Sex-Dependence in the Effect of Pharmaceutical Excipients: Polyoxyethylated Solubilising Excipients Increase Oral Drug Bioavailability in Male but Not Female Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Animals
2.3. Investigation of the Influence of Excipients on the in Vitro Permeability of Ranitidine
2.3.1. Tissue Preparation
2.3.2. Ussing Chamber Set-Up
2.3.3. Transport Study
2.3.4. Calculation
2.4. Pharmacokinetic Experiments
2.5. Methods of Analysis
2.6. Pharmacokinetic Analysis
2.7. Western Blot Analysis to Determine the Influence of Excipients on P-gp Protein Expression
2.7.1. Animal Treatment
2.7.2. Sample Preparation
2.7.3. Protein Analysis
2.8. Real-Time Reverse-Transcription Polymerase Chain Reaction to Determine the Influence of Excipients on the Expression of P-gp Gene
2.8.1. RNA Isolation
2.8.2. RNA Level Analysis
2.8.3. Data Analysis
2.9. Statistical Analysis
3. Results
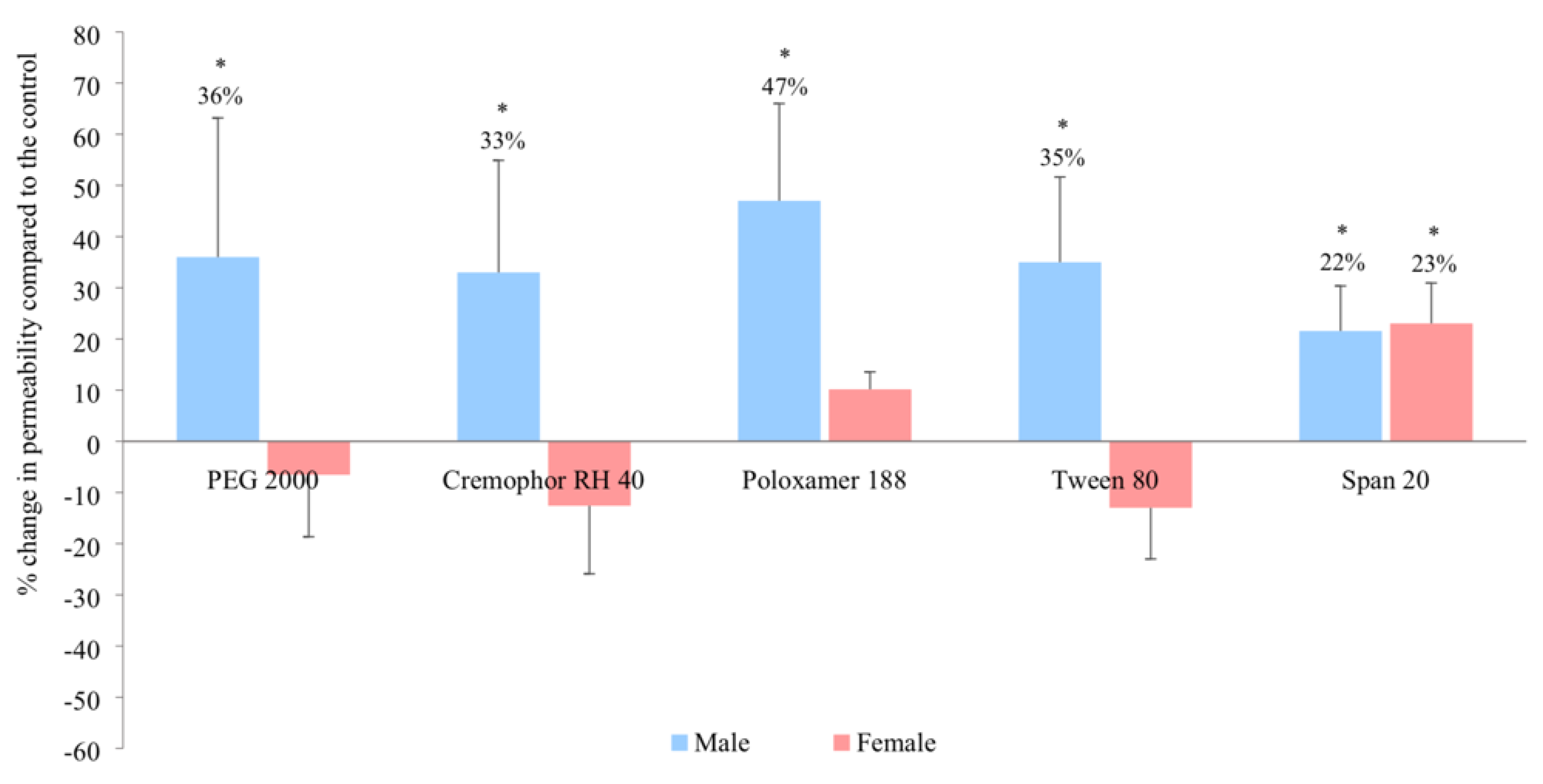
3.1. In Vitro Intestinal Permeability
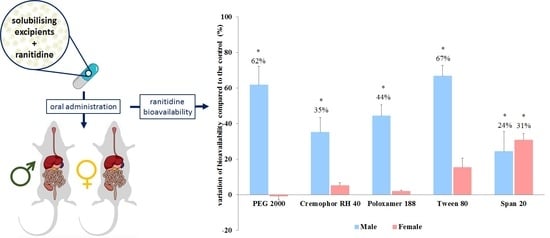
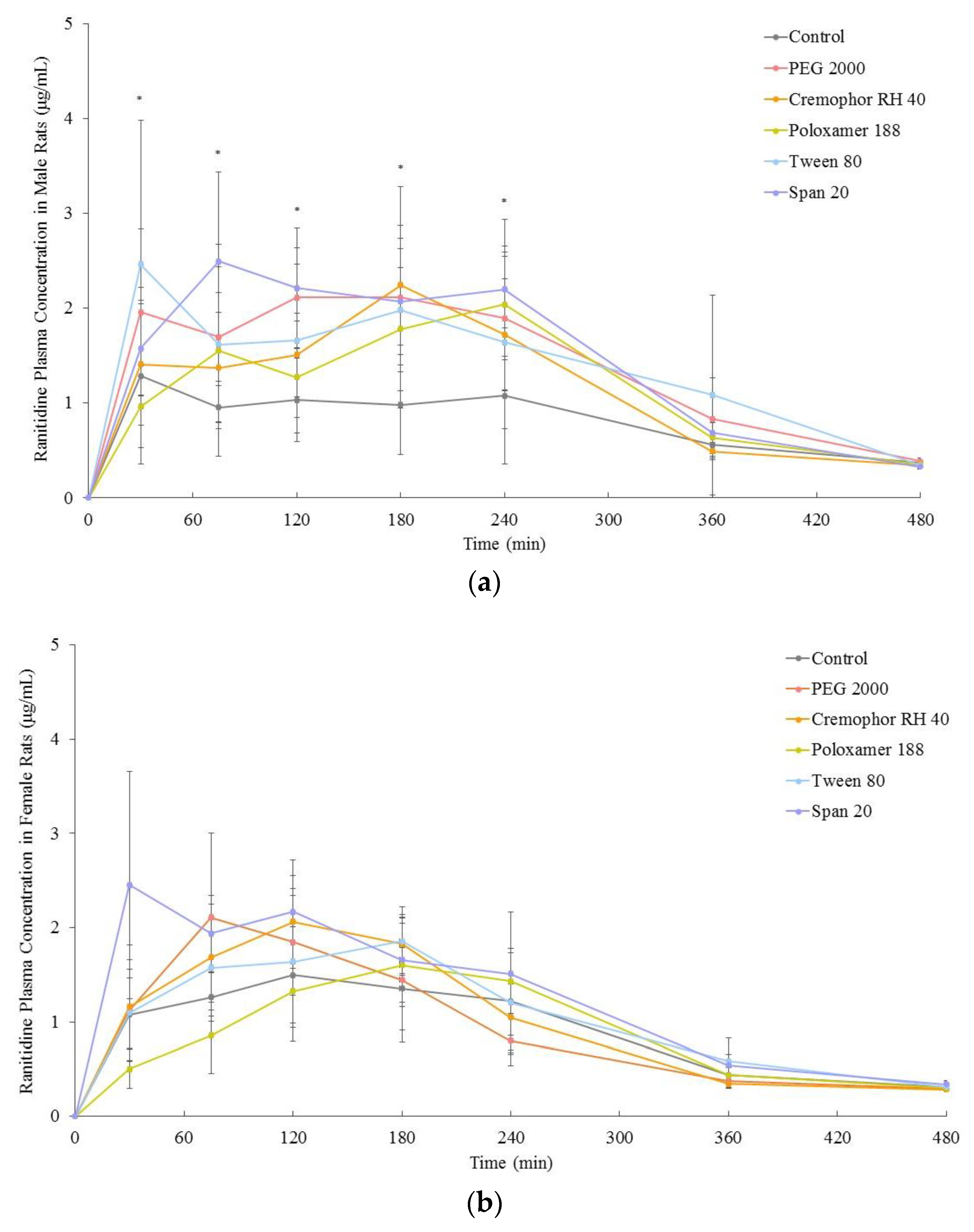
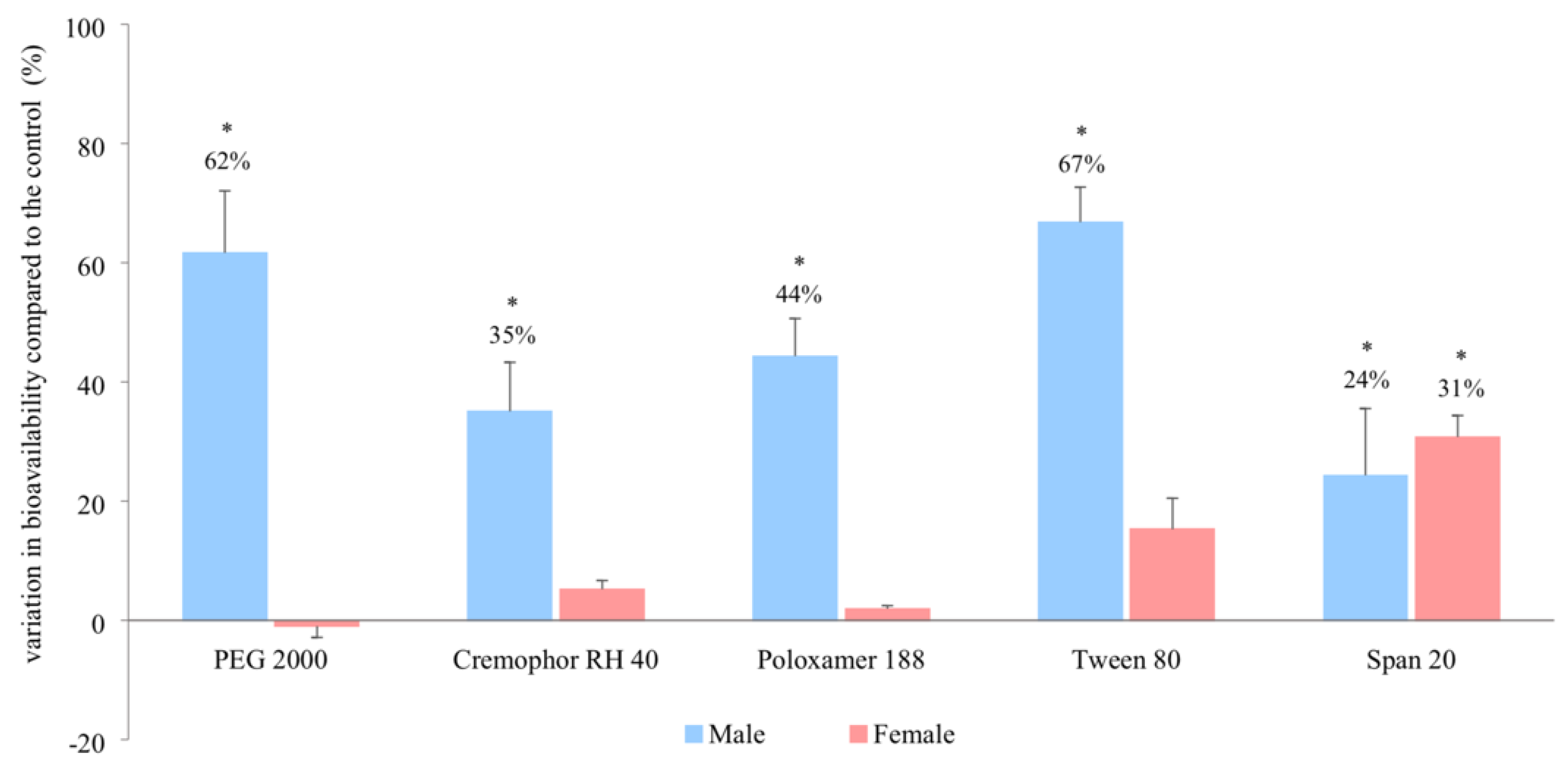
3.2. In Vivo Bioavailability
