Modifications in Vaginal Microbiota and Their Influence on Drug Release: Challenges and Opportunities
Abstract
:1. Introduction
2. Vaginal Microbiota
2.1. Composition of the Vaginal Microbiota
2.2. Functions of Microbiota in the Vaginal Niche
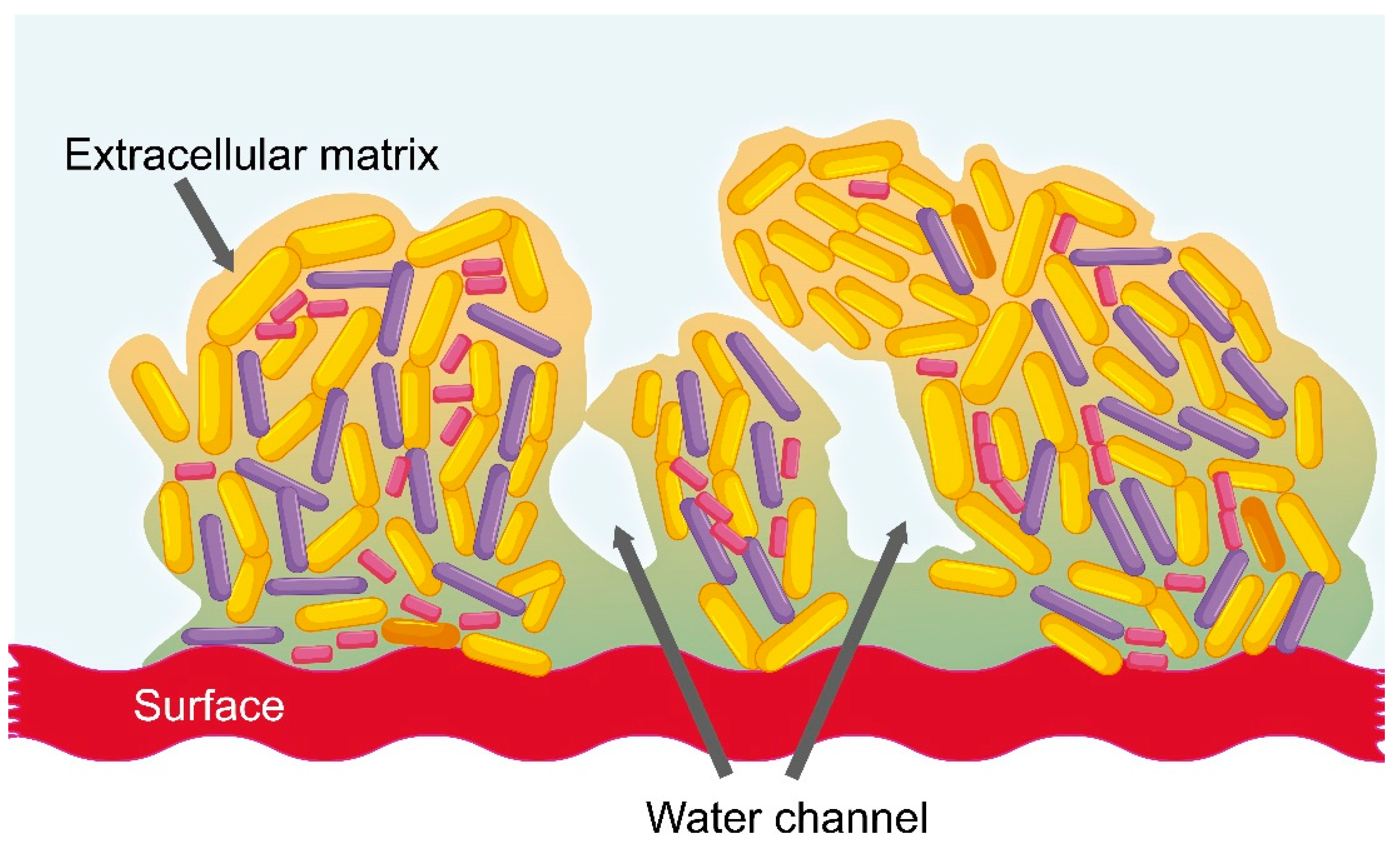
2.3. Vaginal Dysbiosis and the Formation of Bacterial Biofilm
2.4. Current Treatment of the Most Common Vaginal Infections
3. Main Factors That Modify Vaginal Microbiota
3.1. Aerobic Vaginitis
3.2. Bacterial Vaginosis
3.3. Pregnancy
3.4. Age
3.5. Others
4. Physicochemical Changes Produced by Modification in Vaginal Microbiota
5. Effects of Vaginal Microbiota on the Metabolism of Drugs
6. Possible Effects of Changes in Vaginal Microbiota on Drug Release—Challenges and Opportunities
6.1. Influence of pH, Viscosity, Fluid Composition, Drug Metabolism, and other Factors on Drug Delivery
- Ability to bypass first-pass metabolism
- Easy handling, in that self-insertion and/or removal can be performed by patients
- High permeability for low molecular weight drugs
- Relatively large surface area for absorption
- Rich blood supply
- Less potential for pain, tissue damage, or infection compared to parenteral routes.
6.2. Use of Smart Excipients to Take Advantage of Microbiota Modifications and Improve Drug Efficacy
6.3. Feasibility of the Use of Hydrogels to Control the Release of Drugs in Vaginal Microbiota Changes
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leyva-Gómez, G.; Piñón-Segundo, E.; Mendoza-Muñoz, N.; Zambrano-Zaragoza, M.L.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Approaches in polymeric nanoparticles for vaginal drug delivery: A review of the state of the art. Int. J. Mol. Sci. 2018, 19, 1549. [Google Scholar] [CrossRef]
- Dobaria, N.; Mashru, R.; Vadia, N.H. Vaginal drug delivery systems: A Review of Current Status. East Cent. Afr. J. Pharm. Sci. 2007, 10, 3–13. [Google Scholar] [CrossRef]
- Srikrishna, S.; Cardozo, L. The vagina as a route for drug delivery: A review. Int. Urogynecol. J. 2013, 24, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.M.; Palmeira-De-Oliveira, A.; Martinez-De-Oliveira, J.; Palmeira-De-Oliveira, R. Vaginal semisolid products: Technological performance considering physiologic parameters. Eur. J. Pharm. Sci. 2017, 109, 556–568. [Google Scholar] [CrossRef]
- Velloza, J.; Heffron, R. The Vaginal Microbiome and Its Potential to Impact Efficacy of HIV Pre-Exposure Prophylaxis for Women. HIV/AIDS Rep. 2017, 14, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.; Guo, Y.; Pan, H.; Yu, H.; Gu, Z. Niosomes with Sorbitan Monoester as a Carrier for Vaginal Delivery of Insulin: Studies in Rats. Drug Deliv. 2005, 12, 399–407. [Google Scholar] [CrossRef]
- Araos, R.; D’Agata, E.M. The human microbiota and infection prevention. Infect. Hosp. Epidemiol. 2019. [Google Scholar] [CrossRef]
