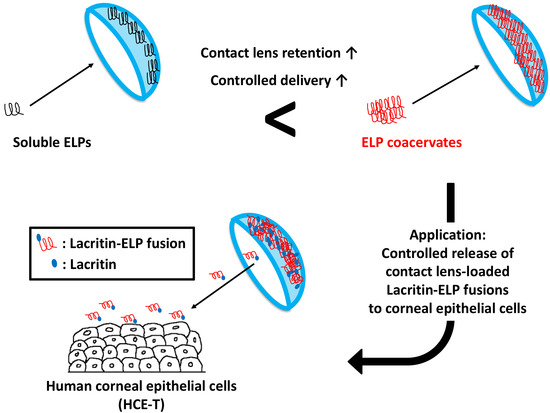
Thermally-Responsive Loading and Release of Elastin-Like Polypeptides from Contact Lenses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis, Expression and Purification of ELPs
2.2. ELPs Inverse Phase Transition Characterization
2.3. Rhodamine Labeling of V96, S96 and LV96, and Decoration of Proclear CompatiblesTM Contact Lenses
2.4. Characterization of Release Kinetics of ELPs from Proclear CompatiblesTM Contact Lenses
2.5. Human Corneal Epithelial Cells-Transformed with SV40 (HCE-T) Uptake Study
2.6. Statistical Analysis
3. Results
3.1. Expression and Purification of ELPs
3.2. ELPs Display Differential Attachment to Commercially Available Contact Lenses
3.3. ELP-Mediated Phase Separation Enhances Attachment to Proclear CompatiblesTM
3.4. Coacervation Prolongs the Retention of ELPs on Proclear CompatiblesTM Contact Lenses
3.5. Co-Incubation of LV96 with Proclear CompatiblesTM Enables Transfer to Cultured HCE-T Cells
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Kearney, C.J.; Mooney, D.J. Macroscale delivery systems for molecular and cellular payloads. Nat. Mater. 2013, 12, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef]
- Hubbell, J.A.; Chilkoti, A. Nanomaterials for Drug Delivery. Science 2012, 337, 303–305. [Google Scholar] [CrossRef]
- MacEwan, S.R.; Chilkoti, A. Elastin-like polypeptides: Biomedical applications of tunable biopolymers. Biopolymers 2010, 94, 60–77. [Google Scholar] [CrossRef]
- Chilkoti, A.; Christensen, T.; MacKay, J.A. Stimulus responsive elastin biopolymers: Applications in medicine and biotechnology. Curr. Opin. Chem. Biol. 2006, 10, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Edman, M.C.; Janga, S.R.; Shi, P.; Dhandhukia, J.; Liu, S.Y.; Louiee, S.G.; Rodgers, K.; MacKay, J.A.; Hamm-Alvarez, S.F. A rapamycin-binding protein polymer nanoparticle shows potent therapeutic activity in suppressing autoimmune dacryoadenitis in a mouse model of Sjogren’s syndrome. J. Control. Release 2013, 171, 269–279. [Google Scholar] [CrossRef]
- Wang, W.; Sreekumar, P.G.; Valluripalli, V.; Shi, P.; Wang, J.; Lin, Y.A.; Cui, H.; Kannan, R.; Hinton, D.R.; Mackay, J.A. Protein polymer nanoparticles engineered as chaperones protect against apoptosis in human retinal pigment epithelial cells. J. Control. Release 2014, 191. [Google Scholar] [CrossRef]
- Janib, S.M.; Pastuszka, M.; Aluri, S.; Folchman-Wagner, Z.; Hsueh, P.Y.; Shi, P.; Yi, A.; Cui, H.; Mackay, J.A. A quantitative recipe for engineering protein polymer nanoparticles. Polym. Chem. 2014, 5, 1614–1625. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.; Zhang, Y.; Christensen, T.; Sagle, L.B.; Chilkoti, A.; Cremer, P.S. Effects of Hofmeister anions on the phase transition temperature of elastin-like polypeptides. J. Phys. Chem. B 2008, 112, 13765–13771. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jashnani, A.; Aluri, S.R.; Gustafson, J.A.; Hsueh, P.Y.; Yarber, F.; McKown, R.L.; Laurie, G.W.; Hamm-Alvarez, S.F.; MacKay, J.A. A thermo-responsive protein treatment for dry eyes. J. Control. Release 2015, 199, 156–167. [Google Scholar] [CrossRef]
