1. Introduction
Onychomycosis is the most common disease of the nail plate and nail bed [
1,
2,
3]. Several antifungal agents are used in the treatment of onychomycosis, among them ketoconazole, an imidazole derivative that acts by blocking the synthesis of ergosterol. Oral treatment of onychomycosis with ketoconazole provides a cure rate of 50% to 70% in fingernail and 15% to 30% in toenail infections [
4,
5]. However, oral treatment with ketoconazole presents several disadvantages, such as long duration, drug interactions, systemic side effects, and high rates of recurrence. As a consequence, the marketed oral formulation (Nizoral, Janssen, Raritan, NJ, USA) was discontinued in 2013 for the treatment of fungal infections. Topical treatment appears safer and multiple topical preparations containing 2% ketoconazole in the form of a gel, cream or lotions are currently on the market. However, the topical treatments available have the disadvantage of low drug permeation through the nail [
4,
6,
7].
Nanostructured lipid carriers (NLC) are colloidal dispersions in which the dispersed phase is composed of both solid and liquid lipids stabilized by an emulsifier [
8,
9]. Lipid nanoparticles have been extensively used for topical application, since their lipid matrix can interact with the
stratum corneum, promoting drug delivery to the skin. Moreover, lipid nanoparticles have been shown to form an occlusive layer on the skin surface significantly increasing tissue hydration and drug skin permeation [
10,
11,
12]. Similarly, it has been shown that lipid nanocarriers are increasing nail plate hydration enhancing drug penetration [
13].
The type of solid lipid selected for the production of NLC has a pivotal role, because it affects a number of physicochemical and biopharmaceutical properties of the particles, such as surface charge, melting point, drug loading and release properties among others [
14,
15]. Plant derived fats are a viable option, since they are made of fatty acids important for maintaining the homeostatic balance of the skin and its annexes [
16,
17]. Ucuùba butter is extracted from the seeds of
Virola surinamensis, a tree found mainly in the Amazon river basin, but present in an area that encompasses all of Central America and part of South America. Traditional uses of this fat span from the production of candles to medical soaps, but Ucuùba butter can be found also as a component of moisturizing creams and shampoos in the Brazilian cosmetic market [
16,
18]. The main fatty acids present in Ucuùba fat are myristic and lauric acid, while oleic, palmitoleic, linoleic acids are present at lower concentrations [
19]. In addition, the presence in the leaves, root, bark and seeds of
Virola surinamensis of phenylpropanoids, propiophenones, lignans, neolignans, polyketides and flavonoids with anti-oxidant, anti-inflammatory and anti-mycotic action has been reported in other studies and has elicited interest in the pharmaceutical properties of this plant [
20,
21,
22].
In several studies related to the optimization of NLC production it was observed that the relative concentration of surfactant and lipids strongly affects the stability of the formulation [
23]. The physical-chemical and biopharmaceutical characteristics of NLC, such as size, entrapment efficiency (EE), physical stability, drug release and morphology, are also strongly influenced by the choice and amount of lipids and surfactant [
23,
24,
25]. Thus, an optimization of NLC production with the aid of design of experiment could be carried out with the objective of modulating the amount of liquid and solid lipids and surfactant to obtain the most desirable properties in terms particle size, entrapment efficiency and stability. Response surface method (RSM) is one of the prevalent approaches in the design of the experiments, which involves the use of different experimental designs to generate polynomial mathematical relationships mapping of the response over the experimental domain with the aim to predict the outcome of a process or select the optimal process parameters [
26]. Box-Behnken design (BBD) is a type of RSM and is an independent, rotatable or nearly rotatable, quadratic design with the process variable combinations at the midpoints of the edges of the process space and its center. A significant advantage of BBD is that it is more cost effective compared to other techniques, such as Central Composite Design, 3-levels factorial design and D-optimal design, as it requires fewer experimental runs and less time for process optimization [
26,
27].
We hypothesized that Ucuùba fat, on account of its physicochemical and biological properties, could be a suitable lipid excipient for the production of NLC loaded with ketoconazole. Thus, the objective of this work was to obtain and optimize ketoconazole-loaded NLC production by high pressure homogenization using Ucuùba fat in the lipid phase. The design of experiment was then applied to select the optimal conditions for the production of Ucuùba fat NLC in terms of encapsulation efficiency (EE), particle size and polydispersity index (PDI) for use as a nail drug delivery system.