- Anahtar, M.N.; Gootenberg, D.B.; Mitchell, C.M.; Kwon, D.S. Cervicovaginal Microbiota and Reproductive Health: The Virtue of Simplicity. Cell Host Microbe 2018, 23, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Karlebach, S.; Gorle, R.; Russell, J.; Ault, K.; Peralta, L.; Schneider, G.M.; Koenig, S.S.K.; et al. Vaginal microbiome of reproductive-age women. Proc. Acad. Natl. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef]
- Van De Wijgert, J.H.H.M.; Borgdorff, H.; Verhelst, R.; Crucitti, T.; Francis, S.; Verstraelen, H.; Jespers, V. The vaginal microbiota: What have we learned after a decade of molecular characterization? PLoS ONE 2014, 9, e105998. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- Dei, M.; Di Maggio, F.; Di Paolo, G.; Bruni, V. Vulvovaginitis in childhood. Best. Pract. Res. Clin. Obstet. Gynaecol. 2010, 24, 129–137. [Google Scholar] [CrossRef]
- Ran, G.; Mladenovi, V. Microbiological aspects of vulvovaginitis in prepubertal girls. Eur. J. Pediatr. 2012, 171, 1203–1208. [Google Scholar]
- Yamamoto, T.; Zhou, X.; Williams, C.J.; Hochwalt, A.; Forney, L.J. Bacterial Populations in the Vaginas of Healthy Adolescent Women. J. Pediatr. Adolesc. Gynecol. 2009, 22, 11–18. [Google Scholar] [CrossRef]
- Al-Baghdadi, O.; Ewies, A.A.A. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: An up-to-date overview. Climacteric 2009, 12, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Hummelen, R.; van der Westen, R.; Reid, G.; Petrova, M.I.; Lievens, E.; Younes, J.A. Women and Their Microbes: The Unexpected Friendship. Trends Microbiol. 2017, 26, 16–32. [Google Scholar]
- Anahtar, M.N.; Byrne, E.H.; Doherty, K.E.; Bowman, B.A.; Yamamoto, H.S.; Soumillon, M.; Padavattan, N.; Ismail, N.; Moodley, A.; Sabatini, M.E.; et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015, 42, 965–976. [Google Scholar] [CrossRef]
- Gong, Z.; Luna, Y.; Yu, P.; Fan, H. Lactobacilli Inactivate Chlamydia trachomatis through Lactic Acid but Not H2O2. PLoS ONE 2014, 9, e107758. [Google Scholar] [CrossRef] [PubMed]
- Tomas, M.S.J.; Tomás, M.S.J.; Ocaña, V.S.; Wiese, B.; Nader-Macías, M.E. Growth and lactic acid production by vaginal Lactobacillus acidophilus CRL 1259, and inhibition of uropathogenic Escherichia coli. J. Med. Microbiol. 2003, 52, 1117–1124. [Google Scholar] [CrossRef]
- A Graver, M.; Wade, J.J. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Shukair, S.A.; Allen, S.A.; Cianci, G.C.; Stieh, D.J.; Anderson, M.R.; Baig, S.M.; Gioia, C.J.; Spongberg, E.J.; Kauffman, S.M.; McRaven, M.D.; et al. Human cervicovaginal mucus contains an activity that hinders HIV-1 movement. Mucosal Immunol. 2013, 6, 427–434. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. Vaginal pH and Microbicidal Lactic Acid When Lactobacilli Dominate the Microbiota. PLoS ONE 2013, 8, e80074. [Google Scholar] [CrossRef]