- Despanie, J.; Dhandhukia, J.P.; Hamm-Alvarez, S.F.; MacKay, J.A. Elastin-like polypeptides: Therapeutic applications for an emerging class of nanomedicines. J. Control. Release 2016, 240, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Sanghi, S.; Kumar, R.; Lumsden, A.; Dickinson, D.; Klepeis, V.; Trinkaus-Randall, V.; Frierson, H.F., Jr.; Laurie, G.W. cDNA and genomic cloning of lacritin, a novel secretion enhancing factor from the human lacrimal gland. J. Mol. Biol. 2001, 310, 127–139. [Google Scholar] [CrossRef]
- McKown, R.L.; Wang, N.N.; Raab, R.W.; Karnati, R.; Zhang, Y.H.; Williams, P.B.; Laurie, G.W. Lacritin and other new proteins of the lacrimal functional unit. Exp. Eye Res. 2009, 88, 848–858. [Google Scholar] [CrossRef]
- Ma, P.; Beck, S.L.; Raab, R.W.; McKown, R.L.; Coffman, G.L.; Utani, A.; Chirico, W.J.; Rapraeger, A.C.; Laurie, G.W. Heparanase deglycanation of syndecan-1 is required for binding of the epithelial-restricted prosecretory mitogen lacritin. J. Cell Biol. 2006, 174, 1097–1106. [Google Scholar] [CrossRef] [Green Version]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- Lang, J.C. Ocular Drug-Delivery Conventional Ocular Formulations. Adv. Drug Deliv. Rev. 1995, 16, 39–43. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular Drug Delivery. AAPS. J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Jwala, J.; Boddu, S.H.S.; Mitra, A.K. Recent Perspectives in Ocular Drug Delivery. Pharm. Res. 2009, 26, 1197–1216. [Google Scholar] [CrossRef]
- Novack, G.D. Ophthalmic Drug Delivery: Development and Regulatory Considerations. Clin. Pharmacol. Ther. 2009, 85, 539–543. [Google Scholar] [CrossRef] [PubMed]
- MSintzel, B.; Bernatchez, S.F.; Tabatabay, C.; Gurny, R. Biomaterials in ophthalmic drug delivery. Eur. J. Pharm. Biopharm. 1996, 42, 358–374. [Google Scholar]
- Guzman-Aranguez, A.; Colligris, B.; Pintor, J. Contact Lenses: Promising Devices for Ocular Drug Delivery. J. Ocul. Pharmacol. Ther. 2013, 29, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.; Lehmussaari, K. Industrial perspective in ocular drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1258–1268. [Google Scholar] [CrossRef]
- Li, C.C.; Chauhan, A. Ocular transport model for ophthalmic delivery of timolol through p-HEMA contact lenses. J. Drug Deliv. Sci. Technol. 2007, 17, 69–79. [Google Scholar] [CrossRef]
- Kim, J.; Chauhan, A. Dexamethasone transport and ocular delivery from poly(hydroxyethyl methacrylate) gels. Int. J. Pharm. 2008, 353, 205–222. [Google Scholar] [CrossRef]
- Gulsen, D.; Chauhan, A. Dispersion of microemulsion drops in HEMA hydrogel: A potential ophthalmic drug delivery vehicle. Int. J. Pharm. 2005, 292, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, Y.; Thomas, J.C.; Tan, G.; John, V.T.; Chauhan, A. Surfactant-laden soft contact lenses for extended delivery of ophthalmic drugs. Biomaterials 2009, 30, 867–878. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Yanez, F.; Barreiro-Iglesias, R.; Concheiro, A. Imprinted soft contact lenses as norfloxacin delivery systems. J. Control. Release 2006, 113, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Chauhan, A. Extended cyclosporine delivery by silicone-hydrogel contact lenses. J. Control. Release 2011, 154, 267–274. [Google Scholar] [CrossRef]
- Hiratani, H.; Alvarez-Lorenzo, C. The nature of backbone monomers determines the performance of imprinted soft contact lenses as timolol drug delivery systems. Biomaterials 2004, 25, 1105–1113. [Google Scholar] [CrossRef]
