Hyaluronic Acid Nanocapsules as a Platform for Needle-Free Vaccination
Abstract
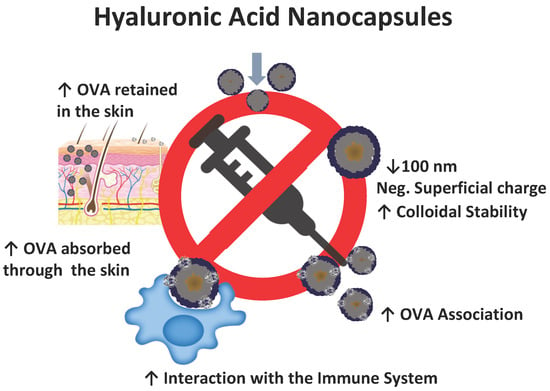
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Characterization of Nanosystems
2.3. Colloidal Stability in Storage and Physiological Conditions
2.4. Ovalbumin Association to the Hyaluronic Acid Nanocapsules
2.5. Cell Viability Studies
2.6. Activation of Complement Cascade Studies
2.7. Skin Permeation Study
2.8. Data Analysis
2.9. Ethical Issues
3. Results and Discussion
3.1. Hyaluronic Acid Nanocapsules Preparation and Characterization
3.2. Colloidal Stability in Storage and Physiological Conditions
3.3. Ovalbumin Association to the Hyaluronic Acid Nanocapsules
3.4. Cell Viability
3.5. Activation of Complement Cascade Studies
3.6. Skin Permeation Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siani, A. Measles outbreaks in Italy: A paradigm of the re-emergence of vaccine-preventable diseases in developed countries. Prev. Med. 2019, 121, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Bragazzi, N.L.; Gianfredi, V.; Villarini, M.; Rosselli, R.; Nasr, A.; Hussein, A.; Martini, M.; Behzadifar, M. Vaccines Meet Big Data: State-of-the-Art and Future Prospects. From the Classical 3Is (“Isolate-Inactivate-Inject”) Vaccinology 1.0 to Vaccinology 3.0, Vaccinomics, and Beyond: A Historical Overview. Front. Public Health 2018, 6, 9. [Google Scholar] [CrossRef]
- Bonam, S.R.; Partidos, C.D.; Halmuthur, S.K.M.; Muller, S. An Overview of Novel Adjuvants Designed for Improving Vaccine Efficacy. Trends Pharmacol. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Schulze, K.; Ebensen, T.; Riese, P.; Prochnow, B.; Lehr, C.-M.; Guzmán, C.A. New Horizons in the Development of Novel Needle-Free Immunization Strategies to Increase Vaccination Efficacy. In How to Overcome the Antibiotic Crisis: Facts, Challenges, Technologies and Future Perspectives; Stadler, M., Dersch, P., Eds.; Springer International Publishing: New York, NY, USA, 2016; pp. 207–234. [Google Scholar] [CrossRef]
- Singh, B.; Maharjan, S.; Sindurakar, P.; Cho, K.H.; Choi, Y.J.; Cho, C.S. Needle-Free Immunization with Chitosan-Based Systems. Int. J. Mol. Sci. 2018, 19, 3639. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- González-Aramundiz, J.V.; Cordeiro, A.S.; Csaba, N.; De la Fuente, M.; Alonso, M. Nanovaccine: Nanocarriers for antigen delivery. Biol. Aujourd’hui 2012, 206, 249–261. [Google Scholar] [CrossRef]
- Kumar, S.; Anselmo, A.C.; Banerjee, A.; Zakrewsky, M.; Mitragotri, S. Shape and size-dependent immune response to antigen-carrying nanoparticles. J. Control. Release 2015, 220, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.P.; Verma, G.; Ke, X.; Thayer, W.M.; Hamerly, T.; Baxter, V.K.; Lee, J.E.; Dinglasan, R.R.; Mao, H.-Q. Critical size limit of biodegradable nanoparticles for enhanced lymph node trafficking and paracortex penetration. Nano Res. 2019, 12, 837–844. [Google Scholar] [CrossRef]
- Lou, B.; De Beuckelaer, A.; Boonstra, E.; Li, D.; De Geest, B.G.; De Koker, S.; Mastrobattista, E.; Hennink, W.E. Modular core-shell polymeric nanoparticles mimicking viral structures for vaccination. J. Control. Release 2019, 293, 48–62. [Google Scholar] [CrossRef] [PubMed]
- González-Aramundiz, J.V.; Presas, E.; Dalmau-Mena, I.; Martínez-Pulgarín, S.; Alonso, C.; Escribano, J.M.; Alonso, M.J.; Csaba, N.S. Rational design of protamine nanocapsules as antigen delivery carriers. J. Control. Release 2017, 245, 62–69. [Google Scholar] [CrossRef]
