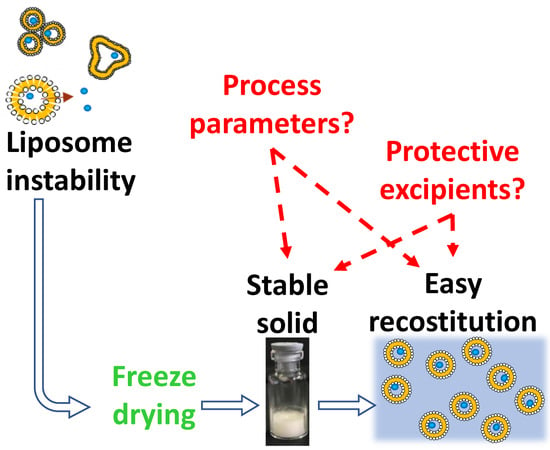
Lyophilization of Liposomal Formulations: Still Necessary, Still Challenging
Abstract
:1. Introduction
2. Thermotropic Behavior of Phospholipid Bilayers
3. Stresses of Liposomal Dispersions Occurring During Freeze-Drying
3.1. Freezing Step
3.2. Drying Steps
4. Stabilization by Excipients
Use of Organic Solvent as Adjuvants
5. Quality by Design
- definition of the target product quality profile,
- determination of the critical quality attributes (CQAs) and critical process parameters (CPPs),
- risk assessment,
- development of an experimental design aiming to investigate the impact of CPPs on CQAs and establish a design space
- design and implementation of a control strategy to ensure a continuous improvement.
6. Quality Attributes of the Freeze-Dried Liposomes
6.1. Criticism of The Lyophilized Products
6.2. Criticisms of The Reconstituted Liposomal Dispersion
7. Conclusion
Funding
Conflicts of Interest
References
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Franzen, U.; Nguyen, T.T.; Vermehren, C.; Gammelgaard, B.; Østergaard, J. Characterization of a liposome-based formulation of oxaliplatin using capillary electrophoresis: Encapsulation and leakage. J. Pharm. Biomed. Anal. 2011, 55, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Jinturkar, K.; Patel, D.; Lalani, J.; Chrougule, M. Recent advances in liposomal dry powder formulations: Preparation and evaluation. Expert Opin. Drug Deliv. 2009, 6, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Wilkhu, J.S.; McNeil, S.E.; Anderson, D.E.; Kirchmeier, M.; Perrie, Y. Development of a solid dosage platform for the oral delivery of bilayer vesicles. Eur. J. Pharm. Sci. 2017, 108, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.M. The care and feeding of a commercial liposomal product: Liposomal amphotericin B (AmBisome). J. Liposome Res. 2017, 27, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An overview of liposome lyophilization and its future potential. J. Control. Rel. 2010, 142, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medicinal Products for Human Use. Guideline on Process Validation for Finished Products—Information and Data to be Provided in Regulatory Submissions; EMA/CHMP/CVMP/QWP/BWP/70278/2012-Rev1,Corr.1; European Medicines Agency: London, UK, 2012. [Google Scholar]
- Committee for Human Medicinal Products. Reflection Paper on the Data Requirements for Intravenous Liposomal Products Developed with Reference to an Innovator Liposomal Product; EMA/CHMP/806058/2009/Rev. 02; European Medicines Agency: London, UK, 2013. [Google Scholar]
- Musazzi, U.M.; Marini, V.; Casiraghi, A.; Minghetti, P. Is the European regulatory framework sufficient to assure the safety of citizens using health products containing nanomaterials? Drug Disc. Today 2017, 22, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Eeman, M.; Deleu, M. From biological membranes to biomimetic model membranes. Base 2010, 14, 719–736. [Google Scholar]
- Hays, L.M.; Crowe, J.H.; Wokers, W.; Rudenko, S. Factors affecting leakage of trapped solutes from phospholipid vescicles during thermotropic phase transition. Cryobiology 2001, 42, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta 1998, 1376, 91–145. [Google Scholar] [CrossRef]
- Arias, J.M.; Tuttolomondo, M.E.; Díaz, S.B.; Altabef, A.B. Molecular view of the structural reorganization of water in DPPC multilamellar membranes induced by L-cysteine methyl ester. J Mol. Struct. 2018, 1156, 360–368. [Google Scholar] [CrossRef]
- Kitayama, H.; Takechi, Y.; Tamai, N.; Matsuki, H.; Yomota, C.; Saito, H. Thermotropic phase behavior of hydrogenated soybean phosphatidylcholine–cholesterol binary liposome membrane. Chem. Pharm. Bull. 2014, 62, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H. Mixed-chain phospholipids: Structures and chain-melting behavior. Lipids 2001, 36, 1077–1097. [Google Scholar] [CrossRef] [PubMed]
