Local Innate Responses to TLR Ligands in the Chicken Trachea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tracheal Organ Culture (TOC)
2.2. Avian Influenza Virus (AIV)
2.3. Stimulation of TOC with TLR Ligands
2.4. cDNA Synthesis and Real-Time PCR
2.5. Stimulation of Chicken Macrophages with Tracheal Supernatants
2.6. Statistical Analysis
3. Results
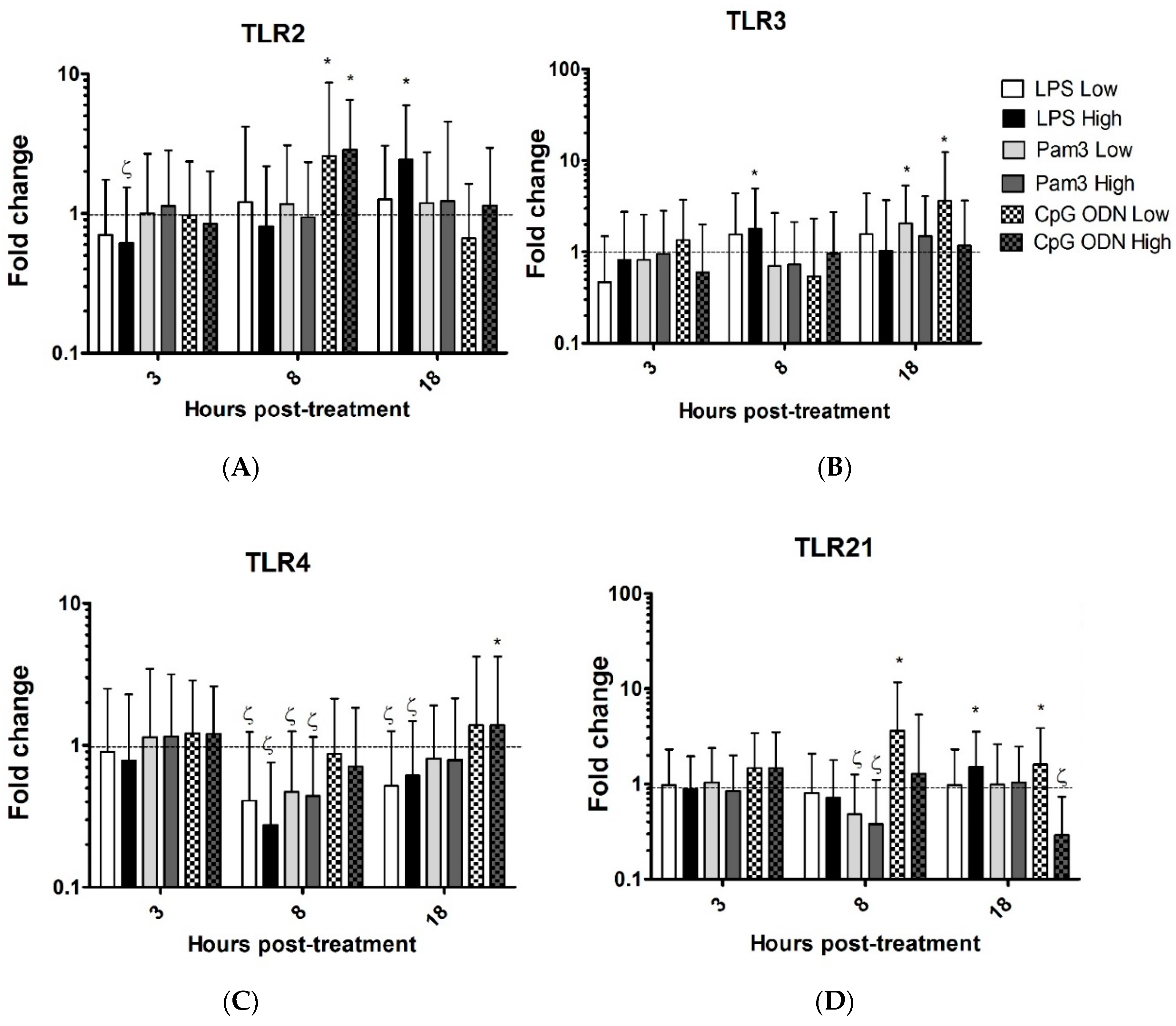
3.1. TLR Ligands Induce the Expression of Innate Response Genes in Chicken Tracheal Organ Cultures
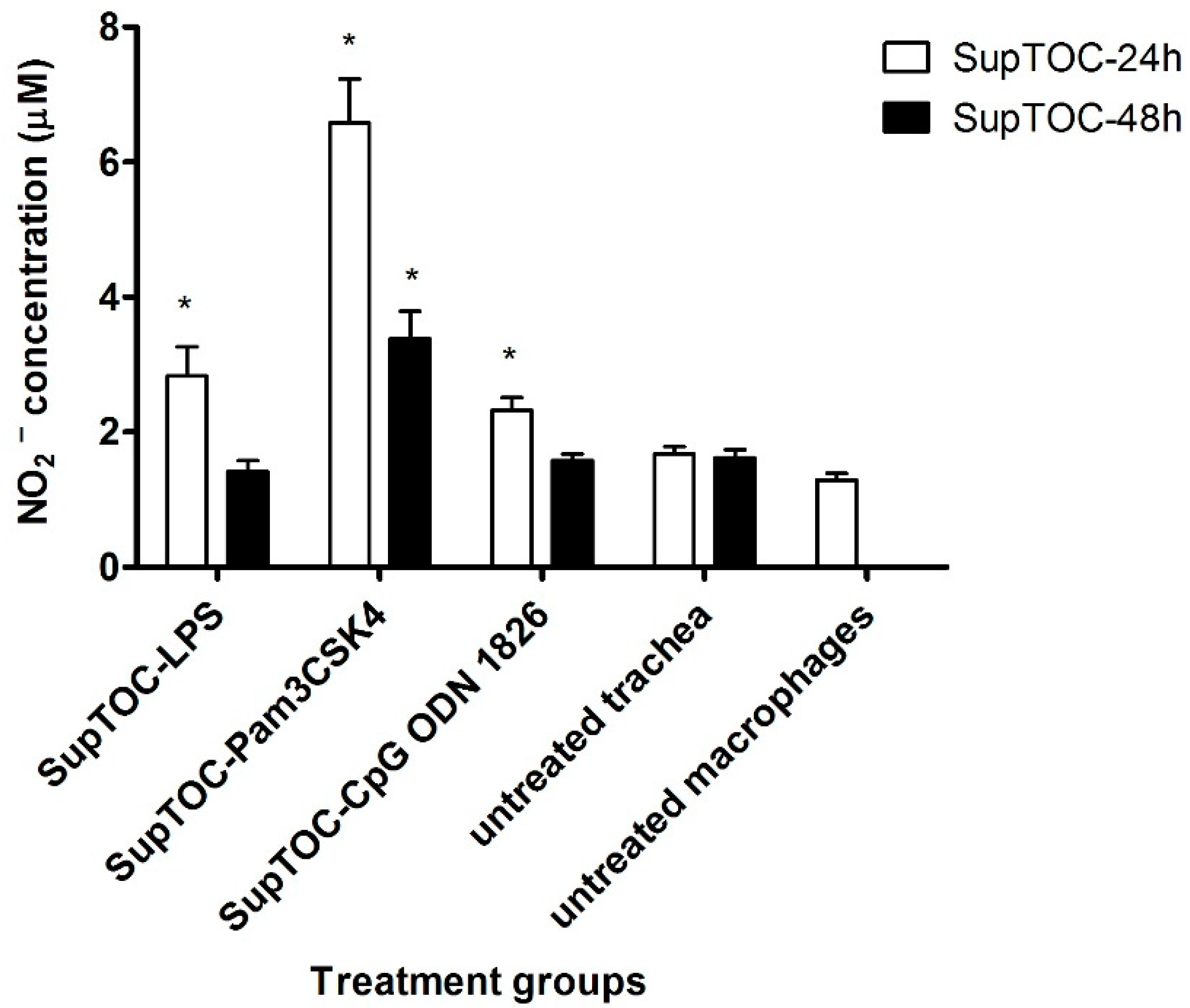
3.2. Supernatants from Chicken Tracheas Treated with TLR Ligands Activate Chicken Macrophages
3.3. Antiviral Compounds Produced by Stimulated TOCs Are Able to Limit AIV Replication in Chicken Macrophages
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Samji, T. Influenza A: Understanding the viral life cycle. Yale J. Biol. Med. 2009, 82, 153–159. [Google Scholar] [PubMed]
- Reemers, S.S.; Groot Koerkamp, M.J.; Holstege, F.C.; van Eden, W.; Vervelde, L. Cellular host transcriptional responses to influenza A virus in chicken tracheal organ cultures differ from responses in in vivo infected trachea. Vet. Immunol. Immunopathol. 2009, 132, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Zaffuto, K.M.; Estevez, C.N.; Afonso, C.L. Primary chicken tracheal cell culture system for the study of infection with avian respiratory viruses. Avian Pathol. 2008, 37, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Gaunson, J.E.; Philip, C.J.; Whithear, K.G.; Browning, G.F. The cellular immune response in the tracheal mucosa to Mycoplasma gallisepticum in vaccinated and unvaccinated chickens in the acute and chronic stages of disease. Vaccine 2006, 24, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Matthijs, M.G.R.; Ariaans, M.P.; Dwars, R.M.; van Eck, J.H.H.; Bouma, A.; Stegeman, A.; Vervelde, L.; Van Eck, J.H.H.; Bouma, A.; Stegeman, A.; et al. Course of infection and immune responses in the respiratory tract of IBV infected broilers after superinfection with E. coli. Vet. Immunol. Immunopathol. 