- González-Aramundiz, J.V.; Lozano, M.V.; Sousa-Herves, A.; Fernandez-Megia, E.; Csaba, N. Polypeptides and polyaminoacids in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 183–201. [Google Scholar] [CrossRef]
- Vicente, S.; Peleteiro, M.; Gonzalez-Aramundiz, J.V.; Diaz-Freitas, B.; Martinez-Pulgarin, S.; Neissa, J.I.; Escribano, J.M.; Sanchez, A.; Gonzalez-Fernandez, A.; Alonso, M.J. Highly versatile immunostimulating nanocapsules for specific immune potentiation. Nanomedicine 2014, 9, 2273–2289. [Google Scholar] [CrossRef]
- Nagula, R.L.; Wairkar, S. Recent advances in topical delivery of flavonoids: A review. J. Control. Release 2019, 296, 190–201. [Google Scholar] [CrossRef]
- Yazdi, A.S.; Röcken, M.; Ghoreschi, K. Cutaneous immunology: Basics and new concepts. Semin. Immunopathology 2016, 38, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ukidve, A.; Dasgupta, A.; Mitragotri, S. Transdermal immunomodulation: Principles, advances and perspectives. Adv. Drug Delivery. Rev. 2018, 127, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Mofidfar, M.; O’Farrell, L.; Prausnitz, M.R. Pharmaceutical jewelry: Earring patch for transdermal delivery of contraceptive hormone. J. Control. Release 2019, 301, 140–145. [Google Scholar] [CrossRef]
- Lee, H.; Song, C.; Baik, S.; Kim, D.; Hyeon, T.; Kim, D.-H. Device-assisted transdermal drug delivery. Adv. Drug Delivery. Rev. 2018, 127, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Nashchekina, Y.A.; Raydan, M. Noninvasive penetration of 5 nm hyaluronic acid molecules across the epidermal barrier (in vitro) and its interaction with human skin cells. Skin Res. Technol. 2018, 24, 129–134. [Google Scholar] [CrossRef]
- Teijeiro-Valiño, C.; Novoa-Carballal, R.; Borrajo, E.; Vidal, A.; Alonso-Nocelo, M.; de la Fuente Freire, M.; Lopez-Casas, P.P.; Hidalgo, M.; Csaba, N.; Alonso, M.J. A multifunctional drug nanocarrier for efficient anticancer therapy. J. Control. Release 2019, 294, 154–164. [Google Scholar] [CrossRef]
- Oyarzun-Ampuero, F.A.; Rivera-Rodriguez, G.R.; Alonso, M.J.; Torres, D. Hyaluronan nanocapsules as a new vehicle for intracellular drug delivery. Eur. J. Pharm. Sci. 2013, 49, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Bussio, J.; Molina-Perea, C.; González-Aramundiz, J. Lower-Sized Chitosan Nanocapsules for Transcutaneous Antigen Delivery. Nanomaterials 2018, 8, 659. [Google Scholar] [CrossRef]
- Neun, B.W.; Dobrovolskaia, M.A. Qualitative Analysis of Total Complement Activation by Nanoparticles. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Humana Press: New York, NY, USA, 2011; Volume 697, pp. 237–245. [Google Scholar]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. Adv. Appl. 2016, 8, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.; Fowler, S. Quantification of Proteins on Western Blots Using ECL. In The Protein Protocols Handbook; Walker, J., Ed.; Humana Press: New York, NY, USA, 2002; pp. 429–437. [Google Scholar] [CrossRef]
- Tiwari, S.; Bahadur, P. Modified hyaluronic acid based materials for biomedical applications. Int. J. Biol. Macromol. 2019, 121, 556–571. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- González-Aramundiz, J.V.; Peleteiro Olmedo, M.; González-Fernández, Á.; Alonso Fernández, M.J.; Csaba, N.S. Protamine-based nanoparticles as new antigen delivery systems. Eur. J. Pharm. Biopharm. 2015, 97, 51–59. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef]