- Heimburg, T. A model for the lipid pretransition: Coupling of ripple formation with the chain-melting transition. Biophys. J. 2000, 78, 1154–1165. [Google Scholar] [CrossRef]
- McElhaney, R.N. The use of differential scanning calorimetry and differential thermal analysis in studies of model and biologic membranes. Chem. Phys. Lipids 1982, 30, 229–259. [Google Scholar] [CrossRef]
- Tardieu, A.; Luzzati, V.; Reman, F.C. Structure and polymorphism of the hydrocarbon chains of lipids: A study of lecithin-water phases. J. Mol. Biol. 1973, 75, 711–733. [Google Scholar] [CrossRef]
- McIntosh, T.J. Differences in hydrocarbon chain tilt between hydrated phosphatidylethanolamine and phosphatidylcholine bilayers. Biophys. J. 1980, 29, 237–245. [Google Scholar] [CrossRef]
- Filippov, A.; Orädd, G.; Lindblom, G. The effect of cholesterol on the lateral diffusion of phospholipids in oriented bilayers. Biophys. J. 2003, 84, 3079–3086. [Google Scholar] [CrossRef]
- Sauvage, F.; Franzè, S.; Bruneau, A.; Alami, M.; Denis, S.; Nicolas, V.; Lesieur, S.; Legrand, F.; Barratt, G.; Messaoudi, S.; et al. Formulation and in vitro efficacy of liposomes containing the Hsp90 inhibitor 6BrCaQ in prostate cancer cells. Int. J. Pharm. 2016, 499, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Stark, B.; Pabst, G.; Prassl, R. Long-term stability of sterically stabilized liposomes by freezing and freeze-drying: Effect of cryoprotectants on structure. Eur. J. Pharm. Sci. 2010, 41, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Gharib, R.; Fourmentin, S.; Charcosset, C.; Greige-Gerges, H. Effect of hydroxypropyl-β–cyclodextrin on lipid membrane fluidity, stability and freeze-drying of liposomes. J. Drug Delivery Sci. Technol. 2018, 44, 101–107. [Google Scholar] [CrossRef]
- Riske, K.A.; Amaral, L.Q.; Lamy-Freund, M.T. Thermal transitions of DMPG bilayers in aqueous solution: SAXS structural studies. Biochim. Biophys. Acta 2001, 1511, 297–308. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Villegas-Opazo, V.A.; Sagredo-Oyarce, D.H.; Sarabia-Vallejos, M.A.; Terraza, C.A. Thermal response analysis of phospholipid bilayers using ellipsometric techniques. Biosens. 2017, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Izutsu, K.I.; Yomota, C.; Kawanishi, T. Stabilization of liposomes in frozen solutions through control of osmotic flow and internal solution freezing by trehalose. J. Pharm. Sci. 2011, 100, 2935–2944. [Google Scholar] [CrossRef] [PubMed]
- Bronshteyn, V.L.; Steponkus, P. Calorimetric studies of freeze-induces dehydration of phospholipids. Biophys. J. 1993, 65, 1853–1865. [Google Scholar] [CrossRef]
- Wolfe, J.; Bryant, G. Freezing, drying, and/or vitrification of membrane–solute–water systems. Cryobiology. 1999, 39, 103–129. [Google Scholar] [CrossRef] [PubMed]
- Nakhla, T.; Marek, M.; Kovalcik, T. Issues associated with large-scale production of liposomal formulations. Drug Delivery Technol. 2002, 2, 1–6. [Google Scholar]
- Wessman, P.; Edwards, K.; Mahlin, D. Structural effects caused by spray- and freeze-drying of liposomes and bilayer disks. J. Pharm. Sci. 2010, 99, 2032–2048. [Google Scholar] [CrossRef] [PubMed]
- Cabane, B.; Blanchon, S.; Neves, C. Recombination of nanometric vesicles during freeze-drying. Langmuir 2006, 22, 1982–1990. [Google Scholar] [CrossRef] [PubMed]
- Ingvarsson, P.T.; Yang, M.; Nielsen, H.M.; Rantanen, J.; Foged, C. Stabilization of liposomes during drying. Expert Opin. Drug Delivery 2011, 8, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Van Winden, E.C. Freeze-drying of liposomes: Theory and practice. Methods Enzymol. 2003, 367, 99–110. [Google Scholar] [PubMed]
- Van Winden, E.C.A.; Crommelin, D.J.A. Short term stability of freeze-dried, lyoprotected liposomes. J. Control. Release 1999, 58, 69–86. [Google Scholar] [CrossRef]
- Henry-Michelland, S.; Ter-Minassian-Saraga, L.; Poly, P.A.; Delattre, J.; Puisieux, F. Lyophilization and rehydration of liposomes. Colloids Surf. 1985, 14, 269–276. [Google Scholar] [CrossRef]