3.3. Relative P-glycoprotein Abundance
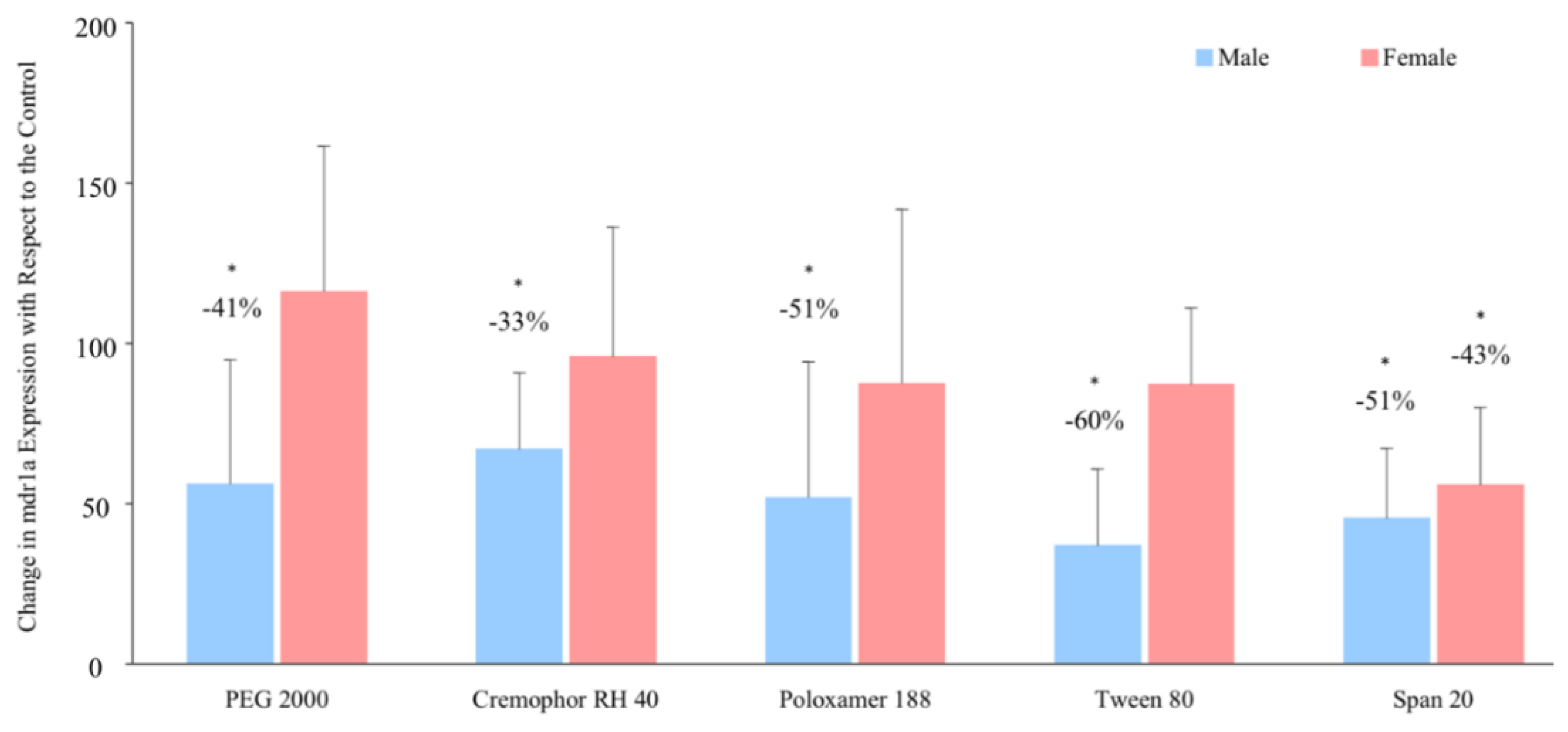
3.4. P-glycoprotein Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rademaker, M. Do Women Have More Adverse Drug Reactions? Am. J. Clin. Dermatol. 2001, 2, 349–351. [Google Scholar] [PubMed]
- Gochfeld, M. Sex Differences in Human and Animal Toxicology. Toxicol. Pathol. 2017, 45, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.; Rosano, G.; Walther, T.; Duarte, J.; Niessner, A.; Kaski, J.; Ceconi, C.; Drexel, H.; Kjeldsen, K.; Savarese, G.; et al. Gender differences in the effects of cardiovascular drugs. Eur. Heart J. Cardiovasc. Pharmacother. 2017, 3, 163–182. [Google Scholar] [CrossRef]
- Coraggio, V.; Guida, F.; Boccella, S.; Scafuro, M.; Paino, S.; Romano, D.; Maione, S.; Luongo, L. Neuroimmune-Driven Neuropathic Pain Establishment: A Focus on Gender Differences. Int. J. Mol. Sci. 2018, 19, 281. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- GAO. Drug Safety: Most Drugs Withdrawn in Recent Years Had Greater Health Risk for Women; United States General Accounting Office: Washington, DC, USA, 2001.
