Behaviour of Tetrabenazine in Acid Medium: Reassessment and Impact on Formulation
Abstract
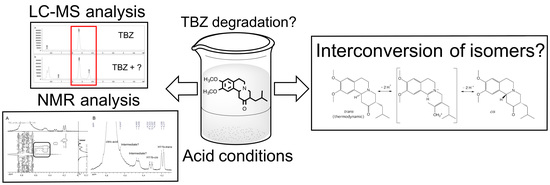
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solvents
2.2. Forced Acid Degradation Studies
2.3. Instrumentation and Chromatographic Separation Conditions
2.3.1. LC-MS Analyses
2.4. Syntheses and Structural Characterization of [1,1-di-2H,3-2H]-Tetrabenazine([1,1-di-2H,3-2H]-TBZ)
2.4.1. Materials and Methods
2.4.2. Synthesis of [1,1-di-2H,3-2H]-Tetrabenazine
2.5. Characterization of TBZ Integrity in ODF
2.5.1. Extraction of TBZ
2.5.2. Condition of Stability Study
3. Results
3.1. Acid-Forced Degradation Studies and LC-MS Analyses
3.2. NMR Studies of TBZ in Acid Medium
3.3. LC-MS Analyses of TBZ Contained in ODF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J.J.; Ondo, W.G.; Dashtipour, K.; Swope, D.M. Tetrabenazine for the treatment of hyperkinetic movement disorders: A review of the literature. Clin. Ther. 2012, 34, 1487–1504. [Google Scholar] [CrossRef] [PubMed]
- Müller, T. Investigational agents for the management of Huntington’s disease. Expert Opin. Investig. Drugs 2017, 26, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kumar, P.; Jamwal, S.; Deshmukh, R.; Gauttam, V. Tetrabenazine: Spotlight on drug review. Ann. Neurosci. 2016, 23, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Caroff, S.N.; Aggarwal, S.; Yonan, C. Treatment of tardive dyskinesia with tetrabenazine or valbenazine: A systematic review. J. Comp. Effect. Res. 2017, 7, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Müller, T. Valbenazine for the treatment of tardive dyskinesia. Expert Rev. Neurother. 2017, 17, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Sarva, H.; Henchcliffe, C. Valbenazine as the first and only approved treatment for adults with tardive dyskinesia. Expert Rev. Clin. Pharmacol. 2018, 11, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Paton, D.M. Deutetrabenazine: Treatment of hyperkinetic aspects of Huntington’s disease, tardive dyskinesia and Tourette syndrome. Drugs Today 2017, 53, 89–102. [Google Scholar] [CrossRef]
- Citrome, L. Deutetrabenazine for tardive dyskinesia: A systematic review of the efficacy and safety profile for this newly approved novel medication—What is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int. J. Clin. Pract. 2017, 71, e13030. [Google Scholar] [CrossRef]
- Cummings, M.A.; Proctor, G.J.; Stahl, S.M. Deuterium tetrabenazine for tardive Ddyskinesia. Clin. Schizophr. Relat. Psychoses 2018, 11, 214–220. [Google Scholar] [CrossRef]
- Dean, M.; Sung, V.W. Review of deutetrabenazine: A novel treatment for chorea associated with Huntington’s disease. Drug Des. Dev. Ther. 2018, 12, 313–319. [Google Scholar] [CrossRef]
- Rodrigues, F.B.; Duarte, G.S.; Costa, J.; Ferreira, J.J.; Wild, E.J. Tetrabenazine versus deutetrabenazine for Huntington’s disease: Twins or distant cousins? Mov. Disord. Clin. Pract. 2017, 4, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Howland, R.H. Deuterated drugs. J. Psychosoc. Nurs. Ment. Health Serv. 2015, 53, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Deuterated drugs draw heavier backing. Nat. Rev. Drug Discov. 2016, 15, 219. [Google Scholar] [CrossRef] [PubMed]
- Bourezg, Z.; Cartiser, N.; Ettouati, L.; Guillon, J.; Lacoudre, A.; Pinaud, N.; Le Borgne, M.; Fessi, H. Structural elucidation of two photolytic degradation products of tetrabenazine. J. Pharm. Biomed. Anal. 2014, 91, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Senta-Loys, Z.; Bourgeois, S.; Valour, J.-P.; Briançon, S.; Fessi, H. Orodispersible films based on amorphous solid dispersions of tetrabenazine. Int. J. Pharm. 2017, 518, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Senta-Loys, Z.; Bourgeois, S.; Pailler-Mattei, C.; Agusti, G.; Briançon, S.; Fessi, H. Formulation of orodispersible films for paediatric therapy: Investigation of feasibility and stability for tetrabenazine as drug model. J. Pharm. Pharmacol. 2017, 69, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Pawar, P.; Khanna, S.; Arora, S. Orally dissolving strips: A new approach to oral drug delivery system. Int. J. Pharm. Investig. 2013, 3, 67–76. [Google Scholar] [CrossRef]
- Dixit, R.P.; Puthli, S.P. Oral strip technology: Overview and future potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef]
- Nagaraju, T.; Gowthami, R.; Rajashekar, M.; Sandeep, S.; Mallesham, M.; Sathish, D.; Shravan Kumar, Y. Comprehensive review on oral disintegrating films. Curr. Drug Deliv. 2013, 10, 96–108. [Google Scholar] [CrossRef]