- Shah, V.H.; Jobanputra, A. Enhanced ungual permeation of terbinafine HCl delivered through liposome-loaded nail lacquer formulation optimized by QbD approach. AAPS PharmSciTech 2018, 19, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, H.; Klose, G.; Heremans, K. FTIR spectroscopy study of the pressure-dependent behaviour of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1-palmitoyl-2-oleolyl-sn-glycero-3-phosphocholine (POPC) at low degrees of hydration. Chem. Phys. Lipids 2013, 170, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kent, B.; Garvey, C.J.; Cookson, D.; Bryant, G. The inverse hexagonal – inverse ribbon – lamellar gel phase transition sequence in low hydration DOPC: DOPE phospholipid mixtures. Chem. Phys. Lipids 2009, 157, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.M.; Zografi, G. The relationship between water vapor absorption and desorption by phospholipids and bilayer phase transitions. J. Pharm. Pharm. Sci. 2007, 96, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H.; Hoekstra, F.A.; Crowe, L.M. Membrane phase transitions are responsible for imbibitional damage in dry pollen. Proc. Nati. Acad. Sci. 1989, 86, 520–523. [Google Scholar] [CrossRef] [Green Version]
- Popova, A.V.; Hincha, D.K. Effects of cholesterol on dry bilayers: Interaction between phophatidylcholine unsaturation and glycolipid or free sugar. Biophys. J. 2007, 93, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Pansare, S.K.; Patel, S.M. Practical considerations for determination of glass transition temperature of a maximally freeze concentrated solution. AAPS PharmSciTech 2016, 17, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.R.; Coombes, A.G.; Perrie, Y. Amino acids as cryoprotectants for liposomal delivery systems. Eur. J. Pharm. Sci. 2007, 30, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.R.; Bramwell, V.W.; Coombes, A.G.; Perrie, Y. Lyophilisation and sterilisation of liposomal vaccines to produce stable and sterile products. Methods 2006, 40, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Greco, K.; Mujat, M.; Galbally-Kinney, K.L.; Hammer, D.X.; Ferguson, R.D.; Iftimia, N.; Mulhall, P.; Sharma, P.; Kessler, W.J.; Pikal, M.J. Accurate prediction of collapse temperature using optical coherence tomography-based freeze-drying microscopy. J. Pharm. Sci. 2013, 102, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Korang-Yeboah, M.; Srinivasan, C.; Siddiqui, A.; Awotwe-Otoo, D.; Cruz, C.N.; Muhammad, A. Application of optical coherence tomography freeze-drying microscopy for designing lyophilization process and its impact on process efficiency and product quality. AAPS PharmSciTech 2018, 19, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Van Winden, E.C.A.; Crommelin, D.J.A. Long term stability of freeze-dried, lyoprotected doxorubicin liposomes. Eur. J. Pharm. Biopharm. 1997, 43, 295–307. [Google Scholar] [CrossRef]
- Wang, D.Q.; Hey, J.M.; Nail, S.L. Effect of collapse on the stability of freeze-dried recombinant Factor VIII and α-amylase. J. Pharm. Sci. 2004, 93, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, B.; Porfire, A.; Van Bockstal, P.J.; Porav, S.; Achim, M.; De Beer, T.; Tomuţă, I. Formulation optimization of freeze-dried long-circulating liposomes and in-line monitoring of the freeze-drying process using an NIR spectroscopy tool. J. Pharm. Sci. 2018, 107, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.M.; Nail, S.L.; Pikal, M.J.; Geidobler, R.; Winter, G.; Hawe, A.; Davagnino, J.; Rambhatla Gupta, S. Lyophilized drug product cake appearance: What is acceptable? J. Pharm. Sci. 2017, 106, 1706–1721. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Deng, Y.; Geng, Y.; Gao, Z.; Zou, J.; Wang, Z. Preparation of submicron unilamellar liposomes by freeze-drying double emulsion. Biochim. Biophys. Acta 2006, 1758, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H.; Hoekstra, F.A.; Nguyen, K.H.N.; Crowe, L.M. Is vitrification involved in depression of the phase transition temperature in dry phospholipids? Biochim. Biophys. Acta 1994, 1280, 187–196. [Google Scholar] [CrossRef]