- Osset, J.; Bartolomé, R.M.; García, E.; Andreu, A. Assessment of the Capacity of Lactobacillus to Inhibit the Growth of Uropathogens and Block Their Adhesion to Vaginal Epithelial Cells. J. Infect. Dis. 2002, 183, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Mastromarino, P.; Brigidi, P.; Macchia, S.; Maggi, L.; Pirovano, F.; Trinchieri, V.; Conte, U.; Matteuzzi, D. Characterization and selection of vaginal Lactobacillus strains for the preparation of vaginal tablets. J. Appl. Microbiol. 2002, 93, 884–893. [Google Scholar] [CrossRef]
- Zárate, G.; Nader-Macias, M.; Nader-Macías, M.; Nader-Macías, M. Influence of probiotic vaginal lactobacilli on in vitro adhesion of urogenital pathogens to vaginal epithelial cells. Lett. Appl. Microbiol. 2006, 43, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Phukan, N.; Parsamand, T.; Simoes-Barbosa, A.; Brooks, A.E.S.; Nguyen, T.N.M. The adherence of Trichomonas vaginalis to host ectocervical cells is influenced by lactobacilli. Sex. Transm. Infect. 2013, 89, 455–459. [Google Scholar] [CrossRef]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.-J.M.; Wells, J.M. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Liver Physiol. 2010, 298, G851–G859. [Google Scholar] [CrossRef]
- Jung, H.-S.; Ehlers, M.M.; Lombaard, H.; Redelinghuys, M.J.; Kock, M.M. Etiology of bacterial vaginosis and polymicrobial biofilm formation. Crit. Rev. Microbiol. 2017, 210, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, M.; Sani, C.; Clemente, A.M.; Iossa, A.; Perissi, E.; Castronovo, G.; Tanturli, M.; Rivero, D.; Cozzolino, F.; Cavalieri, D.; et al. Characterization of cervico-vaginal microbiota in women developing persistent high-risk Human Papillomavirus infection. Sci. Rep. 2017, 7, 10200. [Google Scholar] [CrossRef]
- Balle, C.; Lennard, K.; Dabee, S.; Barnabas, S.L.; Jaumdally, S.Z.; Gasper, M.A.; Maseko, V.; Mbulawa, Z.Z.A.; Williamson, A.-L.; Bekker, L.-G.; et al. Endocervical and vaginal microbiota in South African adolescents with asymptomatic Chlamydia trachomatis infection. Sci. Rep. 2018, 8, 11109. [Google Scholar] [CrossRef]
- Vodstrcil, L.A.; Twin, J.; Garland, S.M.; Fairley, C.K.; Hocking, J.S.; Law, M.G.; Plummer, E.L.; Fethers, K.A.; Chow, E.P.F.; Tabrizi, S.N.; et al. The influence of sexual activity on the vaginal microbiota and Gardnerella vaginalis clade diversity in young women. PLoS ONE 2017, 12, e0171856. [Google Scholar] [CrossRef] [PubMed]
- Hardy, L.; Jespers, V.; Bulck, M.V.D.; Buyze, J.; Mwambarangwe, L.; Musengamana, V.; Vaneechoutte, M.; Crucitti, T. The presence of the putative Gardnerella vaginalis sialidase A gene in vaginal specimens is associated with bacterial vaginosis biofilm. PLoS ONE 2017, 12, e0172522. [Google Scholar] [CrossRef]