2009, 127, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Gaunson, J.E.; Philip, C.J.; Whithear, K.G.; Browning, G.F. Lymphocytic infiltration in the chicken trachea in response to Mycoplasma gallisepticum infection. Microbiology 2000, 146, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.M.; Reed, A.B.W.M.; Thackerab, H.L. Cellular response of the respiratory tract of chickens to infection with Massachusetts 41 and Australian T infectious bronchitis viruses. Avian Dis. 1993, 37, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Toth, T.E. Nonspecific cellular defense of the avian respiratory system: A review. Dev. Comp. Immunol. 2000, 24, 121–139. [Google Scholar] [CrossRef]
- De Geus, E.D.; Jansen, C.A.; Vervelde, L. Uptake of particulate antigens in a nonmammalian lung: Phenotypic and functional characterization of avian respiratory phagocytes using bacterial or viral antigens. J. Immunol. 2012, 188, 4516–4526. [Google Scholar] [CrossRef] [PubMed]
- Klika, E.; Scheuermann, D.W.; De Groodt-Lasseel, M.H.; Bazantova, I.; Switka, A. Pulmonary macrophages in birds (barn owl, Tyto tyto alba), domestic fowl (Gallus gallus f. domestica), quail (Coturnix coturnix), and pigeons (Columbia livia). Anat. Rec. 1996, 246, 87–97. [Google Scholar] [CrossRef]
- Buschiazzo, A.; Alzari, P.M. Structural insights into sialic acid enzymology. Curr. Opin. Chem. Biol. 2008, 12, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-I.; Wang, C.-H.; Liao, J.-W.; Hsu, T.-W.; Kuo, S.-M.; Su, H.-L. The infection of primary avian tracheal epithelial cells with infectious bronchitis virus. Vet. Res. 2010, 41, 6. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.P.S.; Lee, C.W. Species and age related differences in the type and distribution of influenza virus receptors in different tissues of chickens, ducks and turkeys. Virol. J. 2010, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Le Goffic, R.; Balloy, V.; Lagranderie, M.; Alexopoulou, L.; Escriou, N.; Flavell, R.; Chignard, M.; Si-Tahar, M. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006, 2, e53. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Allen, I.C.; Scull, M.A.; Moore, C.B.; Holl, E.K.; McElvania-TeKippe, E.; Taxman, D.J.; Guthrie, E.H.; Pickles, R.J.; Ting, J.P.-Y. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 2009, 30, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Guillot, L.; Le Goffic, R.; Bloch, S.; Escriou, N.; Akira, S.; Chignard, M.; Si-Tahar, M. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 2005, 280, 5571–5580. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Pillai, P.S. Innate immunity to influenza virus infection. Nat. Rev. Immunol. 2014, 14, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Reemers, S.S.; Van Leenen, D.; Groot, M.J.; Van Haarlem, D.; Van De Haar, P.; Van Eden, W.; Vervelde, L. Early host responses to avian influenza A virus are prolonged and enhanced at transcriptional level depending on maturation of the immune system. Mol. Immunol. 2010, 47, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Liniger, M.; Summerfield, A.; Zimmer, G.; McCullough, K.C.; Ruggli, N. Chicken cells sense influenza A virus infection through MDA5 and CARDIF signaling involving LGP2. J. Virol. 2012, 86, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Zhou, H. Functional Analysis of Chicken IRF7 in Response to dsRNA Analog Poly(I:C) by Integrating Overexpression and Knockdown. PLoS ONE 2015, 10, e0133450. [Google Scholar]