- Pereira, C.S.; Lins, R.D.; Chandrasekhar, I.; Freitas, L.C.G.; Hünenberger, P.H. Interaction of the disaccharide trehalose with a phospholipid bilayer: A molecular dynamics study. Biophys. J. 2004, 86, 2273–2285. [Google Scholar] [CrossRef]
- Cacela, C.; Hincha, D.K. Low amounts of sucrose are sufficient to depress the phase transition temperature of dry phosphatidylcholine, but not for lyoprotection of liposomes. Biophys. J. 2006, 90, 2831–2842. [Google Scholar] [CrossRef] [PubMed]
- Kannan, V.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Effect of sucrose as a lyoprotectant on the integrity of paclitaxel-loaded liposomes during lyophilization. J. Liposome Res. 2015, 25, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Wolkers, W.F.; Oldenhof, H.; Tablin, F.; Crowe, J.H. Preservation of dried liposomes in the presence of sugar and phosphate. Biochim. Biophys. Acta 2004, 1661, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Koster, K.L.; Webb, M.S.; Bryant, G.; Lynch, D.V. Interactions between soluble sugars and POPC (1-palmitoyl-2-oleoylphosphatidylcholine) during dehydration: Vitrification of sugars alters the phase behavior of the phospholipid. Biochim. Biophys. Acta 1994, 1193, 143–150. [Google Scholar] [CrossRef]
- Miyajima, K. Role of saccharides for the freeze-thawing and freeze drying of liposome. Adv. Drug Delivery Rev. 1997, 24, 151–159. [Google Scholar] [CrossRef]
- Suzuki, T.; Komatsu, H.; Miyajima, K. Effect of glucose and its oligomers on the stability of freeze-dried liposomes. Biochim. Biophys. Acta 1996, 1278, 176–182. [Google Scholar] [CrossRef]
- Crowe, J.H.; Oliver, A.E.; Hoekstra, F.A.; Crowe, L.M. Stabilization of Dry Membranes by Mixtures of Hydroxyethyl Starch and Glucose: The Role of Vitrification. Cryobiology 1997, 35, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Hayashi, M. The effects of glucose oligomers (maltodextrins) on freeze-drying liposomes. Chem. Pharm. Bull. 1997, 45, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, W.L.J.; Sanders, N.N.; De Smedt, S.C.; Demeester, J.; Frijlink, H.W. Inulin is a promising cryo- and lyoprotectant for PEGylated lipoplexes. J. Controlled Release 2005, 103, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Vincourt, V.; Nguyen, L.; Chaumeil, J.; Dumortier, G. Freeze-drying of ATP entrapped in cationic, low lipid liposomes. Cryobiology 2010, 60, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoven, J.M.; Metselaar, J.M.; Storm, G.; Beijnen, J.H.; Nuijen, B. Cyclodextrin as membrane protectant in spray-drying and freeze-drying of PEGylated liposomes. Int. J. Pharm. 2012, 438, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Wieber, A.; Selzer, T.; Kreuter, J. Physico-chemical characterization of cationic DOTAP liposomes as drug delivery-system for a hydrophilic decapeptide before and after freeze-drying. Eur. J. Pharm. Biopharm. 2012, 80, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Lu, Y.; Qi, J.; Niu, M.; Lian, R.; Wu, W. Solidification of liposomes by freeze-drying: The importance of incorporating gelatin as interior support on enhanced physical stability. Int. J. Pharm. 2015, 478, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Morandi, M.I.; Sommer, M.; Kluzek, M.; Thalmann, F.; Schroder, A.P.; Marques, C.M. DPPC bilayers in solutions of high sucrose content. Biophys. J. 2018, 114, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Strauss, G.; Schurtenberger, P.; Hauser, H. The interaction of saccharides with lipid bilayer vesicles: Stabilization during freeze-thawing and freeze-drying. Biochim. Biophys. Acta 1986, 858, 169–180. [Google Scholar] [CrossRef]
- Crowe, L.M.; Crowe, J.H. Solution effects on the thermotropic phase transition of unilamellar liposomes. Biochim. Biophys. Acta 1991, 1064, 267–274. [Google Scholar] [CrossRef]