- Putting gender on the agenda. Nature 2010, 465, 665. [CrossRef] [PubMed]
- U.S.P. Convention. USP Guideline for Submitting Requests for Revisions to USP-NF. Available online: https://www.usp.org/sites/default/files/usp/document/get-involved/submission-guidelines/excipients_rfr_guideline-28apr16.pdf (accessed on 5 December 2018).
- Goole, J.; Lindley, D.J.; Roth, W.; Carl, S.M.; Amighi, K.; Kauffmann, J.-M.; Knipp, G.T. The effects of excipients on transporter mediated absorption. Int. J. Pharm. 2010, 393, 17–31. [Google Scholar] [CrossRef]
- Koch, K.M.; Parr, A.F.; Tomlinson, J.J.; Sandefer, E.P.; Digenis, G.A.; Donn, K.H.; Powell, J.R. Effect of Sodium Acid Pyrophosphate on Ranitidine Bioavailability and Gastrointestinal Transit Time. Pharm. Res. 1993, 10, 1027–1030. [Google Scholar] [CrossRef]
- Adkin, D.A.; Davis, S.S.; Sparrow, R.A.; Huckle, P.D.; Wilding, I.R. The Effect of Mannitol on the Oral Bioavailability of Cimetidine. J. Pharm. Sci. 1995, 84, 1405–1409. [Google Scholar] [CrossRef]
- Basit, A.W.; Podczeck, F.; Newton, J.M.; Waddington, W.A.; Ell, P.J.; Lacey, L.F. Influence of Polyethylene Glycol 400 on the Gastrointestinal Absorption of Ranitidine. Pharm. Res. 2002, 19, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Schulze, J.D.R.; Waddington, W.A.; Ell, P.J.; Parsons, G.E.; Coffin, M.D.; Basit, A.W. Concentration-Dependent Effects of Polyethylene Glycol 400 on Gastrointestinal Transit and Drug Absorption. Pharm. Res. 2003, 20, 1984–1988. [Google Scholar] [CrossRef]
- Schulze, J.D.; Peters, E.E.; Vickers, A.W.; Staton, J.S.; Coffin, M.D.; Parsons, G.E.; Basit, A.W. Excipient effects on gastrointestinal transit and drug absorption in beagle dogs. Int. J. Pharm. 2005, 300, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Ashiru, D.A.I.; Patel, R.; Basit, A.W. Polyethylene Glycol 400 Enhances the Bioavailability of a BCS Class III Drug (Ranitidine) in Male Subjects but Not Females. Pharm. Res. 2008, 25, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Afonso-Pereira, F.; Murdan, S.; Sousa, J.; Veiga, F.; Basit, A.W. Sex differences in excipient effects: Enhancement in ranitidine bioavailability in the presence of polyethylene glycol in male, but not female, rats. Int. J. Pharm. 2016, 506, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Dou, L.; Murdan, S.; Basit, A.W. An animal’s sex influences the effects of the excipient PEG 400 on the intestinal P-gp protein and mRNA levels, which has implications for oral drug absorption. Eur. J. Pharm. Sci. 2018, 120, 53–60. [Google Scholar] [CrossRef]
- Afonso-Pereira, F.; Dou, L.; Trenfield, S.J.; Madla, C.M.; Murdan, S.; Sousa, J.; Veiga, F.; Basit, A.W. Sex differences in the gastrointestinal tract of rats and the implications for oral drug delivery. Eur. J. Pharm. Sci. 2018, 115, 339–344. [Google Scholar] [CrossRef]
- Freire, A.C.; Basit, A.W.; Choudhary, R.; Piong, C.W.; Merchant, H.A. Does sex matter? The influence of gender on gastrointestinal physiology and drug delivery. Int. J. Pharm. 2011, 415, 15–28. [Google Scholar] [CrossRef]
- Hatton, G.B.; Yadav, V.; Basit, A.W.; Merchant, H.A. Animal Farm: Considerations in Animal Gastrointestinal Physiology and Relevance to Drug Delivery in Humans. J. Pharm. Sci. 2015, 104, 2747–2776. [Google Scholar] [CrossRef]