- Takaoka, A.; Yanai, H. Interferon signalling network in innate defence. Cell. Microbiol. 2006, 8, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Yang, L.; Meng, S.; Xu, L.; Bi, Y.; Jia, X.; Li, J.; Sun, L.; Liu, W. The differential antiviral activities of chicken interferon α (ChIFN-α) and ChIFN-β are related to distinct interferon-stimulated gene expression. PLoS ONE 2013, 8, e59307. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K. The interferon inducible gene: Viperin. J. Interferon. Cytokine Res. 2011, 31, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.C.; Bailey, C.C.; Weyer, J.L.; Radoshitzky, S.R.; Becker, M.M.; Chiang, J.J.; Brass, A.L.; Ahmed, A.A.; Chi, X.; Dong, L.; et al. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PLoS Pathog. 2011, 7, e1001258. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Gibson, M.S.; Wash, R.S.; Ferrara, F.; Wright, E.; Temperton, N.; Kellam, P.; Fife, M. Chicken interferon-inducible transmembrane protein 3 restricts influenza viruses and lyssaviruses in vitro. J. Virol. 2013, 87, 12957–12966. [Google Scholar] [CrossRef] [PubMed]
- St Paul, M.; Brisbin, J.T.; Barjesteh, N.; Villaneueva, A.I.; Parvizi, P.; Read, L.R.; Nagy, E.; Sharif, S. Avian influenza virus vaccines containing Toll-like receptors 2 and 5 ligand adjuvants promote protective immune responses in chickens. Viral Immunol. 2014, 27, 160–166. [Google Scholar] [CrossRef] [PubMed]
- St Paul, M.; Barjesteh, N.; Brisbin, J.T.; Villaneueva, A.I.; Read, L.R.; Hodgins, D.; Nagy, É.; Sharif, S. Effects of ligands for Toll-like receptors 3, 4, and 21 as adjuvants on the immunogenicity of an avian influenza vaccine in chickens. Viral Immunol. 2014, 27, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Barjesteh, N.; Shojadoost, B.; Brisbin, J.T.; Emam, M.; Hodgins, D.C.; Nagy, É.; Sharif, S. Reduction of avian influenza virus shedding by administration of Toll-like receptor ligands to chickens. Vaccine 2015, 33, 4843–4849. [Google Scholar] [CrossRef] [PubMed]