- Crecente-Campo, J.; Alonso, M.J. Engineering, on-demand manufacturing, and scaling-up of polymeric nanocapsules. Bioeng. Transl. Med. 2019, 4, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Aramundiz, J.V.; Peleteiro Olmedo, M.; González-Fernández, A.; Alonso Fernandez, M.J.; Csaba, N.S. Protamine Nanocapsules for the Development of Thermostable Adjuvanted Nanovaccines. Mol. Pharm. 2018. [Google Scholar] [CrossRef]
- Gause, K.T.; Wheatley, A.K.; Cui, J.; Yan, Y.; Kent, S.J.; Caruso, F. Immunological Principles Guiding the Rational Design of Particles for Vaccine Delivery. ACS Nano 2017, 11, 54–68. [Google Scholar] [CrossRef]
- Kawai, M.; Nakamura, T.; Miura, N.; Maeta, M.; Tanaka, H.; Ueda, K.; Higashi, K.; Moribe, K.; Tange, K.; Nakai, Y.; et al. DNA-loaded nano-adjuvant formed with a vitamin E-scaffold intracellular environmentally-responsive lipid-like material for cancer immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2587–2597. [Google Scholar] [CrossRef]
- Abellan-Pose, R.; Teijeiro-Valiño, C.; Santander-Ortega, M.J.; Borrajo, E.; Vidal, A.; Garcia-Fuentes, M.; Csaba, N.; Alonso, M.J. Polyaminoacid nanocapsules for drug delivery to the lymphatic system: Effect of the particle size. Int. J. Pharm. 2016, 509, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Lam, H.T.; Le-Vinh, B.; Phan, T.N.Q.; Bernkop-Schnürch, A. Self-emulsifying drug delivery systems and cationic surfactants: Do they potentiate each other in cytotoxicity? J. Pharm. Pharmacol. 2019, 71, 156–166. [Google Scholar] [CrossRef]
- Gonzalez-Paredes, A.; Torres, D.; Alonso, M.J. Polyarginine nanocapsules: A versatile nanocarrier with potential in transmucosal drug delivery. Int. J. Pharm. 2017, 529, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Feng, X.; Yang, X.; Sun, W.; Jin, Y.; Liu, H.; Ge, K.; Li, Z.; Zhang, J. A simple and powerful co-delivery system based on pH-responsive metal-organic frameworks for enhanced cancer immunotherapy. Biomaterials 2017, 122, 23–33. [Google Scholar] [CrossRef]
- Wusiman, A.; Xu, S.; Ni, H.; Gu, P.; Liu, Z.; Zhang, Y.; Qiu, T.; Hu, Y.; Liu, J.; Wu, Y.; et al. Immunomodulatory effects of Alhagi honey polysaccharides encapsulated into PLGA nanoparticles. Carbohydr. Polym. 2019, 211, 217–226. [Google Scholar] [CrossRef]
- Bass, J.J.; Wilkinson, D.J.; Rankin, D.; Phillips, B.E.; Szewczyk, N.J.; Smith, K.; Atherton, P.J. An overview of technical considerations for Western blotting applications to physiological research. Scand. J. Med. Sci. Sports 2017, 27, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, H.; Chen, Z.; Zheng, Y. Penetration of Lipid Membranes by Gold Nanoparticles: Insights into Cellular Uptake, Cytotoxicity, and Their Relationship. ACS Nano 2010, 4, 5421–5429. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Breen, A.; Pillai, S.C. Toxicity of Nanomaterials: Exposure, Pathways, Assessment, and Recent Advances. ACS Biomater. Sci. Eng. 2018, 4, 2237–2275. [Google Scholar] [CrossRef]
- Li, J.C.; Mao, H.L.; Kawazoe, N.; Chen, G.P. Insight into the interactions between nanoparticles and cells. Biomater. Sci. 2017, 5, 173–189. [Google Scholar] [CrossRef]
- Giang, J.; Seelen, M.A.J.; van Doorn, M.B.A.; Rissmann, R.; Prens, E.P.; Damman, J. Complement Activation in Inflammatory Skin Diseases. Front. Immunol. 2018, 9, 639. [Google Scholar] [CrossRef]
- Ghebrehiwet, B. The complement system: An evolution in progress. F1000Research 2016, 5, 2840. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, Y.; Wang, L.; Zhang, W.; Chen, X.; Yang, X.; Xu, J.; Ma, G. Engineering Biomaterial-Associated Complement Activation to Improve Vaccine Efficacy. Biomacromolecules 2013. [Google Scholar] [CrossRef] [PubMed]