- Mai, Y.; Afonso-Pereira, F.; Murdan, S.; Basit, A.W. Excipient-mediated alteration in drug bioavailability in the rat depends on the sex of the animal. Eur. J. Pharm. Sci. 2017, 107, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Patel, N.; Lin, S. Solubility and dissolution enhancement strategies: Current understanding and recent trends. Drug Dev. Ind. Pharm. 2015, 41, 875–887. [Google Scholar] [CrossRef]
- Clarke, L.L. A guide to Ussing chamber studies of mouse intestine. Am. J. Physiol. Liver Physiol. 2009, 296, G1151–G1166. [Google Scholar] [CrossRef]
- Polentarutti, B.I.; Peterson, A.L.; Sjöberg, Å.K.; Anderberg, E.K.I.; Utter, L.M.; Ungell, A.B. Evaluation of Viability of Excised Rat Intestinal Segments in the Ussing Chamber: Investigation of Morphology, Electrical Parameters, and Permeability Characteristics. Pharm. Res. 1999, 16, 446–454. [Google Scholar] [CrossRef]
- Mai, Y.; Murdan, S.; Awadi, M.; Basit, A.W. Establishing an in vitro permeation model to predict the in vivo sex-related influence of PEG 400 on oral drug absorption. Int. J. Pharm. 2018, 542, 280–287. [Google Scholar] [CrossRef]
- Ashiru, D.A.; Patel, R.; Basit, A.W. Simple and universal HPLC-UV method to determine cimetidine, ranitidine, famotidine and nizatidine in urine: Application to the analysis of ranitidine and its metabolites in human volunteers. J. Chromatogr. B 2007, 860, 235–240. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- MacLean, C.; Moenning, U.; Reichel, A.; Fricker, G. Closing the gaps: a full scan of the intestinal expression of p-glycoprotein, breast cancer resistance protein, and multidrug resistance-associated protein 2 in male and female rats. Drug Metab Dispos. 2008, 37, 1249–1254. [Google Scholar] [CrossRef]
- Cornaire, G.; Hermann, P.; Cloarec, A.; Arellano, C.; Woodley, J.; Houin, G. Impact of excipients on the absorption of P-glycoprotein substrates in vitro and in vivo. Int. J. Pharm. 2004, 278, 119–131. [Google Scholar] [CrossRef]
- Shen, L.; Black, E.D.; Witkowski, E.D.; Lencer, W.I.; Guerriero, V.; Schneeberger, E.E.; Turner, J.R. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J. Cell Sci. 2006, 119, 2095–2106. [Google Scholar] [CrossRef]
- Tayrouz, Y.; Ding, R.; Burhenne, J.; Riedel, K.; Weiss, J.; Hoppe-Tichy, T.; Haefeli, W.E.; Mikus, G. Pharmacokinetic and pharmaceutic interaction between digoxin and Cremophor RH40. Clin. Pharmacol. Ther. 2003, 73, 397–405. [Google Scholar] [CrossRef]
- Rege, B.D.; Kao, J.P.; E Polli, J. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur. J. Pharm. Sci. 2002, 16, 237–246. [Google Scholar] [CrossRef]
- Hodaei, D.; Baradaran, B.; Valizadeh, H.; Mohammadnezhad, L.; Zakeri-Milani, P. The effect of Tween excipients on expression and activity of P-glycoprotein in Caco-2 cells. Pharma. Ind. 2013, 76, 788–794. [Google Scholar]
- Schuetz, E.G.; Furuya, K.N.; Schuetz, J.D. Interindividual variation in expression of P-glycoprotein in normal human liver and secondary hepatic neoplasms. J. Pharmacol. Exp. Ther. 1995, 275, 1011–1018. [Google Scholar]
- Potter, J.M.; McWhinney, B.C.; Sampson, L.; Hickman, P.E. Area-Under-the-Curve Monitoring of Prednisolone for Dose Optimization in a Stable Renal Transplant Population. Ther. Drug Monit. 2004, 26, 408–414. [Google Scholar] [CrossRef]
- Amin, M.D.L. P-glycoprotein Inhibition for Optimal Drug Delivery. Drug Target Insights 2013, 7, 27–34. [Google Scholar] [CrossRef]