- Hardy, L.; Jespers, V.; Dahchour, N.; Mwambarangwe, L.; Musengamana, V.; Vaneechoutte, M.; Crucitti, T. Unravelling the Bacterial Vaginosis-Associated Biofilm: A Multiplex Gardnerella vaginalis and Atopobium vaginae Fluorescence In Situ Hybridization Assay Using Peptide Nucleic Acid Probes. PLoS ONE 2015, 10, e0136658. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Parvaiz, F.; Manzoor, S. Bacterial Vaginosis: An insight into the prevalence, alternative regimen treatments and it’s associated resistance patterns. Microb. Pathog. 2019, 127, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Cerca, N.; Crucitti, T.; Vaneechoutte, M.; Hardy, L.; Jespers, V. Bacterial biofilms in the vagina. Res. Microbiol. 2017, 168, 865–874. [Google Scholar]
- Ortega-Peña, S.; Hernández-Zamora, E. Biopelículas microbianas y su impacto en áreas médicas: fisiopatología, diagnóstico y tratamiento. Bol. Med. Hosp. Infant. Mex. 2018, 75, 79–88. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Biofilm-Related Infections: Bridging the Gap between Clinical Management and Fundamental Aspects of Recalcitrance toward Antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Verstraelen, H.; Swidsinski, A. The biofilm in bacterial vaginosis: Implications for epidemiology, diagnosis and treatment: 2018 update. Curr. Opin. Infect. Dis. 2019, 32, 38–42. [Google Scholar] [CrossRef]
- Reiter, S.; Kellogg Spadt, S. Bacterial vaginosis: A primer for clinicians. Postgrad. Med. 2019, 131, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Castro, J.; Palmeira-De-Oliveira, A.; Martinez-De-Oliveira, J.; Cerca, N. Bacterial Vaginosis Biofilms: Challenges to Current Therapies and Emerging Solutions. Front. Microbiol. 2016, 6, 683640. [Google Scholar] [CrossRef]
- Menard, J.-P. Antibacterial treatment of bacterial vaginosis: current and emerging therapies. Int. J. Women’s Health 2011, 3, 295–305. [Google Scholar] [CrossRef]
- Donders, G.G.; Vereecken, A.; Bosmans, E.; DeKeersmaecker, A.; Salembier, G.; Spitz, B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: Aerobic vaginitis. BJOG Int. J. Obstet. Gynaecol. 2002, 109, 34–43. [Google Scholar] [CrossRef]
- Donders, G.G. Definition and classification of abnormal vaginal flora. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Tempera, G.; Abbadessa, G.; Bonfiglio, G.; Cammarata, E.; Cianci, A.; Corsello, S.; Raimondi, A.; Ettore, G.; Nicolosi, D.; Furneri, P.M. Topical Kanamycin: An Effective Therapeutic Option in Aerobic Vaginitis Topical. J. Chemoterapy. 2006, 18, 409–414. [Google Scholar] [CrossRef]
- Kaambo, E.; Africa, C.; Chambuso, R.; Passmore, J.-A.S. Vaginal Microbiomes Associated With Aerobic Vaginitis and Bacterial Vaginosis. Front. Public Health 2018, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.; Bellen, G.; Grinceviciene, S.; Ruban, K.; Vieira-Baptista, P. Aerobic vaginitis: No longer a stranger. Res. Microbiol. 2017, 168, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, A.; Phillips, I.; Fox, A.; Barlow, D. Anaerobic vaginosis (non-specific vaginitis): Clinical, microbiological, and therapeutic findings. Lancet 1983, 322, 1379–1382. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The Human Microbiome: At the interface of health and disease. Nat. Rev. Microbiol. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Hill, G.B. The microbiology of bacterial vaginosis. Am. J. Obstet. Gynecol. 1993, 169, 450–454. [Google Scholar] [CrossRef]
