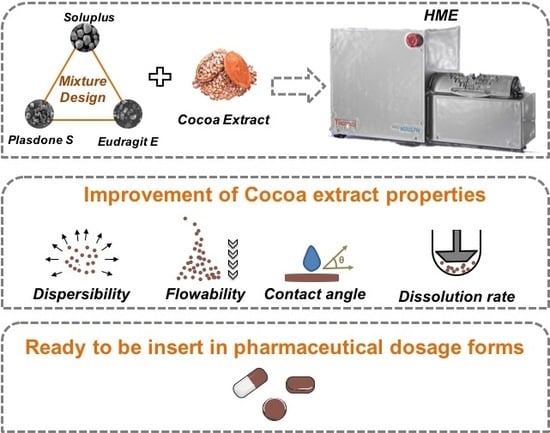
Dissolution Enhancement in Cocoa Extract, Combining Hydrophilic Polymers through Hot-Melt Extrusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mixture Design
2.3. Hot-melt extrusion (HME) Preparation
2.4. Drug Determination
2.5. Morphological Analysis
2.6. Thermogravimetric Analyses (TGA)
2.7. X-Ray Powder Diffraction (XRPD)
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
2.9. Flow Measurements
2.10. Contact Angle Determination
2.11. Dissolution Studies
2.12. Statistical Analysis
3. Results and Discussion
3.1. Thermal Stability of Theobromine (TB) and Cocoa extract (CE)
3.2. Physicochemical Characterization
3.3. Flow Evaluation
3.4. Wettability
3.5. Dissolution Rate
3.6. Prediction of the Optimized Formulation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ellam, S.; Williamson, G. Cocoa and human health. Annu. Rev. Nutr. 2013, 33, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Neufingerl, N.; Zebregs, Y.E.M.P.; Schuring, E.A.H.; Trautwein, E.A. Effect of cocoa and theobromine consumption on serum HDL-cholesterol concentrations: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Baggott, M.J.; Childs, E.; Hart, A.B.; De Bruin, E.; Palmer, A.A.; Wilkinson, J.E.; de Wit, H. Psychopharmacology of theobromine in healthy volunteers. Psychopharmacology 2013, 228, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Simons, F.E.R.; Becker, A.B.; Simons, K.J.; Gillespie, C.A. The bronchodilator effect and pharmacokinetics of theobromine in young patients with asthma. J. Allergy Clin. Immunol. 1985, 76, 703–707. [Google Scholar] [CrossRef]
- Alves, V.M.L.; Sá-Barreto, L.C.L.; Souza, G.H.B.; Cunha-Filho, M.S.S. Co-processed extracts of Cassia angustifolia Vahl, Fabaceae, and Maytenus ilicifolia (Schrad.) Planch., Celastraceae, for production of high load tablets. Rev. Bras. Farmacogn. 2011, 21, 510–517. [Google Scholar] [CrossRef] [Green Version]
- Belščak-Cvitanović, A.; Benković, M.; Komes, D.; Bauman, I.; Horžić, D.; Dujmić, F.; Matijasec, M. Physical properties and bioactive constituents of powdered mixtures and drinks prepared with cocoa and various sweeteners. J. Agric. Food Chem. 2010, 58, 7187–7195. [Google Scholar] [CrossRef] [PubMed]
- Sanphui, P.; Nangia, A. Salts and Co-crystals of Theobromine and their phase transformations in water. J. Chem. Sci. 2014, 126, 1249–1264. [Google Scholar] [CrossRef]
- Pinho, L.A.G.; Lima, S.G.B.; Malaquias, L.F.B.; Pires, F.Q.; Sá-Barreto, L.L.; Cardozo-Filho, L.; Gratieri, T.; Gelfuso, G.; Cunha-Filho, M. Improvements of theobromine pharmaceutical properties using solid dispersions prepared with newfound technologies. Chem. Eng. Res. Des. 2017, 132, 1193–1201. [Google Scholar] [CrossRef]
- Takabe, H.; Warnken, Z.N.; Zhang, Y.; Davis, D.A.; Smyth, H.D.C.; Kuhn, J.G.; Weitman, S.; Williams, R.O., III. A Repurposed Drug for Brain Cancer: Enhanced Atovaquone Amorphous Solid Dispersion by Combining a Spontaneously Emulsifying Component with a Polymer Carrier. Pharmaceutics 2018, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Douroumis, D. An in-vitro–in-vivo taste assessment of bitter drug: Comparative electronic tongues study. J. Pharm. Pharmacol. 2015, 67, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Lowinger, M.B.; Barrett, S.E.; Zhang, F.; Williams, R.O., III. Sustained Release Drug Delivery Applications of Polyurethanes. Pharmaceutics 2018, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Gloria, A.; Raucci, M.G.; De Santis, R.; Ambrosio, L. Bio-inspired composite and cell instructive platforms for bone regeneration. Int. Mater. Rev. 2012, 57, 256–275. [Google Scholar] [CrossRef]