- Peer, D.; Florentin, A.; Margalit, R. Hyaluronan is a key component in cryoprotection and formulation of targeted unilamellar liposomes. Biochim. Biophys. Acta 2003, 1612, 76–82. [Google Scholar] [CrossRef]
- Garipova, V.R.; Gennari, C.G.M.; Selmin, F.; Cilurzo, F.; Moustafine, R.I. Mucoadhesive interpolyelectrolyte complexes for the buccal delivery of clobetasol. Polym. 2018, 10, 85. [Google Scholar] [CrossRef]
- Crowe, J.H.; Leslie, S.B.; Crowe, L.M. Is vitrification sufficient to preserve liposomes during freeze-drying? Cryobiology 1994, 31, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Kassem, M.A.; Aboul-Einien, M.H.; El Taweel, M.M. Dry gel containing optimized felodipine-loaded transferosomes: A promising transdermal delivery system to enhance drug bioavailability. AAPS PharmSciTech 2018, 19, 2155–2173. [Google Scholar] [CrossRef] [PubMed]
- Skrabanja, A.T.P.; De Meere, A.L.J.; De Ruiter, R.A.; Van Den Oetelaar, J.M. Lyophilization of biotechnology products. PDA J. Pharm. Sci. Technol. 1994, 48, 311–317. [Google Scholar] [PubMed]
- Arpicco, S.; Lerda, C.; Dalla Pozza, E.; Costanzo, C.; Tsapis, N.; Stella, B.; Donadelli, M.; Dando, I.; Fattal, E.; Cattel, L.; et al. Hyaluronic acid-coated liposomes for active targeting of gemcitabine. Eur. J. Pharm. Biopharm. 2013, 85, 373–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatnagar, B.S.; Martin, S.W.H.; Hodge, T.S.; Das, T.K.; Joseph, L.; Teagarden, D.L.; Shalaev, E.Y.; Suryanarayanan, R. Investigation of PEG crystallization in frozen and freeze-dried PEGylated recombinant human growth hormone–sucrose systems: Implications on storage stability. J. Pharm. Sci. 2011, 100, 3062–3075. [Google Scholar] [CrossRef] [PubMed]
- Popova, A.V.; Hincha, D.K. Effects of flavonol glycosides on liposome stability during freezing and drying. Biochim. Biophys. Acta 2016, 1858, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Fujioka, H.; Nakamura, Y. Cryoprotective effect of gelatin and albumin on recombinant human tumor necrosis factor liposome. Chem. Pharm. Bull. 1993, 41, 2138–2140. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, X.; Shen, B.; Xie, Y.; Shen, C.; Lu, Y.; Qi, J.; Yuan, H.; Wu, W. Enhanced stability of liposomes against solidification stress during freeze-drying and spray-drying by coating with calcium alginate. J. Drug Delivery Sci. Technol. 2015, 30, 163–170. [Google Scholar] [CrossRef]
- Teagarden, D.L.; Baker, D.S. Practical aspects of lyophilization using non-aqueous co-solvent systems. Eur. J. Pharm. Sci. 2002, 15, 115–133. [Google Scholar] [CrossRef]
- Kunz, C.; Gieseler, H. Factors influencing the retention of organic solvents in products freeze-dried from co-solvent system. J. Pharm. Sci. 2018, 107, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Kasrain, K.; DeLuca, P.P. Thermal analysis of the tertiary butyl alcohol-water system and its implication on freeze-drying. Pharm. Res. 1995, 12, 484–490. [Google Scholar] [CrossRef]
- Kasrain, K.; DeLuca, P.P. The effect of tertiary butyl alcohol on the resistance of the dry product layer during primary drying. Pharm. Res. 1995, 12, 491–495. [Google Scholar] [CrossRef]
- Cui, J.X.; Li, C.L.; Deng, Y.J.; Wang, Y.L.; Wang, W. Freeze-drying of liposomes using tertiary butyl alcohol/water cosolvent systems. Int. J. Pharm. 2006, 312, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yu, S.; Fu, D. Preparation of liposomes containing zedoary turmeric oil using freeze-drying of liposomes via TBA/water cosolvent systems and evaluation of the bioavailability of the oil. J. Drug Targeting 2010, 18, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Vessot, S.; Andrieu, J. A Review on freeze drying of drugs with tert-butanol (TBA) + water systems: Characteristics, advantages, drawbacks. Drying Technol. 2012, 30, 377–385. [Google Scholar] [CrossRef]
- Robert, J.L. Pharmaceutical Development. In Proceedings of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH Q8 (R2), Kuala Lumpur, Malaysia, July 2010. [Google Scholar]