- Freitas, A.C.; Chaban, B.; Bocking, A.; Rocco, M.; Yang, S.; Hill, J.E.; Money, D.M. The vaginal microbiome of pregnant women is less rich and diverse, with lower prevalence of Mollicutes, compared to non-pregnant women. Sci. Rep. 2017, 7, 9212. [Google Scholar] [CrossRef]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014, 2, 1–19. [Google Scholar]
- Prince, A.L.; Chu, D.M.; Seferovic, M.D.; Antony, K.M.; Ma, J.; Aagaard, K.M. The Perinatal Microbiome and Pregnancy: Moving Beyond the Vaginal Microbiome. Cold Spring Harb. Perspect. Med. 2015, 5, 1–23. [Google Scholar] [CrossRef]
- Nuriel-ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Petrova, M.I.; Broek, M.V.D.; Balzarini, J.; Vanderleyden, J.; Lebeer, S. Vaginal microbiota and its role in HIV transmission and infection. FEMS Microbiol. Rev. 2013, 37, 762–792. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Fadrosh, D.; Chang, K.; Silver, M.; Viscidi, R.P.; Burke, A.E.; Ravel, J.; Gravitt, P.E. Association between the vaginal microbiota, menopause status and signs of vulvovaginal atrophy. Menopause 2014, 21, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Fettweis, J.M.; Brooks, J.P.; Jefferson, K.K.; Buck, G.A. The Changing Landscape of the Vaginal Microbiome. Clin. Lab. Med. 2014, 34, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.F.; Sobel, J.D.; Akins, R.A.; Hassan, S.S.; Chaiworapongsa, T.; Kusanovic, J.P.; Romero, R. The vaginal microbiome: New information about genital tract flora using molecular based techniques. BJOG: Int. J. Obstet. Gynaecol. 2011, 118, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Galhardo, C.L.; Soares, J.M.; Simões, R.S.; A Haidar, M.; De Lima, G.R.; Baracat, E.C. Estrogen effects on the vaginal pH, flora and cytology in late postmenopause after a long period without hormone therapy. Clin. Exp. Obstet. Gynecol. 2006, 33, 85–89. [Google Scholar]
- Muhleisen, A.L.; Herbst-Kralovetz, M.M. Menopause and the vaginal microbiome. Maturitas 2016, 91, 42–50. [Google Scholar] [CrossRef]
- Long, C.-Y.; Liu, C.-M.; Hsu, S.-C.; Wu, C.-H.; Wang, C.-L.; Tsai, E.-M. A randomized comparative study of the effects of oral and topical estrogen therapy on the vaginal vascularization and sexual function in hysterectomized postmenopausal women. Menopause 2006, 13, 737–743. [Google Scholar] [CrossRef]
- Robinson, D.; Cardozo, L. Urogenital effects of hormone therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 91–104. [Google Scholar] [CrossRef]
- Brotman, R.M.; He, X.; Gajer, P.; Fadrosh, D.; Sharma, E.; Mongodin, E.F.; Ravel, J.; Glover, E.D.; Rath, J.M. Association between cigarette smoking and the vaginal microbiota: A pilot study. BMC Infect. Dis. 2014, 14, 471. [Google Scholar] [CrossRef] [PubMed]