4. Discussion
The main purpose of this study was the development and optimization of Ucuùba fat NLC, using ketoconazole as a drug model for the treatment onychomycosis. Since the introduction at the beginning of the new millennium of the Quality-by-Design approach, the pharmaceutical sector has started using the design of the experiment as a statistical tool to maximize process knowledge and efficiently optimize product quality characteristics [
38,
39]. This approach has been applied to the manufacturing process of several dosage forms, including as tablets [
40], pellets [
41], semisolid formulations [
42] and spray-dried powders to be used in dry powder inhalers [
43].
Design of Experiment (DoE) was deemed suitable for the development and optimization of innovative and complex dosage forms, such as nanoparticles. In fact, scale-up manufacturing issues and poor process control have been indicated as some of the critical underlying problems that hampered the transfer of nanomedicines to the clinic [
44]. At the same time, the potential of nanoformulations, such as lipid nanovectors, and their inclusion in oral [
45,
46] or transmucosal medicinal products have not yet been fully studied [
47,
48,
49].
For these reasons, high-pressure homogenization, a robust and easy to scale up method, was selected for the manufacturing of NLC in this study [
50] and a BBD was applied to understand and optimize of Ucuùba fat NLC manufacturing process. The BBD results in a mathematical model which describes as closely as possible effects of the factors and their levels on the critical quality attributes of the product over the whole design space, and also predicts the responses [
51].
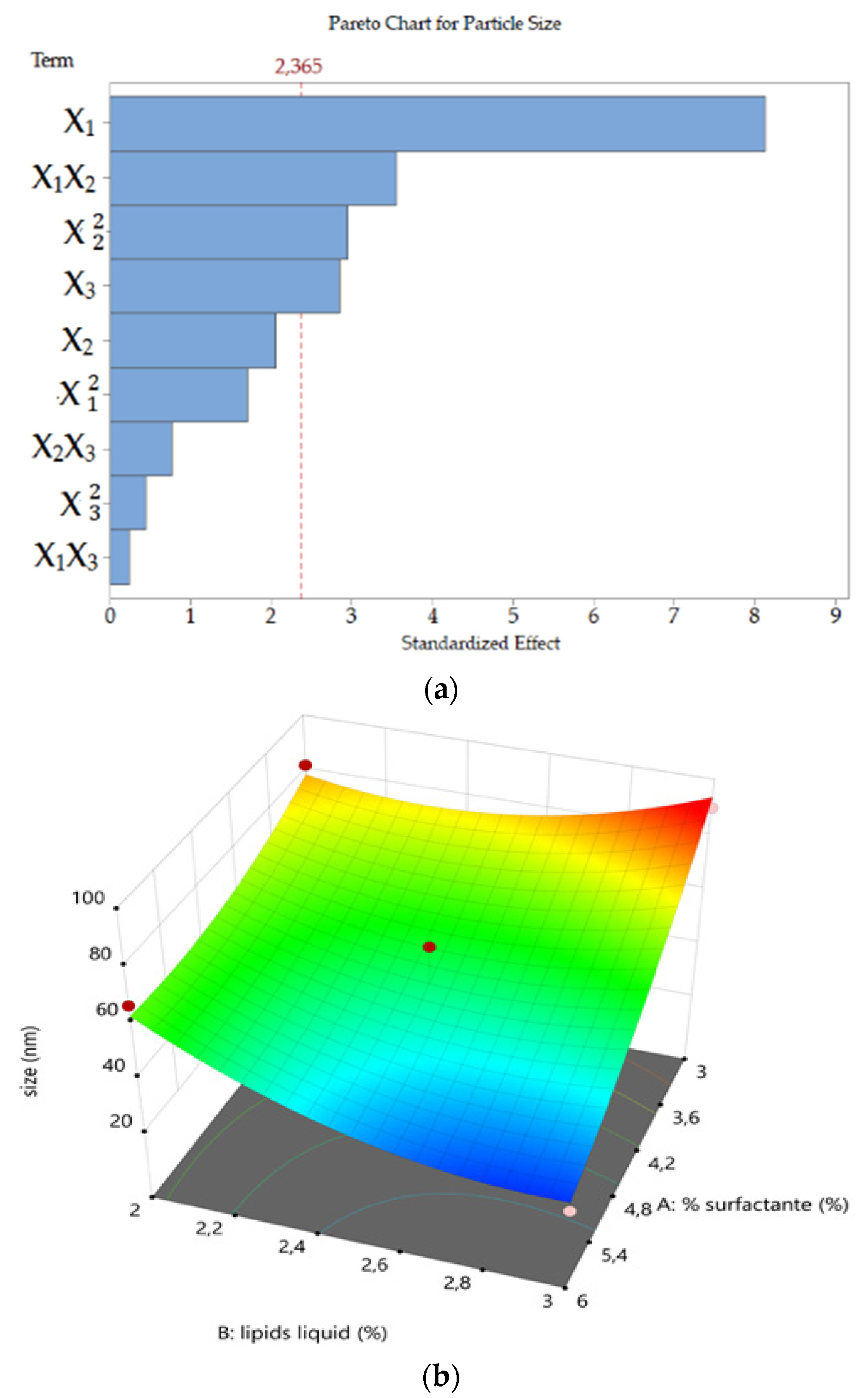
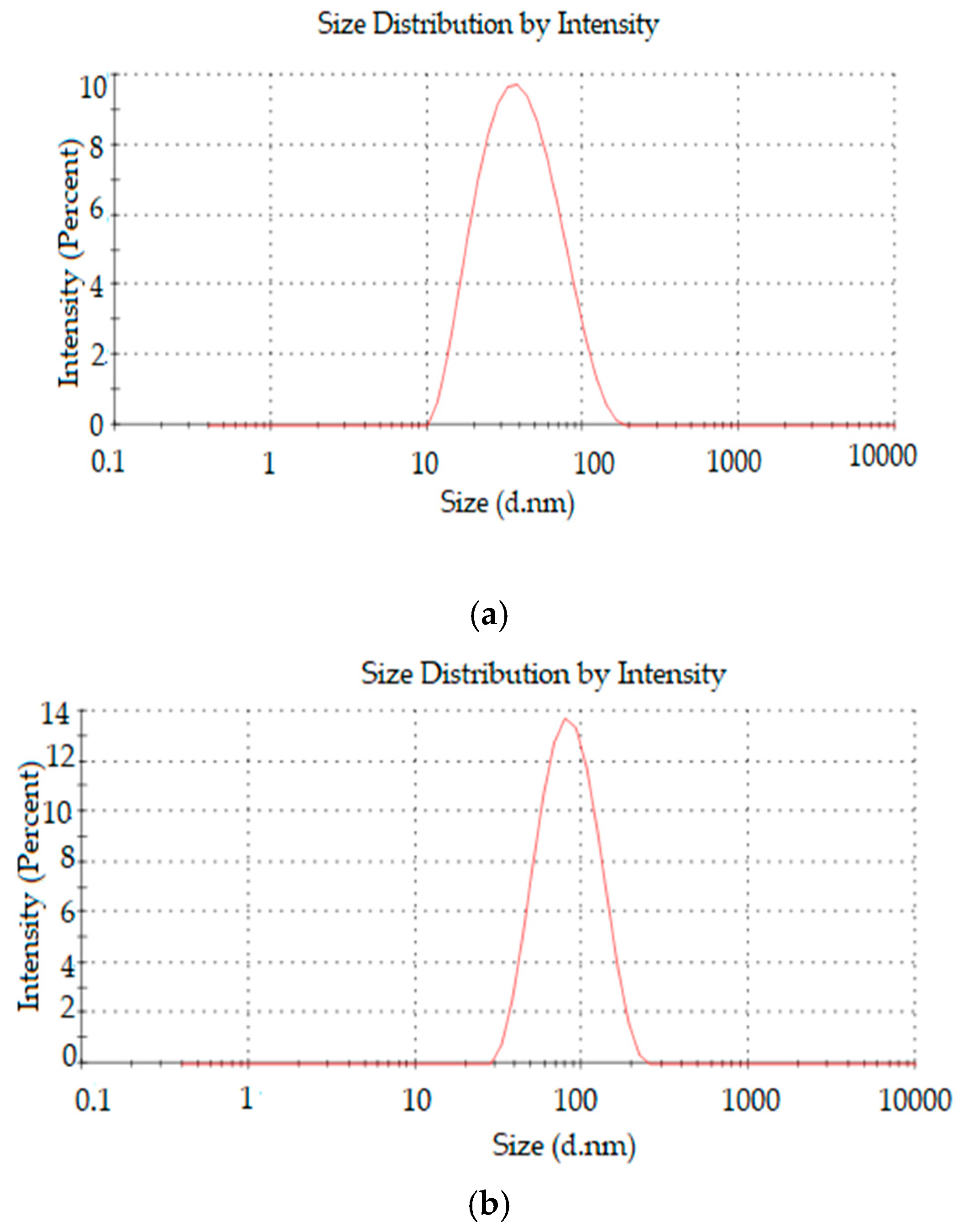
The high-pressure homogenization manufacturing process led to homogeneous, clear and transparent yellow-brownish dispersions. Particle size (Y1) analysis confirmed that it was possible to obtain dispersions below 100 nm over the experimental design space. Particle size was strongly influenced by TPGS and Ucuùba fat concentrations. The liquid lipid Capryol™ 90 influenced the particle size only in combination with TPGS (X1X2, p = 0.0092) probably because it acts as co-surfactant in this formulation.
In other studies, surfactant concentration was also a significant factor in decreasing particle size. Indeed, increasing the amount of surfactant in the system usually results in smaller particles until a saturation point is reached where the amount of surfactant does not affect the average particle size [
25,
52]. In particular, TPGS is a vitamin E derivative and a nonionic surfactant, it is able to stabilize nanoemulsions by lowering interfacial tension with steric repulsion between nanoparticles. As a consequence, bigger amounts of surfactant result in smaller particle size [
53]. Interestingly, TPGS has recently been shown to release tocopherol and tocopherol succinate by enzymatic cleavage of ester bonds [
54]. Alpha-tocopherol has been demonstrated to be able to increase amphotericin B activity against
Candida albicans [
55]. Therefore, the increase in surfactant concentration could not only contribute to particle size decrease, but high TPGS concentrations could result in enhanced antifungal activity of ketoconazole.
Although Capryol™ 90 and TPGS contributed to the decrease in particle size, Ucuùba fat acted in the opposite direction. It was observed, in this DoE, that Ucuùba fat content increase is always related to an increase in particle size (see Equation (6)). The physicochemical characteristics of Ucuùba fat can actually affect nanoparticles size. For example, the viscosity of the lipid phase influences the average particle size of the NLC during the pre-emulsion and homogenization stages at high pressure. The lower the viscosity of the two phases, the smaller the particles formed [
56]. An increase in the amount of Ucuùba fat in the lipid phase increases the viscosity of the system and causes greater resistance to the shear forces applied during homogenization, resulting in larger particles.