- Sylvester, B.; Porfire, A.; Achim, M.; Rus, L.; Tomuţă, I. A step forward towards the development of stable freeze-dried liposomes: A quality by design approach (QbD). Drug Dev. Ind. Pharm. 2018, 44, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Kamal, N.; Cutie, A.J.; Habib, M.J.; Zidan, A.S. QbD approach to investigate product and process variabilities for brain targeting liposomes. J. Liposome Res. 2015, 25, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Porfire, A.; Muntean, D.M.; Rus, L.; Sylvester, B.; Tomuţă, I. A quality by design approach for the development of lyophilized liposomes with simvastatin. Saudi Pharm. J. 2017, 25, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Ueda, H.; Nakagaki, M. Effect of water on lamellar structure of DPPC/sugar systems. Biochim. Biophys. Acta Biomembr. 1997, 1328, 197–206. [Google Scholar] [CrossRef]
- Tang, X.; Pikal, M.J. Design of freeze-drying processes for pharmaceuticals: Practical advice. Pharm. Res. 1997, 14, 1151–1160. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Guide to inspections of lyophilization of parenterals. Available online: https://www.fda.gov/ICECI/Inspections/InspectionGuides/ucm074909.htm (accessed on 10 August 2018).
- Dawson, P.J.; Hockley, D.J. Scanning electron microscopy of freeze-dried preparations: Relationship of morphology to freeze-drying parameters. Dev. Biol. Stand. 1992, 74, 185–192. [Google Scholar] [PubMed]
- Wahl, V.; Khinast, J.; Paudel, A. Lyophilized protein powders: A review of analytical tools for rootcause analysis of lot-to-lot variability. TrAC Trends Anal. Chem. 2016, 82, 468–491. [Google Scholar] [CrossRef]
- Selmin, F.; Gennari, C.G.M.; Minghetti, P.; Marotta, L.A.; Viviani, B.; Vagdama, P.; Montanari, L.; Cilurzo, F. Enhanced hydration stability of Bombyx mori silk fibroin/PEG 600 composite scaffolds for tissue engineering. Polym. Adv. Technol. 2014, 25, 532–538. [Google Scholar] [CrossRef]
- Brouckaert, D.; De Meyer, L.; Vanbillemont, B.; Van Bockstal, P.J.; Lammens, J.; Mortier, S.; Corver, J.; Vervaet, C.; Nopens, I.; De Beer, T. The potential of near-infrared chemical imaging as process analytical technology tool for continuous freeze-drying. Anal. Chem. 2018, 90, 4354–4362. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Williams, D. Morphological and compressional mechanical properties of freeze-dried mannitol, sucrose, and trehalose cakes. J. Pharm. Sci. 2013, 102, 4246–4255. [Google Scholar] [CrossRef] [PubMed]
- Hackl, E.V.; Ermolina, I. Using texture analysis technique to assess the freeze-dried cakes in vials. J. Pharm. Sci. 2016, 105, 2073–2085. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Lee, S.L.; Tyner, K. Liposomal drug product development and quality: Current US experience and perspective. AAPS J. 2017, 19, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Payton, N.M.; Wempe, M.F.; Xu, Y.; Anchordoquy, T.J. Long term storage of lyophilized liposomal formulations. J. Pharm. Sci. 2014, 103, 3869–3878. [Google Scholar] [CrossRef] [PubMed]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of nanoparticle tracking analysis (NTA) by nanosight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [PubMed]
- De Morais Ribeiro, L.N.; Muniz Couto, V.; Fraceto, L.F.; de Paula, E. Use of nanoparticle concentration as a tool to understand the structural properties of colloids. Sci. Rep. 2018, 8, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Franzé, S.; Marengo, A.; Stella, B.; Minghetti, P.; Arpicco, S.; Cilurzo, F. Hyaluronan-decorated liposomes as drug delivery systems for cutaneous administration. Int. J. Pharm. 2018, 535, 333–339. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Liposome Drug Products Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation Guidance for Industry. 2018. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm070570.pdf (accessed on 21 August 2018).