- Bagaitkar, J.; DeMuth, D.R.; A Scott, D. Tobacco use increases susceptibility to bacterial infection. Tob. Induc. Dis. 2008, 4, 12. [Google Scholar] [CrossRef]
- Nelson, T.M.; Borgogna, J.C.; Michalek, R.D.; Roberts, D.W.; Rath, J.M.; Glover, E.D.; Ravel, J.; Shardell, M.D.; Yeoman, C.J.; Brotman, R.M. Cigarette smoking is associated with an altered vaginal tract metabolomic profile. Sci. Rep. 2018, 8, 852. [Google Scholar] [CrossRef]
- Zhou, X.; Suzuki, H.; Schütte, U.; A Hansmann, M.; Davis, C.C.; Brown, C.J.; Pierson, J.D.; Forney, L.J. The vaginal bacterial communities of Japanese women resemble those of women in other racial groups. FEMS Immunol. Med. Microbiol. 2010, 58, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Da, M.; Zhang, W.; Qi, Q.; Zhang, C.; Han, S. Role of Lactobacillus in cervical cancer. Cancer Manag. Res. 2018, 10, 1219–1229. [Google Scholar] [CrossRef]
- Matos, A.; Da Silva, A.P.; Medeiros, R.; Bicho, M.; Bicho, M.C. Microenvironment in Vagina as a Key-Player on Cervical Cancer: Interaction of Polymorphic Genetic Variants and Vaginal Microbiome as Co-Factors. Cerv. Cancer Screen. Treat. Prev. Univers. Protoc. Ultim. Control 2018. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Lehne, B.; Bhatia, R.; Lyons, D.; Paraskevaidis, E.; Li, J.V.; et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep. 2015, 5, 16865. [Google Scholar] [CrossRef]
- Braundmeier, A.G.; Lenz, K.M.; Inman, K.S.; Chia, N.; Jeraldo, P.; Walther-António, M.R.S.; Miller, M.E.B.; Yang, F.; Creedon, D.J.; Nelson, H.; et al. Individualized medicine and the microbiome in reproductive tract. Front. Physiol. 2015, 6, 97. [Google Scholar] [CrossRef]
- Mashburn, J. Etiology, Diagnosis, and Management of Vaginitis. J. Midwifery Women’s Health 2006, 51, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Mills, B.B. Vaginitis: Beyond the Basics. Obstet. Gynecol. Clin. 2017, 44, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.; ElWerdany, M.; Hadoura, E.; Mahmood, T. Vaginal discharge. Obstet. Gynaecol. Reprod. Med. 2016, 26, 317–323. [Google Scholar] [CrossRef]
- Sousa, T.; Paterson, R.; Moore, V.; Carlsson, A.; Abrahamsson, B.; Basit, A.W. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int. J. Pharm. 2008, 363, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.D.; Sheth, A.N.; Read, T.D.; Frisch, M.B.; Mehta, C.C.; Martin, A.; Haaland, R.E.; Patel, A.S.; Pau, C.-P.; Kraft, C.S.; et al. The Female Genital Tract Microbiome Is Associated With Vaginal Antiretroviral Drug Concentrations in Human Immunodeficiency Virus–Infected Women on Antiretroviral Therapy. J. Infect. Dis. 2017, 216, 990–999. [Google Scholar] [CrossRef]
- Klatt, N.R.; Cheu, R.; Birse, K.; Zevin, A.S.; Perner, M.; Noël-Romas, L.; Grobler, A.; Westmacott, G.; Xie, I.Y.; Butler, J.; et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 2017, 356, 938–945. [Google Scholar] [CrossRef]