- Barjesteh, N.; Brisbin, J.T.; Behboudi, S.; Nagy, É.; Sharif, S. Induction of antiviral responses against avian influenza virus in embryonated chicken eggs with Toll-like receptor ligands. Viral Immunol. 2015, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barjesteh, N.; Behboudi, S.; Brisbin, J.T.; Villanueva, A.I.; Nagy, E.; Sharif, S. TLR ligands induce antiviral responses in chicken macrophages. PLoS ONE 2014, 9, e105713. [Google Scholar] [CrossRef] [PubMed]
- Szretter, K.J.; Balish, A.L.; Katz, J.M. Influenza: Propagation, quantification, and storage. Curr. Protoc. Microbiol. 2006. [Google Scholar] [CrossRef]
- Coox, J.K.A.; Poultry, H.; With, E.; September, F.A. The use of chicken tracheal organ cultures for the isolation and assay of avian infectious bronchitis virus. Arch. Virol. 1976, 118, 109–118. [Google Scholar]
- Abd El Rahman, S.; El-Kenawy, A.A.; Neumann, U.; Herrler, G.; Winter, C. Comparative analysis of the sialic acid binding activity and the tropism for the respiratory epithelium of four different strains of avian infectious bronchitis virus. Avian Pathol. 2009, 38, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.; Herrler, G.; Neumann, U. Infection of the tracheal epithelium by infectious bronchitis virus is sialic acid dependent. Microbes Infect. 2008, 10, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Lima-Junior, D.S.; Costa, D.L.; Carregaro, V.; Cunha, L.D.; Silva, A.L.N.; Mineo, T.W.P.; Gutierrez, F.R.S.; Bellio, M.; Bortoluci, K.R.; Flavell, R.A.; et al. Inflammasome-derived IL-1β production induces nitric oxide-mediated resistance to Leishmania. Nat. Med. 2013, 19, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003, 73, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.A.; Heggen, C.L.; Hussain, I. Avian macrophage: Effector functions in health and disease. Dev. Comp. Immunol. 