study of the Effectiveness of the Containment Level-4 (CL-4) Chemical Shower in Decontaminating Dover Positive-Pressure Suits. Appl. Biosaf. 2011, 16, 112–117. [Google Scholar] [CrossRef]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hume, A.J.; Ames, J.; Rennick, L.J.; Duprex, W.P.; Marzi, A.; Tonkiss, J.; Mühlberger, E. Inactivation of RNA Viruses by Gamma Irradiation: A Study on Mitigating Factors. Viruses 2016, 8, 204. https://0-doi-org.brum.beds.ac.uk/10.3390/v8070204
Hume AJ, Ames J, Rennick LJ, Duprex WP, Marzi A, Tonkiss J, Mühlberger E. Inactivation of RNA Viruses by Gamma Irradiation: A Study on Mitigating Factors. Viruses. 2016; 8(7):204. https://0-doi-org.brum.beds.ac.uk/10.3390/v8070204
Chicago/Turabian StyleHume, Adam J., Joshua Ames, Linda J. Rennick, W. Paul Duprex, Andrea Marzi, John Tonkiss, and Elke Mühlberger. 2016. "Inactivation of RNA Viruses by Gamma Irradiation: A Study on Mitigating Factors" Viruses 8, no. 7: 204. https://0-doi-org.brum.beds.ac.uk/10.3390/v8070204