- Akhtar, N.; Ahad, A.; Khan, F.; Allaham, A.; Talegaonkar, S. The Ameliorated Pharmacokinetics of VP-16 in Wistar Rats: A Possible Role of P-Glycoprotein Inhibition by Pharmaceutical Excipients. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 191–199. [Google Scholar] [CrossRef]
- Al-Ali, A.A.A.; Steffansen, B.; Holm, R.; Nielsen, C.U. Nonionic surfactants increase digoxin absorption in Caco-2 and MDCKII MDR1 cells: Impact on P-glycoprotein inhibition, barrier function, and repeated cell exposure. Int. J. Pharm. 2018, 551, 270–280. [Google Scholar] [CrossRef]
- Zakeri, P.; Valizadeh, H. Intestinal transporters: Enhanced absorption through P-glycoprotein-related drug interactions. Expert Opin rug Metab Toxicol. 2014, 10, 859–871. [Google Scholar] [CrossRef]
- Al-Ali, A.A.A.; Quach, J.R.C.; Bundgaard, C.; Steffansen, B.; Holm, R.; Nielsen, C.U. Polysorbate 20 alters the oral bioavailability of etoposide in wild type and mdr1a deficient Sprague-Dawley rats. Int. J. Pharm. 2018, 543, 352–360. [Google Scholar] [CrossRef]
- Doige, C.A.; Yu, X.; Sharom, F.J. The effects of lipids and detergents on ATPase-active P-glycoprotein. Biochim. Biophys. Acta (BBA) Biomembr. 1993, 1146, 65–72. [Google Scholar] [CrossRef]
- Matsaridou, I.; Barmpalexis, P.; Salis, A.; Nikolakakis, I. The Influence of Surfactant HLB and Oil/Surfactant Ratio on the Formation and Properties of Self-emulsifying Pellets and Microemulsion Reconstitution. AAPS PharmSciTech 2012, 13, 1319–1330. [Google Scholar] [CrossRef]
- Maskarinec, S.A.; Hannig, J.; Lee, R.C.; Lee, K.Y.C. Direct observation of poloxamer 188 insertion into lipid monolayers. Biophys. J. 2002, 82, 1453–1459. [Google Scholar] [CrossRef]
- Wan, L.S.; Lee, P.F. CMC of polysorbates. J. Pharm. Sci. 1974, 63, 136–137. [Google Scholar] [CrossRef]
- Peltonen, L.; Hirvonen, J.; Yliruusi, J. The Behavior of Sorbitan Surfactants at the Water–Oil Interface: Straight-Chained Hydrocarbons from Pentane to Dodecane as an Oil Phase. J. Colloid Interface Sci. 2001, 240, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Li-Blatter, X.; Nervi, P.; Seelig, A. Detergents as intrinsic P-glycoprotein substrates and inhibitors. Biochim. Biophys. Acta 2009, 1788, 2335–2344. [Google Scholar] [CrossRef] [PubMed]
- Ferté, J. Analysis of the tangled relationships between P-glycoprotein-mediated multidrug resistance and the lipid phase of the cell membrane. Eur. J. Biochem. 2000, 267, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Hodaei, D.; Baradaran, B.; Valizadeh, H.; Zakeri-Milani, P. Effects of polyethylene glycols on intestinal efflux pump expression and activity in Caco-2 cells. Braz. J. Pharm. Sci. 2015, 51, 745–753. [Google Scholar] [CrossRef]
- Zhao, W.; Alama, T.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Effects of 2 Polyoxyethylene Alkyl Ethers on the Function of Intestinal P-glycoprotein and Their Inhibitory Mechanisms. J. Pharm. Sci. 2016, 105, 3668–3679. [Google Scholar] [CrossRef] [PubMed]
- Denson, L.A.; Bohan, A.; Held, M.A.; Boyer, J.L. Organ-specific alterations in RAR alpha:RXR alpha abundance regulate rat Mrp2 (Abcc2) expression in obstructive cholestasis. Gastroenterology 2002, 123, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Freedman, L.P. Multimeric Coactivator Complexes for Steroid/Nuclear Receptors. Trends Endocrinol. Metab. 1999, 10, 403–407. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Shimano, H.; Yahagi, N.; Ide, T.; Amemiya-Kudo, M.; Matsuzaka, T.; Nakakuki, M.; Tomita, S.; Okazaki, H.; Tamura, Y.; et al. Polyunsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. J. Biol. Chem. 2002, 277, 1705–1711. [Google Scholar] [CrossRef]