2000, 24, 103–119. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, Z. The essential adaptors of innate immune signaling. Protein Cell 2013, 4, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, S.; Akira, S. Toll-like receptors and Type I interferons. J. Biol. Chem. 2007, 282, 15319–15323. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.C.Y.; Barr, I.; Hansbro, P.M.; Wark, P.A. Human influenza is more effective than avian influenza at antiviral suppression in airway cells. Am. J. Respir. Cell Mol. Biol. 2011, 44, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.C.-Y.; Parsons, K.; Barr, I.; Lowther, S.; Middleton, D.; Hansbro, P.M.; Wark, P.A. Critical role of constitutive type I interferon response in bronchial epithelial cell to influenza infection. PLoS ONE 2012, 7, e32947. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Jorns, C.; Stertz, S.; Boisson-Dupuis, S.; Thimme, R.; Weidmann, M.; Casanova, J.-L.; Haller, O.; Kochs, G. Induction of MxA gene expression by influenza A virus requires type I or type III interferon signaling. J. Virol. 2007, 81, 7776–7785. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, F.; Dickensheets, H.; Gamero, A.M.; Vogel, S.N.; Donnelly, R.P. An essential role for IFN-B in the induction of IFN-stimulated gene expression by LPS in macrophages. J. Leukoc. Biol. 2014, 96, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Boo, K.H.; Yang, J.S. Intrinsic cellular defenses against virus infection by antiviral type I interferon. Yonsei Med. J. 2010, 51, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Grandvaux, N.; Servant, M.J.; tenOever, B.; Sen, G.C.; Balachandran, S.; Barber, G.N.; Lin, R.; Hiscott, J. Transcriptional Profiling of Interferon Regulatory Factor 3 Target Genes: Direct Involvement in the Regulation of Interferon-Stimulated Genes. J. Virol. 