- Cunha-Filho, M.; Araújo, M.R.; Gelfuso, G.M.; Gratieri, T. FDM 3D printing of modified drug-delivery systems using hot melt extrusion: A new approach for individualized therapy. Ther. Deliv. 2017, 8, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Malaquias, L.F.B.; Schulte, H.L.; Chaker, J.A.; Karan, K.; Durig, T.; Marreto, R.N.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. Hot Melt Extrudates Formulated Using Design Space: One Simple Process for Both Palatability and Dissolution Rate Improvement. J. Pharm. Sci. 2018, 107, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Chuah, A.M.; Jacob, B.; Jie, Z.; Ramesh, S.; Mandal, S.; Puthan, J.K.; Deshpande, P.; Vaidyanathan, V.V.; Gelling, R.W.; Patel, G.; et al. Enhanced bioavailability and bioefficacy of an amorphous solid dispersion of curcumin. Food Chem. 2014, 156, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Piao, J.; Cho, H.J.; Kang, W.S.; Kim, H.Y. Improvement in antiproliferative activity of Angelica gigas Nakai by solid dispersion formation via hot-melt extrusion and induction of cell cycle arrest and apoptosis in HeLa cells. Biosci. Biotechnol. Biochem. 2015, 79, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kang, Q.; Liu, N.; Zhang, Q.; Zhang, Y.; Li, H.; Zhao, B.; Chen, Y.; Lan, Y.; Ma, Q.; et al. Enhanced dissolution rate and oral bioavailability of Ginkgo biloba extract by preparing solid dispersion via hot-melt extrusion. Fitoterapia 2015, 102, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Viana, C.; Zemolin, G.M.; Müller, L.S.; Molin, T.R.D.; Seiffert, H.; De Carvalho, L.M. Liquid chromatographic determination of caffeine and adrenergic stimulants in food supplements sold in brazilian e-commerce for weight loss and physical fitness. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2016, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, I.; Sá-Barreto, L.C.L.; Lima, E.M.; Cunha-Filho, M.S.S. Preformulation studies of itraconazole associated with benznidazole and pharmaceutical excipients. Thermochim. Acta 2014, 575, 29–33. [Google Scholar] [CrossRef]
- Carr, R.L. Evaluating flow properties of solids. Chem. Eng. 1965, 72, 163–168. [Google Scholar]
- Alves-Silva, I.; Marreto, R.N.; Gelfuso, G.M.; Sá-Barreto, L.C.L.; Lima, E.M.; Cunha-Filho, M.S.S. Preparation of benznidazole pellets for immediate drug delivery using the extrusion spheronization technique. Drug Dev. Ind. Pharm. 2017, 43, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A. The concept of dissolution efficiency. J. Pharm. Pharmacol. 1975, 27, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Gabbay Alves, T.V.; Silva da Costa, R.; Aliakbarian, B.; Casazza, A.A.; Perego, P.; Pinheiro Arruda, M.S.; Carréra Silva Júnior, J.O.; Converti, A.; Ribeiro Costa, R.M. Bioactive compounds and antioxidant potential for polyphenol-rich cocoa extract obtained by agroindustrial residue. Nat. Prod. Res. 2017, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Talvani, A.; Bahia, M.T.; Sá-Barreto, L.C.L.; Lima, E.M.; Cunha-Filho, M.S.S. Carvedilol:decomposition kinetics and compatibility with pharmaceutical excipients. J. Therm. Anal. Calorim. 2014, 115, 2501–2506. [Google Scholar] [CrossRef]
- Findoráková, L.; Svoboda, R. Kinetic analysis of the thermal decomposition of Zn(II) 2-chlorobenzoate complex with caffeine. Thermochim. Acta 2012, 543, 113–117. [Google Scholar] [CrossRef]
- Izutsu, K.; Hiyama, Y.; Yomota, C.; Kawanishi, T. Near-infrared analysis of hydrogen-bonding in glass- and rubber-state amorphous saccharide solids. AAPS PharmSciTech 2009, 10, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Krantz, M.; Zhang, H.; Zhu, J. Characterization of powder flow: Static and dynamic testing. Powder Technol. 2009, 194, 239–245. [Google Scholar] [CrossRef]