- Jung, H.J.; Abou-Jaoude, M.; Carbia, B.E.; Plummer, C.; Chauhan, A. Glaucoma therapy by extended release of timolol from nanoparticle loaded silicone-hydrogel contact lenses. J. Control. Release 2013, 165, 82–89. [Google Scholar] [CrossRef]
- Ciolino, J.B.; Stefanescu, C.F.; Ross, A.E.; Salvador-Culla, B.; Cortez, P.; Ford, E.M.; Wymbs, K.A.; Sprague, S.L.; Mascoop, D.R.; Rudina, S.S.; Trauger, S.A.; Cade, F.; Kohane, D.S. In vivo performance of a drug-eluting contact lens to treat glaucoma for a month. Biomaterials 2014, 35, 432–439. [Google Scholar] [CrossRef]
- Espandar, L.; Caldwell, D.; Watson, R.; Blanco-Mezquita, T.; Zhang, S.; Bunnell, B. Application of adipose-derived stem cells on scleral contact lens carrier in an animal model of severe acute alkaline burn. Eye Contact Lens 2014, 40, 243–247. [Google Scholar] [CrossRef]
- Leader, B.; Baca, Q.J.; Golan, D.E. Protein therapeutics: A summary and pharmacological classification. Nat. Rev. Drug Discov. 2008, 7, 21–39. [Google Scholar] [CrossRef]
- Frokjaer, S.; Otzen, D.E. Protein drug stability: A formulation challenge. Nat. Rev. Drug Discov. 2005, 4, 298–306. [Google Scholar] [CrossRef]
- Laukkanen, A.; Valtola, L.; Winnik, F.M.; Tenhu, H. Formation of colloidally stable phase separated poly(N-vinylcaprolactam) in water: A study by dynamic light scattering, microcalorimetry, and pressure perturbation calorimetry. Macromolecules 2004, 37, 2268–2274. [Google Scholar] [CrossRef]
- Maeda, Y.; Higuchi, T.; Ikeda, I. Change in hydration state during the coil-globule transition of aqueous solutions of poly(N-isopropylacrylamide) as evidenced by FTIR spectroscopy. Langmuir 2000, 16, 7503–7509. [Google Scholar] [CrossRef]
- Debelle, L.; Alix, A.J.; Jacob, M.P.; Huvenne, J.P.; Berjot, M.; Sombret, B.; Legrand, P. Bovine elastin and kappa-elastin secondary structure determination by optical spectroscopies. J. Biolog. Chem. 1995, 270, 26099–26103. [Google Scholar] [CrossRef]
- Baker, E.N.; Hubbard, R.E. Hydrogen bonding in globular proteins. Prog. Biophys. Mol. Biol. 1984, 44, 97–179. [Google Scholar] [CrossRef]
- Hansen, A.S.; Du, L.; Kjaergaard, H.G. Positively Charged Phosphorus as a Hydrogen Bond Acceptor. J. Phys. Chem. Lett. 2014, 5, 4225–4231. [Google Scholar] [CrossRef] [PubMed]
- Court, J.L.; Redman, R.P.; Wang, J.H.; Leppard, S.W.; Obyrne, V.J.; Small, S.A.; Lewis, A.L.; Jones, S.A.; Stratford, P.W. A novel phosphorylcholine-coated contact lens for extended wear use. Biomaterials 2001, 22, 3261–3272. [Google Scholar] [PubMed]
- Sreekumar, P.G.; Li, Z.; Wang, W.; Spee, C.; Hinton, D.R.; Kannan, R.; MacKay, J.A. Intra-vitreal alphaB crystallin fused to elastin-like polypeptide provides neuroprotection in a mouse model of age-related macular degeneration. J. Control. Release 2018, 283, 94–104. [Google Scholar] [CrossRef]
- Wang, W.; Despanie, J.; Shi, P.; Edman-Woolcott, M.C.; Lin, Y.A.; Cui, H.; Heur, J.M.; Fini, M.E.; Hamm-Alvarez, S.F.; MacKay, J.A. Lacritin-mediated regeneration of the corneal epithelia by protein polymer nanoparticles. J. Mater. Chem. B 2014, 2, 8131–8141. [Google Scholar] [CrossRef] [Green Version]