2002, 76, 5532–5539. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Farzan, M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat. Rev. Immunol. 2013, 13, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Brass, A.L.; Huang, I.-C.; Benita, Y.; John, S.P.; Krishnan, M.N.; Feeley, E.M.; Ryan, B.J.; Weyer, J.L.; van der Weyden, L.; Fikrig, E.; et al. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell 2009, 139, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Sha, Q.; Truong-Tran, A.Q.; Plitt, J.R.; Beck, L.A.; Schleimer, R.P. Activation of airway epithelial cells by toll-like receptor agonists. Am. J. Respir. Cell Mol. Biol. 2004, 31, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Karpala, A.J.; Lowenthal, J.W.; Bean, A.G. Activation of the TLR3 pathway regulates IFNB production in chickens. Dev. Comp. Immunol. 2008, 32, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Blankley, S.; Graham, C.M.; Howes, A.; Bloom, C.I.; Berry, M.P.R.; Chaussabel, D.; Pascual, V.; Banchereau, J.; Lipman, M.; O’Garra, A. Identification of the key differential transcriptional responses of human whole blood following TLR2 or TLR4 ligation in vitro. PLoS ONE 2014, 9, e97702. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kaiser, V.; Beier, E.; Bechheim, M.; Guenthner-biller, M.; Ablasser, A.; Berger, M.; Endres, S.; Hartmann, G. Self-priming determines high type I IFN production by plasmacytoid dendritic cells. Eur. J. Immunol. 2015, 44, 807–818. [Google Scholar] [CrossRef] [PubMed]
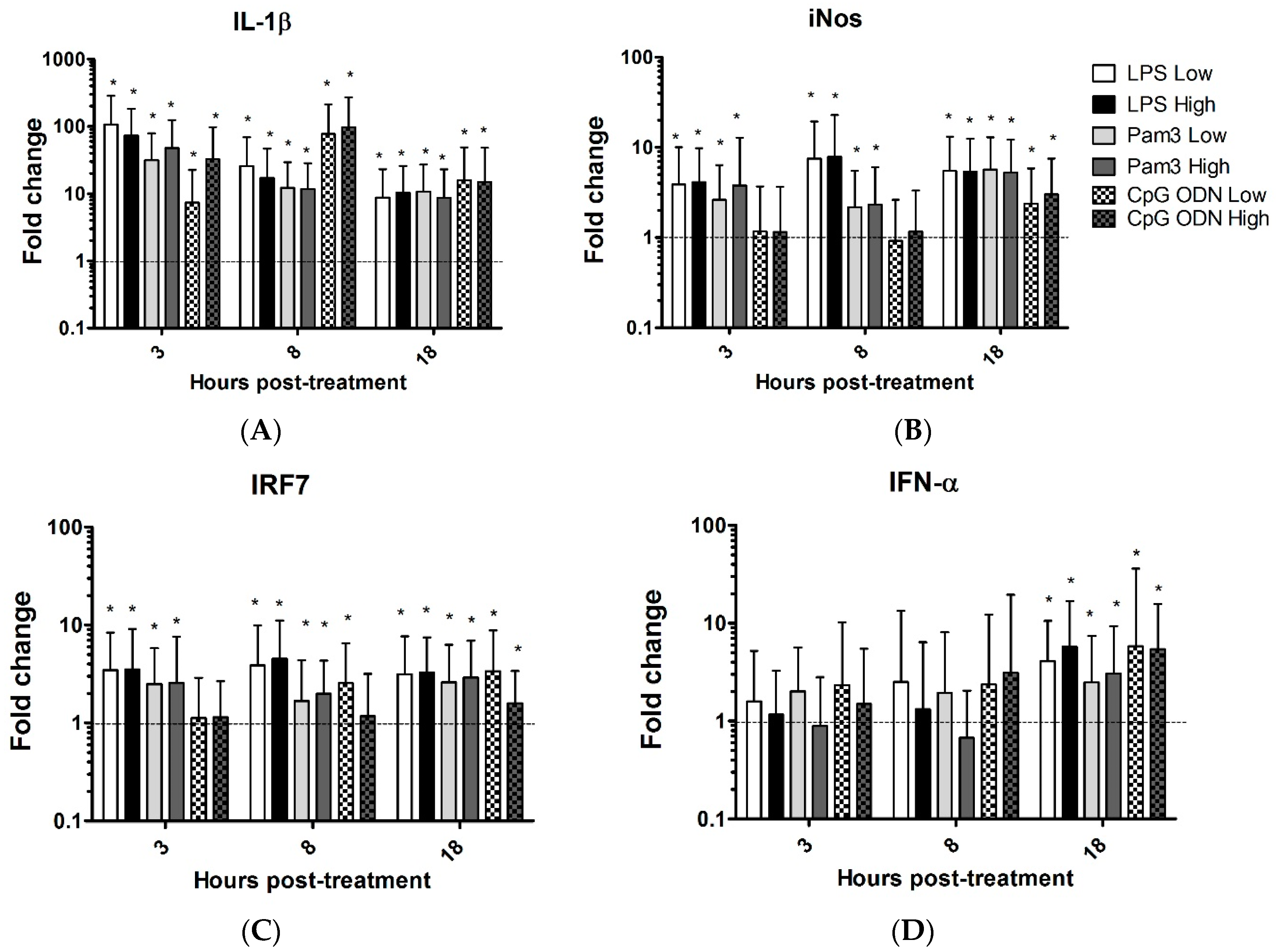
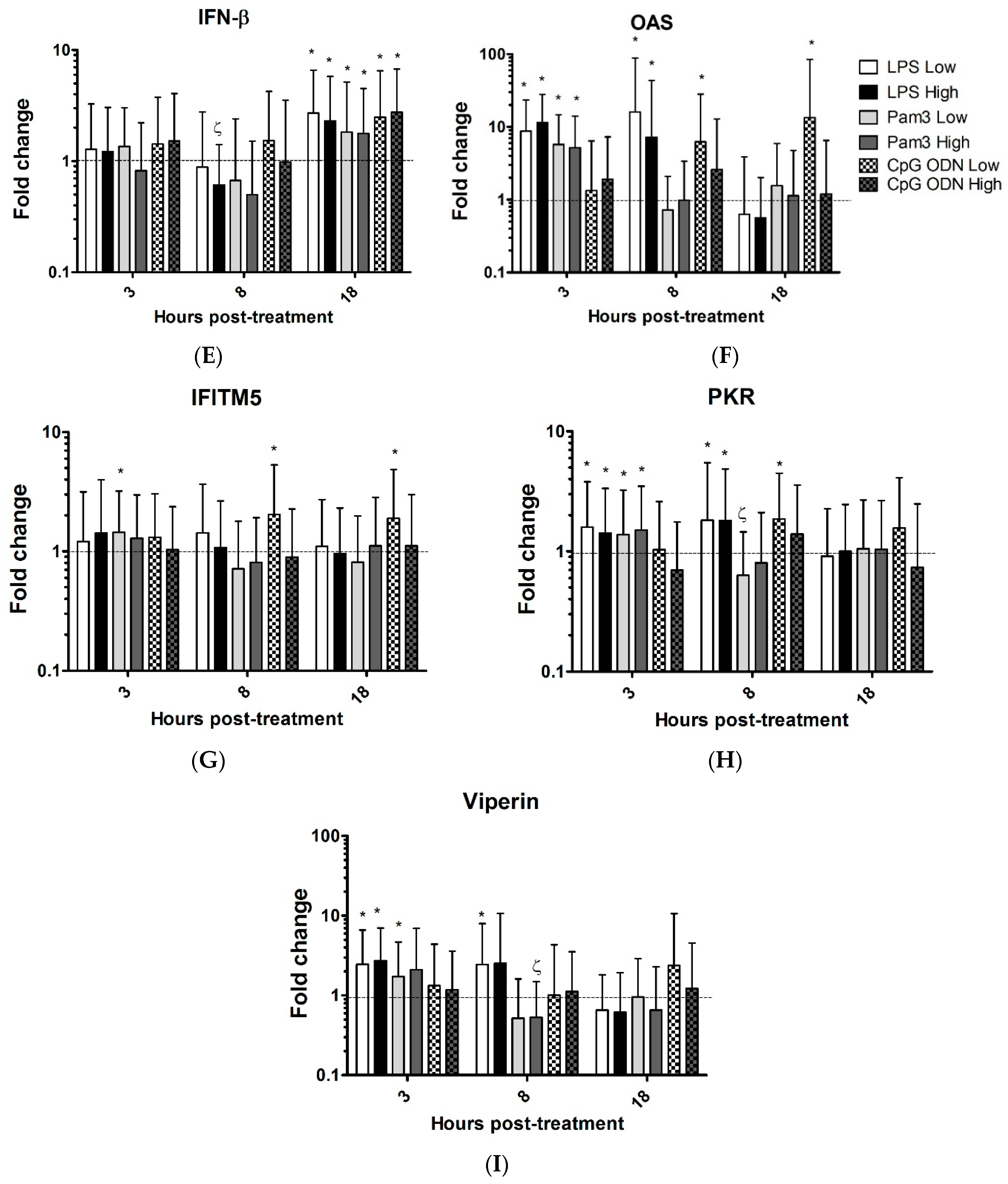

| Genes | LPS Low | LPS Highv | Pam Low | Pam High | CpG ODN LOW | CpG ODN High | ||||||||||||||||||