- Maddineni, S.; Battu, S.K.; Morott, J.; Majumdar, S.; Murthy, S.N.; Repka, M.A. Influence of process and formulation parameters on dissolution and stability characteristics of Kollidon® VA 64 hot-melt extrudates. AAPS PharmSciTech 2015, 16, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tang, N.; Lian, R.; Qi, J.; Wu, W. Understanding the relationship between wettability and dissolution of solid dispersion. Int. J. Pharm. 2014, 465, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Kwon, S.H.; Lee, S.E.; Jang, W.S.; Byeon, J.C.; Jeong, H.M.; Park, J.S. Use of acidifier and solubilizer in tadalafil solid dispersion to enhance the in vitro dissolution and oral bioavailability in rats. Int. J. Pharm. 2017, 526, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, C.; Millqvist-Fureby, A.; Schuleit, M. Surface composition and contact angle relationships for differently prepared solid dispersions. Eur. J. Pharm. Biopharm. 2008, 70, 478–485. [Google Scholar] [CrossRef] [PubMed]










| Samples | CE % (w/w) | Polymer % (w/w) | Temperature (°C) | Rotation (rpm) | Drug Content (%) | Strip Aspect | ||
|---|---|---|---|---|---|---|---|---|
| Sol | PVP | EuE | ||||||
| HME-Sol | 30 | 70 | 0 | 0 | 185 | 50 | 98.4 ± 0.3 |  |
| HME-PVP | 30 | 0 | 70 | 0 | 170 | 100 | 101.5 ± 0.5 |  |
| HME-EuE | 30 | 0 | 0 | 70 | 150 | 100 | 103.7 ± 0.5 |  |
| HME-Sol-EuE | 30 | 35 | 35 | 0 | 160 | 100 | 90.1 ± 0.2 |  |
| HME-PVP-EuE | 30 | 0 | 35 | 35 | 160 | 100 | 94.1 ± 0.7 |  |
| HME-Sol-PVP | 30 | 35 | 35 | 0 | 170 | 100 | 99.0 ± 0.2 |  |
| HME-Sol-EuE-PVP | 30 | 23.3 | 23.3 | 23.3 | 150 | 100 | 98.3 ± 0.3 |  |
| Sample | Repose Angle (° ± SD) | Spatula Angle (° ± SD) | Compressibility (%) | Flowability (%) | Dispersibility (%) | ||
|---|---|---|---|---|---|---|---|
| CE | 53.4 ± 1.4 |  | 65.3 ± 1.5 |  | 50.5 ± 1.7 | 49.0 ± 0.0 | 37.8 ± 3.2 |
| HME Sol | 31.5 ± 1.4 |  | 37.3 ± 1.1 |  | 20.2 ± 1.7 | 85.5 ± 1.0 | 7.2 ± 0.6 |
| HME PVP | 42.1 ± 1.0 |  | 53.4 ± 1.4 |  | 26.8 ± 0.9 | 71.5 ± 1.2 | 5.7 ± 0.3 |
| HME EuE | 34.7 ± 0.9 |  | 39.4 ± 1.0 |  | 18.2 ± 0.0 | 83.5 ± 1.4 | 11.8 ± 2.7 |
| HME PVP-Sol | 33.6 ± 1.6 |  | 42.1 ± 2.6 |  | 20.2 ± 1.7 | 81.5 ± 1.3 | 7.2 ± 0.2 |
| HME PVP-EuE | 33.6 ± 0.7 |  | 32.6 ± 1.0 |  | 18.2 ± 2.6 | 86.0 ± 1.3 | 9.2 ± 1.0 |
| HME Sol-EuE | 35.0 ± 0.5 |  | 34.7 ± 0.3 |  | 18.2 ± 0.0 | 84.0 ± 0.6 | 9.9 ± 1.0 |
| HME EuE-Sol-PVP | 32.6 ± 1.0 |  | 32.3 ± 1.2 |  | 15.9 ± 3.3 | 88.0 ± 1.6 | 7.5 ± 1.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinho, L.A.G.; Souza, S.G.; Marreto, R.N.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. Dissolution Enhancement in Cocoa Extract, Combining Hydrophilic Polymers through Hot-Melt Extrusion. Pharmaceutics 2018, 10, 135. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10030135
Pinho LAG, Souza SG, Marreto RN, Sa-Barreto LL, Gratieri T, Gelfuso GM, Cunha-Filho M. Dissolution Enhancement in Cocoa Extract, Combining Hydrophilic Polymers through Hot-Melt Extrusion. Pharmaceutics. 2018; 10(3):135. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10030135
Chicago/Turabian StylePinho, Ludmila A. G., Saulo G. Souza, Ricardo N. Marreto, Livia L. Sa-Barreto, Tais Gratieri, Guilherme M. Gelfuso, and Marcilio Cunha-Filho. 2018. "Dissolution Enhancement in Cocoa Extract, Combining Hydrophilic Polymers through Hot-Melt Extrusion" Pharmaceutics 10, no. 3: 135. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10030135