- Rincon, A.C.; Molina-Martinez, I.T.; de las Heras, B.; Alonso, M.; Bailez, C.; Rodriguez-Cabello, J.C.; Herrero-Vanrell, R. Biocompatibility of elastin-like polymer poly(VPAVG) microparticles: In vitro and in vivo studies. J. Biomed. Mater. Res. A 2006, 78, 343–351. [Google Scholar] [CrossRef] [PubMed]
- George, E.M.; Mahdi, F.; Logue, O.C.; Robinson, G.G.; Bidwell, G.L., 3rd. Corneal Penetrating Elastin-Like Polypeptide Carriers. J. Ocul. Pharmacol. Ther. 2016, 32, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.J.; Chauhan, A. Temperature sensitive contact lenses for triggered ophthalmic drug delivery. Biomaterials 2012, 33, 2289–2300. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Muller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]






| Label | Amino Acid Composition | *MW (kDa) | Tt (°C) at 25 μM | Phase Diagram | |
|---|---|---|---|---|---|
| Slope, m [°C/log10(μM)] | y-intercept, b [°C] | ||||
| S96 | G(VPGSG)96Y | 38.4 | 57.6 | −1.669 | 59.31 |
| V96 | G(VPGVG)96Y | 39.5 | 31.6 | −3.252 | 36.06 |
| LV96 | **Lacritin-G(VPGVG)96Y | 52.3 | 26.8 | −1.192 | 28.56 |
| Brand Name | Manufacturer | Polymer | Monomer | ELP Attachment |
|---|---|---|---|---|
| Proclear CompatiblesTM | CooperVision | Omafilcon A | pHEMA/PC | + |
| Dailies AquaComfort PlusTM | CIBA Vision | Nelfilcon A | HPMC/PEG/PVA | − |
| Acuvue Oasys® | Johnson & Johnson | Senofilcon A | pHEMA + DMA + mPDMS + siloxane macromer + TEGDMA + PVP | − |
| Acuvue Advanced Plus® | Johnson & Johnson | Galyfilcon A | pHEMA + DMA + mPDMS + siloxane macromer + EGDMA + PVP | − |
| Parameters | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
|---|---|---|---|---|---|
| ELP | V96 | V96 | V96 | S96 | S96 |
| Label Temp (°C) | 37 | 37 | 4 | 37 | 4 |
| Release Temp (°C) | 37 | 4 | 4 | 37 | 4 |
| Percent Fast (%) | 16.8 (15.6~18.0) | 75.0 (63.7~86.2) | 35.1 (27.9~42.2) | 63.3 (59.3~67.3) | 55.9 (52.1~59.6) |
| kfast (h−1) | 2.9 (2.0~3.9) | 0.1 (0.06~0.2) | 3.3 (0.0~6.7) | 3.4 (2.0~4.7) | 2.4 (1.4~3.7) |
| t1/2,fast (h) | 0.2 (0.18~0.35) | 5.8 (4.0~10.9) | 0.2 (0.1~inf.) | 0.2 (0.1~0.3) | 0.3 (0.2~0.5) |
| kslow (h−1) | 0.0002 (0.0~0.0004) | 0.009 (0.004~0.01) | 0.007 (0.005~0.009) | 0.005 (0.003~0.007) | 0.006 (0.005~0.007) |
| t1/2,slow (h) | 4615 (1815~inf.) | 78.3 (49.8~183.7) | 96.2 (76.9~128.4) | 137.1 (101.6~210.7) | 112.5 (95.9~136.0) |
| AUC0-120h | 9938 | 2565 | 5156 | 3245 | 4660 |
| AUC0-Inf | 418564 | 3525 | 9302 | 7608 | 9436 |
| R2 | 0.89 | 0.88 | 0.81 | 0.82 | 0.93 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Lee, C.; Pastuszka, M.; Laurie, G.W.; MacKay, J.A. Thermally-Responsive Loading and Release of Elastin-Like Polypeptides from Contact Lenses. Pharmaceutics 2019, 11, 221. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11050221
Wang W, Lee C, Pastuszka M, Laurie GW, MacKay JA. Thermally-Responsive Loading and Release of Elastin-Like Polypeptides from Contact Lenses. Pharmaceutics. 2019; 11(5):221. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11050221
Chicago/Turabian StyleWang, Wan, Changrim Lee, Martha Pastuszka, Gordon W. Laurie, and J. Andrew MacKay. 2019. "Thermally-Responsive Loading and Release of Elastin-Like Polypeptides from Contact Lenses" Pharmaceutics 11, no. 5: 221. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11050221