| Phospholipids | Acyl Chains | Tm (°C) | Tp (°C) | Ref. |
|---|---|---|---|---|
| DLPC | 12:0/12:0 | −1 | - | [12,13] |
| DMPC | 14:0/14:0 | 24 | 22.0 | [13] |
| DPPC | 16:0/16:0 | 40.5 | 35.5 | [14] |
| DSPC | 18:0 | 49.1 | 54.5 | [13] |
| DOPC | 18:1 | −18 | 9.0 | [13] |
| HSPC | 16:0/18:0 | 53.6 | 47.8 | [15] |
| EPC | Mixed chains | −15 to −20 | - | [13] |
| Protectant | Liposome Components | Effect | Ref. | |
|---|---|---|---|---|
| Reconstitution (Protectant:lipids Ratio) | Avoiding Leakage (Substance) | |||
| Mannitol | POPC or DSPC: ls-PG: DSPE-PEG2000 | Y (5-10:1 w/w) | - | [23] |
| Glucose | POPC or DSPC: ls-PG: DSPE-PEG2000 DOPC or DPPC or EPC EPC | Y (5-10:1 w/w) - N ( (0-500 mg/mL) | - Ineffective (calcein) Ineffective in the range (CF) | [23] |
| [60] | ||||
| [61] | ||||
| Lactose | POPC or DSPC: ls-PG: DSPE-PEG2000 | Y (5-10:1 w/w) | - | [23] |
| DPPC:DPPG:CHOL | Y (5% w/w in; 15% out) | Effective (doxorubicin) | [48] | |
| SPC or SPC:SPS or SPC:SPS:CHOL | Y (5% w/w) | - | [52] | |
| Maltose | DOPC or EPC DPPC | - - | Ineffective (calcein) Effective (calcein) | [60] |
| DPPC | - | Effective ( > 5:1 mol/mol) | [62] | |
| DPPC:DPPG:CHOL | Y (5% w/w in; 15% out) | Effective (doxorubicin) | [48] | |
| Sucrose | DPPC:DPPG:CHOL | Y (5% w/w in; 15% out) | Effective (doxorubicin) | [48] |
| DOTAP:DOPE DOTAP:DOPE:DSPE-PEG | Y (57:1 w/w) Y (51:1 w/w) | - - | [63] | |
| EPC | Y (0-500 mg/mL) | ≥50 mg/mL (CF) | [61] | |
| SPC:CHOL:DOTAP EPC:CHOL:DOTAP HSPC:CHOL:DOTAP | Y (3-15:1 w/w) Y (3-15:1 w/w) Y (20-25:1 w/w) | Ineffective (ATP) Ineffective (ATP) Not tested | [64] | |
| DPPC:CHOL:DSPE-PEG | Y (6:1 w/w) | Ineffective (prednisolone) | [65] | |
| DOTAP:CHOL | Y (1.6-2.7:1 w/w) | Ineffective (decapeptide) | [66] | |
| SPC SPC:SPS SPC:SPS:CHOL | Y (5% w/w) Y (5% w/w) Y (5% w/w) | - - - | [52] | |
| Trehalose | DPPC EPC DSPC:DSPE-PEG2000:ls-PG POPC:DSPE-PEG2000:ls-PG DPPC:DPPG:CHOL DOTAP:DOPE DOTAP:DOPE:DSPE-PEG SPC or EPC:CHOL:DOTAP HSPC:CHOL:DOTAP DPPC:CHOL:DSPE-PEG DOTAP:CHOL EPC:CHOL | Y (> 0.5:1) Y (> 0.3:1 w/w) | Ineffective (CF) Ineffective (CF) | [53] |
| Y (5-10:1 w/w) Y (5-10:1 w/w) | - - | [23] | ||
| Y (5% w/w in; 15% out) | Effective (doxorubicin) | [48] | ||
| Y (57:1 w/w) Y (51:1 w/w) | - - | [63] | ||
| Y (5-15:1 w/w) Y (5-25:1 w/w) | Effective (< 10:1 w/w) (ATP) | [60] | ||
| Y (6:1 w/w) | Ineffective (prednisolone) | [65] | ||
| Y (1.6-3.7:1 w/w) | Ineffective (decapeptide) | [66] | ||
| Y (2-8:1 mol/mol) N (10:1 mol/mol) | Effective at 4:1 mol:mol (ibuprofen) | [44] | ||
| Maltotriose | DOPC or EPC DPPC | - - | Ineffective (calcein) Effective (calcein) | [60] |
| Maltotetraose | DOPC or EPC DPPC | - - | Ineffective (calcein) Effective (calcein) | |
| Maltoexaose | DOPC or EPC DPPC | - - | Ineffective (calcein) Effective (calcein) | |