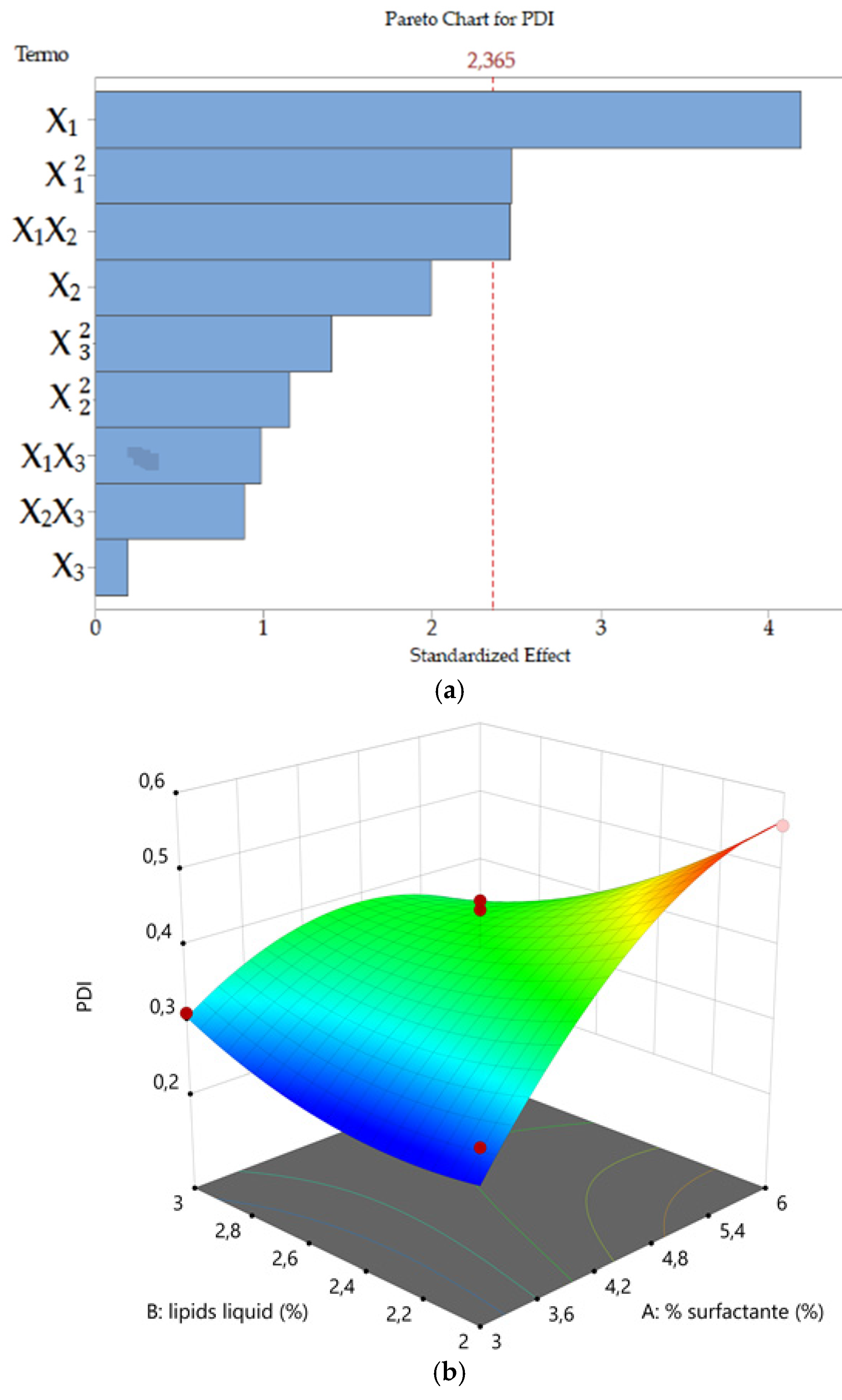
PDI (
Y2) values ranged from 0.248 to 0.558. This parameter reflects the homogeneity of the nanoparticle populations, values lower than 0.3 indicates relatively narrow particle size distribution and values even lower associated with monodisperse particles are generally desirable. On the contrary, higher values are associated with polydisperse particle size distribution [
57]. In our DoE, formulations with high surfactant concentration showed PDI values above 0.4 and multimodal size distribution. The correlation among surfactant concentration and increasing PDI value could be explained when considering that an excess of TPGS tends to self-assemble into micelles. Furthermore, it is possible that during the Ucuùba fat NLC preparation with high pressure homogenizer the temperature increase could have decreased the TPGS critical micelle concentration (CMC) and favored the production of different micelle populations leading to PDI increase [
29].