- Simon, J.A.; Lin, F.; Hulley, S.B.; Blanche, P.J.; Waters, D.; Shiboski, S.; Rotter, J.I.; Nickerson, D.A.; Yang, H.; Saad, M.; et al. Phenotypic Predictors of Response to Simvastatin Therapy Among African-Americans and Caucasians: The Cholesterol and Pharmacogenetics (CAP) Study. Am. J. Cardiol. 2006, 97, 843–850. [Google Scholar] [CrossRef]
- Wilson, I.D.; Nicholson, J.K. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl. Res. 2017, 179, 204–222. [Google Scholar] [CrossRef]
- Hussain, A.; Ahsan, F. The vagina as a route for systemic drug delivery. J. Control. Release 2005, 103, 301–313. [Google Scholar] [CrossRef]
- Manallack, D.T. The pK(a) Distribution of Drugs: Application to Drug Discovery. Perspect. Med. Chem. 2007, 1, 25–38. [Google Scholar]
- Allen, V.; Lee, W.; Chandra, S.; Fanning, C.; Young, D. The effect of vaginal pH on labor induction with vaginal misoprostol. J. Matern Neonatal Med. 2007, 17, 387–391. [Google Scholar]
- Kurian, M.; Rao, B.; Rao, A.A.S. Effect of vaginal pH on efficacy of dinoprostone gel for labour induction. Int. J. Reprod. Contracept. Obstet. Gynecol. 2016, 5, 1196–1201. [Google Scholar] [CrossRef]
- De Araújo Pereira, R.R.; Bruschi, M.L. Vaginal mucoadhesive drug delivery systems. Drug Dev. Ind. Pharm. 2012, 38, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Lete, I.; Luis Duenas, J.; V Esplugues, J.; Marti-Cabrera, M. Is the Vagina an Adequate Route for the Administration of Hormonal Contraceptives? Curr. Drug Metab. 2011, 11, 839–849. [Google Scholar] [CrossRef]
- Nakano, F.Y.; Leão, R.D.B.F.; Esteves, S.C. Insights into the role of cervical mucus and vaginal pH in unexplained infertility. MedicalExpress 2015, 2. [Google Scholar] [CrossRef]
- Ensign, L.M.; Tang, B.C.; Wang, Y.-Y.; Tse, T.A.; Hoen, T.; Cone, R.; Hanes, J. Mucus-Penetrating Nanoparticles for Vaginal Drug Delivery Protect Against Herpes Simplex Virus. Sci. Transl. Med. 2012, 4, 138. [Google Scholar] [CrossRef]
- Acartürk, F.; Parlatan, Z.I.; Saracoĝlu, Ö.F. Comparison of vaginal aminopeptidase enzymatic activities in various animals and in humans. J. Pharm. Pharmacol. 2001, 53, 1499–1504. [Google Scholar]
- Olmsted, S.S.; Meyn, L.A.; Rohan, L.C.; Hillier, S.L. Glycosidase and proteinase activity of anaerobic gram-negative bacteria isolated from women with bacterial vaginosis. Sex. Trans. Dis. 2003, 30, 257–261. [Google Scholar] [CrossRef]