- Bax, A.; Davis, D.G. Practical aspects of two-dimensional transverse NOE spectroscopy. J. Magn. Reson. 1985, 63, 207–213. [Google Scholar] [CrossRef]
- Desvaux, H.; Berthault, P.; Birlirakis, N.; Goldman, M.; Piotto, M. Improved versions of off-resonance ROESY. J. Magn. Reson. Ser. A 1995, 113, 47–52. [Google Scholar] [CrossRef]
- Openshaw, H.T.; Whittaker, N. 277. The synthesis of emetine and related compounds. Part V. A stereochemically favourable synthesis of emetine. J. Chem. Soc. 1963, 1461–1471. [Google Scholar] [CrossRef]
- Oppolzer, W. Camphor derivatives as chiral auxiliaries in asymmetric synthesis. Tetrahedron 1987, 43, 1969–2004. [Google Scholar] [CrossRef]
- Salunke, S.; Brandys, B.; Giacoia, G.; Tuleu, C. The STEP (Safety and Toxicity of Excipients for Paediatrics) database: Part 2—The pilot version. Int. J. Pharm. 2013, 457, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Trivino, A.; Prasad, D.; Chauhan, H. Investigation and correlation of drug polymer miscibility and molecular interactions by various approaches for the preparation of amorphous solid dispersions. Eur. J. Pharm. Sci. 2015, 71, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Krill, S.L.; Wang, Z.; Telang, C. Miscibility/stability considerations in binary solid dispersion systems composed of functional excipients towards the design of multi-component amorphous systems. J. Pharm. Sci. 2009, 98, 4711–4723. [Google Scholar] [CrossRef] [PubMed]
- Barra, M.; Chen, N. Kinetic studies on the thermal cis−trans isomerization of 1,3-diphenyltriazene in aqueous solution. Effects of acids and bases. J. Org. Chem. 2000, 65, 5739–5744. [Google Scholar] [CrossRef]


| Stress Condition | Solvent | Time | Temp. (°C) | Visual Observation | tr TBZ (min) | tr Unknown (min) | Ratio (%) |
|---|---|---|---|---|---|---|---|
| No | ACN | One week | 4 | limpid colorless | 1.847 | - | - |
| No | ACN + H2O | 70 h | 70 | light yellow | 1.853 | 2.336 | 79:21 |
| Acid | HCl 0.1 M | 70 h | 70 | dark yellow-orange | 1.837 | 2.314 | 77:23 |
| Acid | HCl 1 M | 16 h | 70 | dark orange | 1.977 | 2.562 | 84:16 |
| Acid | HCl 1 M | 70 h | 70 | dark brown | 1.850 | 2.332 | 78:22 |
| Acid | Citric acid 1.5 M | 70 h | 70 | light yellow | 1.852 | 2.334 | 79:21 |
| Formulation | Polymer Matrix (%) | TBZ (%) | Citric Acid (%) | Glycerol (%) | Sorbitol (%) |
|---|---|---|---|---|---|
| HPMC-ODF (F1) | 50.00 | 15.00 | 15.00 | 12.00 | 8.00 |
| PVP-ODF (F2) | 54.35 | 16.30 | 16.30 | 13.05 | - |
| PUL-ODF (F3) | 54.35 | 16.30 | 16.30 | 13.05 | - |
| HEC-ODF (F4) | 56.80 | 17.05 | 17.05 | - | 9.10 |
| Sample | Visual Observation | TBZ (%) | Unknown (%) |
|---|---|---|---|
| ODF at T = 0, ambient temperature (n = 3) | |||
| F1 | Homogeneous, transparent, slightly yellow | 78.4 ± 0.9 | 17.4 ± 0.3 |
| F2 | Homogeneous, transparent, no colour | 83.2 ± 2.3 | 14.2 ± 2.2 |
| F3 | Homogeneous, transparent, no colour | 81.2 ± 0.5 | 17.2 ± 0.3 |
| F4 | Homogeneous, transparent, no colour | 82.3 ± 0.9 | 14.6 ± 0.2 |
| ODF stored at 40 °C, 75% HR after 3 months (n = 3) | |||
| F1 | Homogeneous, transparent, yellow | 74.2 ± 0.7 | 17.2 ± 0.4 |
| F2 | Homogeneous, opaque area, slightly yellow | 91.2 ± 0.6 | 4.4 ± 0.5 |
| F3 | Homogenenous, transparent, slightly yellow | 79.7 ± 0.3 | 15.7 ± 0.1 |
| F4 | Heterogeneous, opaque area, slightly yellow | 81.0 ± 0.3 | 14.2 ± 0.3 |
| ODF stored at 40 °C, 75% HR after 6 months (n = 3) | |||
| F1 | Homogeneous, transparent, yellow | 80.1 ± 0.1 | 16.3 ± 0.1 |
| F2 | Homogeneous, opaque area, slightly yellow | 89.5 ± 0.7 | 6.5 ± 0.6 |
| F3 | Homogenenous, transparent, slightly yellow | 84.3 ± 0.1 | 14.1 ± 0.6 |
| F4 | Heterogeneous, opaque area, slightly yellow | 92.65 ±0.7 | 6.0 ± 0.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ettouati, L.; Senta-Loys, Z.; Bourgeois, S.; Fenet, B.; Le Borgne, M.; Fessi, H. Behaviour of Tetrabenazine in Acid Medium: Reassessment and Impact on Formulation. Pharmaceutics 2019, 11, 44. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11010044
Ettouati L, Senta-Loys Z, Bourgeois S, Fenet B, Le Borgne M, Fessi H. Behaviour of Tetrabenazine in Acid Medium: Reassessment and Impact on Formulation. Pharmaceutics. 2019; 11(1):44. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11010044
Chicago/Turabian StyleEttouati, Laurent, Zoé Senta-Loys, Sandrine Bourgeois, Bernard Fenet, Marc Le Borgne, and Hatem Fessi. 2019. "Behaviour of Tetrabenazine in Acid Medium: Reassessment and Impact on Formulation" Pharmaceutics 11, no. 1: 44. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11010044