- Lee, S.D.; Wasan, K.M.; Calcagni, A.; Avery, M.; McCush, F.; Chen, C. The in Vitro Plasma Distribution of a Novel Cholesteryl Ester Transfer Protein Inhibitor, Torcetrapib, Is Influenced by Differences in Plasma Lipid Concentrations. Pharm. Res. 2006, 23, 1025–1030. [Google Scholar] [CrossRef]
- Chawla, A. Nuclear Receptors and Lipid Physiology: Opening the X-Files. Science 2001, 294, 1866–1870. [Google Scholar] [CrossRef]
- Bookout, A.L.; Jeong, Y.; Downes, M.; Yu, R.T.; Evans, R.M.; Mangelsdorf, D.J. Anatomical Profiling of Nuclear Receptor Expression Reveals a Hierarchical Transcriptional Network. Cell 2006, 126, 789–799. [Google Scholar] [CrossRef]
- Sachs-Barrable, K.; Thamboo, A.; Lee, S.D.; Wasan, K.M. Lipid excipients Peceol and Gelucire 44/14 decrease P-glycoprotein mediated efflux of rhodamine 123 partially due to modifying P-glycoprotein protein expression within Caco-2 cells. J. Pharm. Pharm. Sci. 2007, 10, 319–331. [Google Scholar]


| Gene | Primer (5′ – 3′) | Amplicon (bp) | GenBank® Accession | |
|---|---|---|---|---|
| mdr1a | Forward | CACCATCCAGAACGCAGACT | 159 | NM_133401 |
| Reverse | ACATCTCGCATGGTCACAGTT | |||
| mdr1b | Forward | AACGCAGACTTGATCGTGGT | 144 | NM_012623 |
| Reverse | AGCACCTCAAATACTCCCAGC | |||
| anti-beta actin | Forward | GCAGGAGTACGATGAGTCCG | 74 | NM_031144 |
| Reverse | ACGCAGCTCAGTAACAGTCC | |||
| Pharmacokinetic Parameters | Control (i.e., No Excipient) | PEG 2000 | Cremophor RH 40 | Poloxamer 188 | Tween 80 | Span 20 |
|---|---|---|---|---|---|---|
| Male | ||||||
| AUC0–480 (μg.min/mL) | 391 ± 108 | 680 ± 167 * | 563 ± 94 * | 561 ± 112 * | 670 ± 146 * | 546 ± 80 * |
| AUC0–infinity/∞ (μg.min/mL) | 485 ± 109 | 784 ± 171 * | 655 ± 85 * | 700 ± 77 * | 809 ± 212 * | 603 ± 91 * |
| cmax (μg/mL) | 2.0 ± 0.8 | 3.5 ± 0.7 * | 3.4 ± 0.6 * | 3.9 ± 0.7 * | 3.8 ± 0.9 * | 3.8 ± 0.5 * |
| tmax (min) | 146 ± 110 | 150 ± 79 | 155 ± 61 | 195 ± 72 | 160 ± 128 | 157 ± 95 |
| Female | ||||||
| AUC0–480 (μg.min/mL) | 437 ± 59 | 454 ± 74 | 490 ± 27 | 422 ± 61 | 507 ± 33 | 613 ± 76 * |
| AUC0–infinity/∞ (μg.min/mL) | 517 ± 47 | 512 ± 66 | 544 ± 26 | 527 ± 61 | 596 ± 52 | 676 ± 79 * |
| cmax (μg/mL) | 2.5 ± 0.2 | 1.8 ± 0.7 | 2.1 ± 0.3 | 1.9 ± 0.5 | 2.6 ± 0.4 | 3.2 ± 0.6 |
| tmax (min) | 127 ± 75 | 107 ± 42 | 98 ± 38 | 190 ± 45 | 150 ± 35 | 70 ± 65 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, Y.; Dou, L.; Madla, C.M.; Murdan, S.; Basit, A.W. Sex-Dependence in the Effect of Pharmaceutical Excipients: Polyoxyethylated Solubilising Excipients Increase Oral Drug Bioavailability in Male but Not Female Rats. Pharmaceutics 2019, 11, 228. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11050228
Mai Y, Dou L, Madla CM, Murdan S, Basit AW. Sex-Dependence in the Effect of Pharmaceutical Excipients: Polyoxyethylated Solubilising Excipients Increase Oral Drug Bioavailability in Male but Not Female Rats. Pharmaceutics. 2019; 11(5):228. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11050228
Chicago/Turabian StyleMai, Yang, Liu Dou, Christine M. Madla, Sudaxshina Murdan, and Abdul W. Basit. 2019. "Sex-Dependence in the Effect of Pharmaceutical Excipients: Polyoxyethylated Solubilising Excipients Increase Oral Drug Bioavailability in Male but Not Female Rats" Pharmaceutics 11, no. 5: 228. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11050228