- Peleteiro, M.; Presas, E.; González-Aramundiz, J.V.; Sánchez-Correa, B.; Simón-Vázquez, R.; Csaba, N.; Alonso, M.J.; González-Fernández, Á. Polymeric Nanocapsules for Vaccine Delivery: Influence of the Polymeric Shell on the Interaction With the Immune System. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- HogenEsch, H.; O’Hagan, D.T.; Fox, C.B. Optimizing the utilization of aluminum adjuvants in vaccines: You might just get what you want. npj Vaccines 2018, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Figueroa, M.J.; González-Aramundiz, J.V. Passive and Iontophoretic Transdermal Penetration of Chlorpromazine. Pharm. Dev. Technol. 2008, 13, 271–275. [Google Scholar] [CrossRef]
- Jung, E.C.; Maibach, H.I. Animal models for percutaneous absorption. J. Appl. Toxicol. 2015, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Figueroa, M.J.; Abarca-Riquelme, J.M.; González-Aramundiz, J.V. Influence of protamine shell on nanoemulsions as a carrier for cyclosporine-A skin delivery. Pharm. Dev. Technol. 2019, 24, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.D.; Meinardi, M.M.H.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.B.; Jones, S.A. Hyaluronic acid: A unique topical vehicle for the localized delivery of drugs to the skin. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 308–318. [Google Scholar] [CrossRef]
- Yang, J.-A.; Kim, E.-S.; Kwon, J.H.; Kim, H.; Shin, J.H.; Yun, S.H.; Choi, K.Y.; Hahn, S.K. Transdermal delivery of hyaluronic acid—Human growth hormone conjugate. Biomaterials 2012, 33, 5947–5954. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jeong, H.; Han, S.; Beack, S.; Hwang, B.W.; Shin, M.; Oh, S.S.; Hahn, S.K. Hyaluronate and its derivatives for customized biomedical applications. Biomaterials 2017, 123, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Kim, K.S.; Yun, S.H.; Hahn, S.K. Enhancing the transdermal penetration of nanoconstructs: Could hyaluronic acid be the key? Nanomedicine 2014, 9, 743–745. [Google Scholar] [CrossRef] [PubMed]






| Nanosystem | Size (nm) | PDI | Potential z (mv) | % OVA Association |
|---|---|---|---|---|
| HA-NCs blank | 85 ± 4 | 0.175 | −19 ± 1 | |
| OVA loaded HA-NCs | 95 ± 5 | 0.132 | −22 ± 2 | |
| OVA loaded HA-NCs (isolated) | 93 ± 4 | 0.128 | −20 ± 1 | 67 ± 5 |
| Treatment | Amount/Concentration | Normalized Cleaved Protein |
|---|---|---|
| Positive control | 10 μg CVF | 1 * |
| Negative control | dPBS | 0.35 ± 0.04 |
| HA-NCs | 2 mg/mL | 0.82 ± 0.15 * |
| 1.75 mg/mL | 0.72 ± 0.13 * | |
| 1.25 mg/mL | 0.73 ± 0.17 * | |
| 0.75 mg/mL | 0.42 ± 0.07 | |
| 0.5 mg/mL | 0.45 ± 0.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bussio, J.I.; Molina-Perea, C.; González-Aramundiz, J.V. Hyaluronic Acid Nanocapsules as a Platform for Needle-Free Vaccination. Pharmaceutics 2019, 11, 246. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11050246
Bussio JI, Molina-Perea C, González-Aramundiz JV. Hyaluronic Acid Nanocapsules as a Platform for Needle-Free Vaccination. Pharmaceutics. 2019; 11(5):246. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11050246
Chicago/Turabian StyleBussio, Juan I., Carla Molina-Perea, and José Vicente González-Aramundiz. 2019. "Hyaluronic Acid Nanocapsules as a Platform for Needle-Free Vaccination" Pharmaceutics 11, no. 5: 246. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11050246