| Maltoheptaose | DOPC or EPC DPPC | - - | Ineffective (calcein) effective (calcein,>90%) | |
| DPPC | - | Ineffective (calcein, 75%) | [62] | |
| HES | EPC | Y (0-500 mg/mL) | Ineffective (CF) | [61] |
| Dextran (1.5 kDa) | DOTAP:DOPE DOTAP:DOPE:DSPE-PEG | Y (57:1 w/w) Y (51:1 w/w) | - - | [63] |
| Dextran (5 kDa) | DOTAP:DOPE | Y (57:1 w/w) | - | |
| Dextran (40 kDa) | DOTAP:DOPE:DSPE-PEG | Y (57:1 w/w) | - | |
| Dextran (480 KDa) | DPPC or EPC | Y (5-15:1 w/w) | Ineffective (CF) | [53] |
| Inulin (1.8 kDa) | DOTAP:DOPE DOTAP:DOPE:DSPE-PEG | Y (57:1 w/w) Y (51:1 w/w) | - - | [63] |
| Inulin (4 kDa) | DOTAP:DOPE DOTAP:DOPE:DSPE-PEG | Y (57:1 w/w) Y (51:1 w/w) | - - | [63] |
| HP-β-cyclodextrin | DPPC:CHOL:DSPE-PEG | Y (6:1 w/w) | Effective (prednisolone) | [65] |
| Quercetin-3-O-glucoside | EPC EPC:EPE EPC:DLPE | Y (30 mol %) N (30 mol %) Y (30 mol %) | Ineffective (CF) | [42] |
| Quercetin-3-O-rhamnoside | EPC EPC:EPE EPC:DLPE | Y (30 mol %) N (30 mol %) Y (30 mol %) | Ineffective (CF) | [42] |
| Kaempferol-3-O--glucoside | EPC or EPC:EPE or EPC:DLPE | Y (30 mol/mol) | Ineffective (CF) | [42] |
| Kaempferol-7-O--glucoside | EPC or EPC:EPE or EPC:DLPE | Y (30 mol/mol) | Ineffective (CF) | [42] |
| Arginine | EPC:CHOL | Y (4:1 mol/mol, only) | Effective (ibuprofen) | [44] |
| Histidine | EPC:CHOL | Y (4:1 mol/mol) | Effective (ibuprofen) | [44] |
| Lysine | EPC:CHOL | Y (2-4:1 mol/mol) | Effective (4:1 mol:mol) (ibuprofen) | [44] |
| Gelatin | SPC:CHOL | Y (5-20% w/v in) | Effective (>10%, 94.2% CF) | [67] |
| Compound | Tc (°C) | Tg’ (°C) | Wg’ (%) |
|---|---|---|---|
| Glucose | −40 | −43 | 29.1 |
| Fructose | −48 | −42 | 49.0 |
| Sorbitol | −27 | −43 | 18.7 |
| Inositol | −27 | ||
| Sucrose | −32 | −32.0 | 35.9 |
| Lactose | −32 | −28.0 | 40.8 |
| Maltose | −32 | −29.5 | 20.0 |
| Raffinose | −26 | −26.5 | |
| Threalose | −29.5 | 16.7 | |
| Dextran | −9 | −9 | |
| HPβCD (hydroxypropyl-β-cyclodextrin) | −8 | −8 | |
| Poly(vinyl pyrrolidone) | −23 | −19.5 | |
| Poly(ethylene glycol) | −13 | −13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franzé, S.; Selmin, F.; Samaritani, E.; Minghetti, P.; Cilurzo, F. Lyophilization of Liposomal Formulations: Still Necessary, Still Challenging. Pharmaceutics 2018, 10, 139. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10030139
Franzé S, Selmin F, Samaritani E, Minghetti P, Cilurzo F. Lyophilization of Liposomal Formulations: Still Necessary, Still Challenging. Pharmaceutics. 2018; 10(3):139. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10030139
Chicago/Turabian StyleFranzé, Silvia, Francesca Selmin, Elena Samaritani, Paola Minghetti, and Francesco Cilurzo. 2018. "Lyophilization of Liposomal Formulations: Still Necessary, Still Challenging" Pharmaceutics 10, no. 3: 139. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10030139