| 3 h | 8 h | 18 h | 3 h | 8 h | 18 h | 3 h | 8 h | 18 h | 3 h | 8 h | 18 h | 3 h | 8 h | 18 h | 3 h | 8 h | 18 h | |||||||
| IL-1B | 107↑ | 26↑ | 8.7↑ | 73↑ | 17↑ | 10↑ | 32↑ | 12↑ | 11↑ | 48↑ | 12↑ | 8.7↑ | 7.3↑ | 77↑ | 16 ↑ | 33↑ | 97↑ | 15↑ | ||||||
| iNOS | 3.9↑ | 7.5↑ | 5.5↑ | 4.1↑ | 7.9↑ | 5.4↑ | 2.6↑ | 2.2↑ | 5.7↑ | 3.8↑ | 2.3↑ | 5.3↑ | 2.4↑ | 3↑ | ||||||||||
| IRF7 | 3.5↑ | 3.9↑ | 3.2↑ | 3.5↑ | 4.5↑ | 3.3↑ | 2.5↑ | 1.7↑ | 2.6↑ | 2.6↑ | 2↑ | 2.9↑ | 2.6↑ | 3.4↑ | 1.6↑ | |||||||||
| IFN-α | 4 ↑ | 5.7↑ | 1.9↑ | 2.5↑ | 3.0↑ | 5.8↑ | 5.4↑ | |||||||||||||||||
| IFN-β | 2.7↑ | 1.7↓ | 2.3↑ | 1.8↑ | 2.5↑ | 2.8↑ | ||||||||||||||||||
| OAS | 8.7↑ | 16↑ | 12↑ | 7.3↑ | 5.7↑ | 5.2↑ | 6.3↑ | 13.4↑ | ||||||||||||||||
| IFITM5 | 1.5↑ | 1.9↑ | 2↑ | |||||||||||||||||||||
| PKR | 1.6↑ | 1.8↑ | 1.4↑ | 1.8↑ | 1.4↑ | 1.7↓ | 1.5↑ | 1.9↑ | ||||||||||||||||
| viperin | 2.5↑ | 2.5↑ | 2.7↑ | 1.7↑ | 2↓ | |||||||||||||||||||
| TLR2 | 1.7↓ | 2.4↑ | 2.6↑ | 2.9↑ | ||||||||||||||||||||
| TLR3 | 1.8↑ | 2.0↑ | 3.6↑ | |||||||||||||||||||||
| TLR4 | 2.5↓ | 2↓ | 3.3↓ | 1.7↓ | 2.0↓ | 2.5↓ | 1.3↑ | |||||||||||||||||
| TLR21 | 1.5↑ | 2↓ | 2.5↓ | 3.5↑ | 1.6↑ | 3.3↓ | ||||||||||||||||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barjesteh, N.; Alkie, T.N.; Hodgins, D.C.; Nagy, É.; Sharif, S. Local Innate Responses to TLR Ligands in the Chicken Trachea. Viruses 2016, 8, 207. https://0-doi-org.brum.beds.ac.uk/10.3390/v8070207
Barjesteh N, Alkie TN, Hodgins DC, Nagy É, Sharif S. Local Innate Responses to TLR Ligands in the Chicken Trachea. Viruses. 2016; 8(7):207. https://0-doi-org.brum.beds.ac.uk/10.3390/v8070207
Chicago/Turabian StyleBarjesteh, Neda, Tamiru Negash Alkie, Douglas C. Hodgins, Éva Nagy, and Shayan Sharif. 2016. "Local Innate Responses to TLR Ligands in the Chicken Trachea" Viruses 8, no. 7: 207. https://0-doi-org.brum.beds.ac.uk/10.3390/v8070207