- Katz, D.F.; Yuan, A.; Gao, Y. Vaginal drug distribution modeling. Adv. Drug Deliv. Rev. 2015, 92, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Alexander, N.J.; Baker, E.; Kaptein, M.; Karck, U.; Miller, L.; Zampaglione, E. Why consider vaginal drug administration? Fertil. Steril. 2004, 82, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N.; Huang, W.M. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Wilkinson, E.M.; Ilhan, Z.E.; Herbst-Kralovetz, M.M. Microbiota–drug interactions: Impact on metabolism and efficacy of therapeutics. Maturitas 2018, 112, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Aboud, H.M.; Hassan, A.H.; Ali, A.A.; Abdel-Razik, A.-R.H. Novel in situ gelling vaginal sponges of sildenafil citrate-based cubosomes for uterine targeting. Drug Deliv. 2018, 25, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Brown, M.B. Polymeric gels for intravaginal drug delivery. J. Control. Release 2018, 270, 145–157. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]


| Woman’s lifecycle | Predominant Microorganisms | References |
|---|---|---|
| Childhood | Gram-negative anaerobic bacteria, such as Bacteroides, Fusobacterium, Veillonella Gram-positive anaerobic bacteria, such as Actinomyces, Bifidobacterium, Peptococcus, Peptostreptococcus, and Propionibacterium Aerobic bacteria such as Staphylococcus aureus, Staphylococccus epidermidis, Streptococcus viridans, and Enterococcus faecalis | [12,13] |
| Prepuberal | Low abundance of lactobacilli, Gardnerella vaginalis, and Prevotella bivia | [13] |
| Puberty | Predominant species are Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners, and Lactobacillus jensenii | [14] |
| Adult | Similar to puberty, Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners, and Lactobacillus jensenii | [14] |
| Menopause | Predominant species are Lactobacillus crispatus, Lactobacillus iners, Gardnerella vaginalis, and Prevotella and a lower abundance of Candida, Mobiluncus, Staphylococcus, Bifidobacterium, and Gemella | [15] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyva-Gómez, G.; Del Prado-Audelo, M.L.; Ortega-Peña, S.; Mendoza-Muñoz, N.; Urbán-Morlán, Z.; González-Torres, M.; González-Del Carmen, M.; Figueroa-González, G.; Reyes-Hernández, O.D.; Cortés, H. Modifications in Vaginal Microbiota and Their Influence on Drug Release: Challenges and Opportunities. Pharmaceutics 2019, 11, 217. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11050217
Leyva-Gómez G, Del Prado-Audelo ML, Ortega-Peña S, Mendoza-Muñoz N, Urbán-Morlán Z, González-Torres M, González-Del Carmen M, Figueroa-González G, Reyes-Hernández OD, Cortés H. Modifications in Vaginal Microbiota and Their Influence on Drug Release: Challenges and Opportunities. Pharmaceutics. 2019; 11(5):217. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11050217
Chicago/Turabian StyleLeyva-Gómez, Gerardo, María L. Del Prado-Audelo, Silvestre Ortega-Peña, Néstor Mendoza-Muñoz, Zaida Urbán-Morlán, Maykel González-Torres, Manuel González-Del Carmen, Gabriela Figueroa-González, Octavio D. Reyes-Hernández, and Hernán Cortés. 2019. "Modifications in Vaginal Microbiota and Their Influence on Drug Release: Challenges and Opportunities" Pharmaceutics 11, no. 5: 217. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11050217