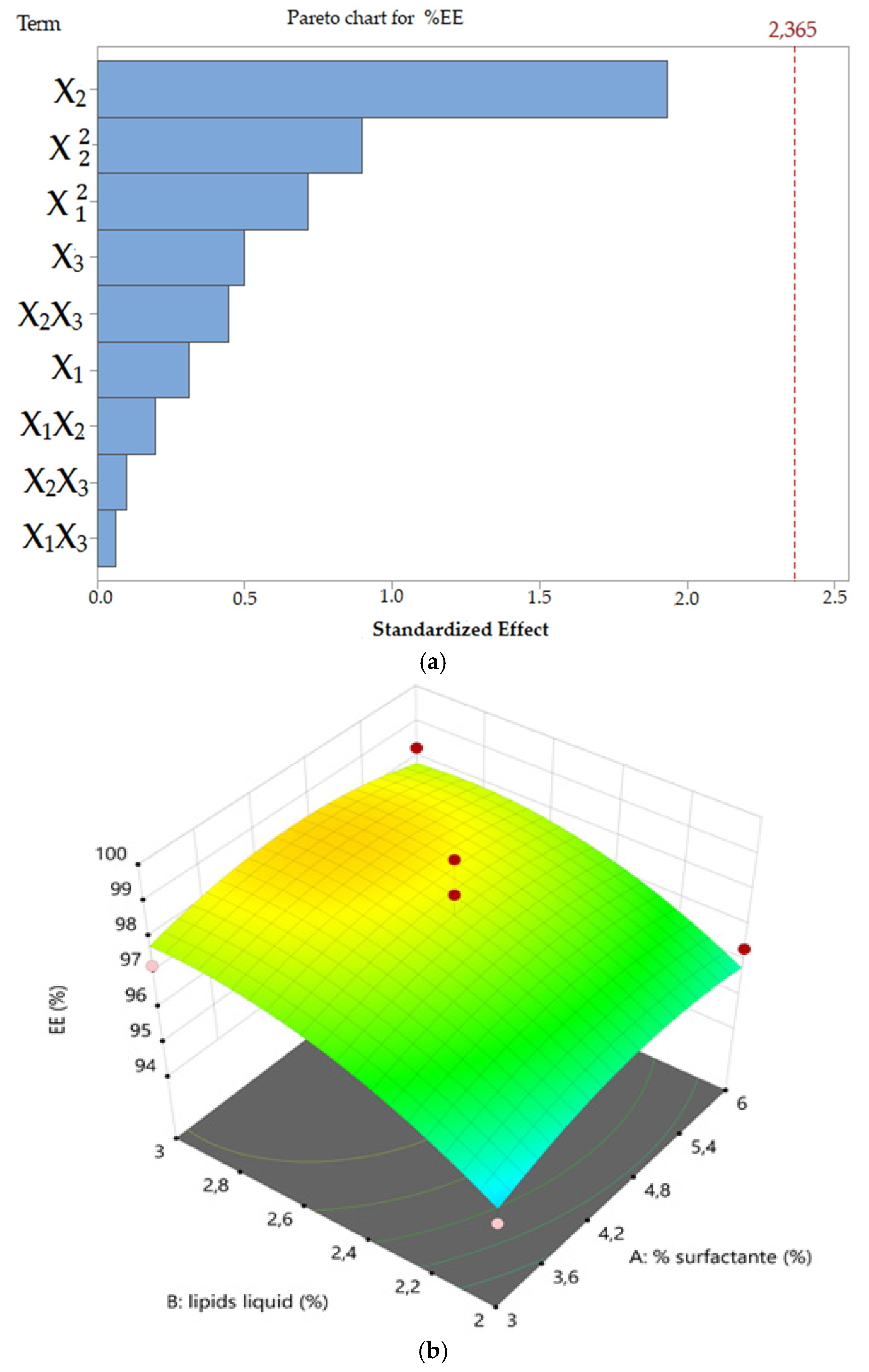
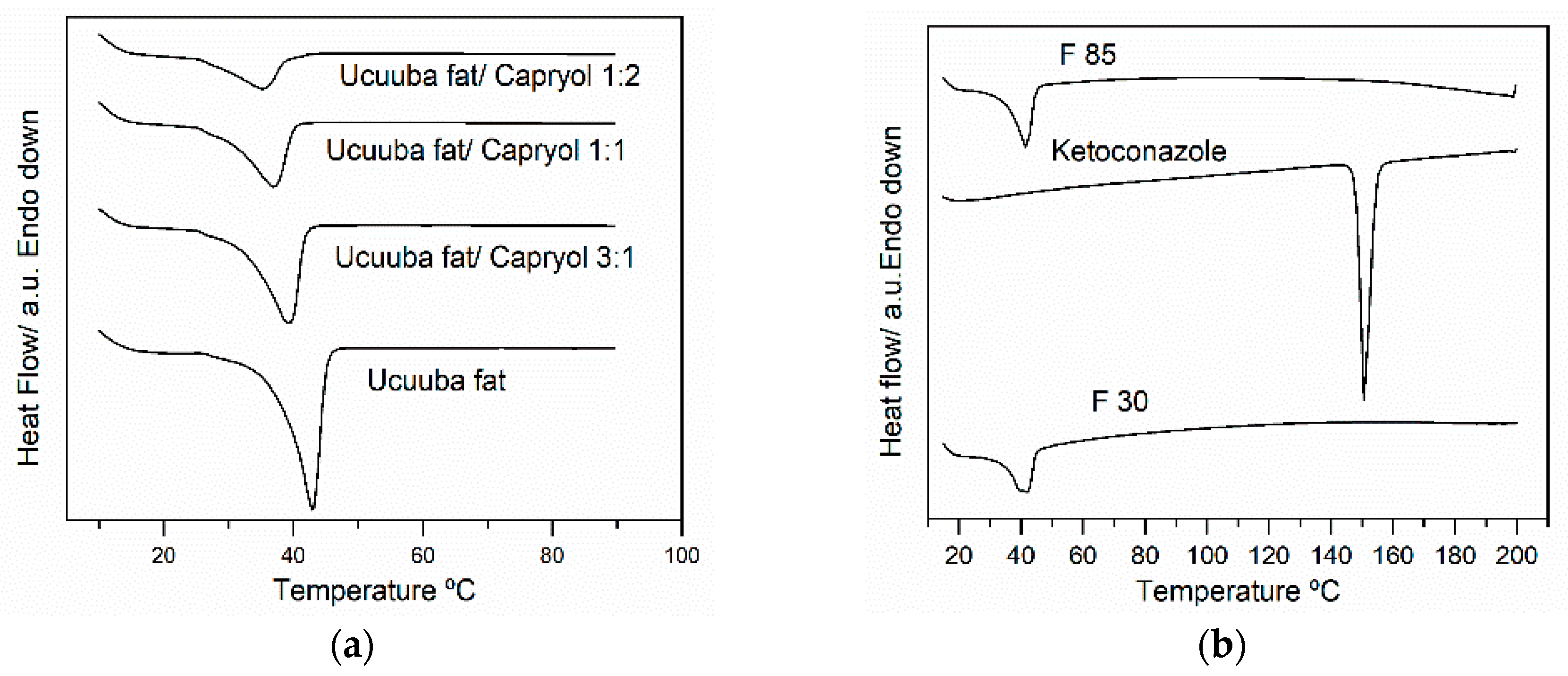
Concerning ketoconazole EE (
Y3) was found to range from 93.91 to 99.66%. In this study, the
EE was not found to be dependent on the process parameters explored. This result was probably obtained because the percentage of ketoconazole was maintained constant in all formulations (0.5%
w/
v ketoconazole) and Ucuùba fat NLC offered sufficient lipid matrix to fully solubilize and encapsulate the ketoconazole inside the nanoparticles. The lipid phase in NLC was a mixture of solid and liquid lipids and this peculiar feature is the reason for its higher encapsulation efficiency and better storage stability when compared to solid lipid nanoparticles (SLN) [
58]. In particular, the DSC thermograms of Ucuùba fat was found to be affected by the mixing with Capryol™ 90 and it was observed that increasing the ratio of Capryol™ 90 a broadening of the melting peak of the solid fat was observed, possibly indicating the formation of several polymorphic forms. Similarly, the effect of the addition of α-tocopherol on the crystalline organization of Compritol
® ATO 888, for NLC production was studied and it was observed that the addition of 30% α-tocopherol decreases the melting point of Compritol from 71 to 50.3 °C. The authors attributed this melting point decrease to the appearance of less organized polymorphic arrangements in the solid lipid, due to the addition of α-tocopherol [
59].
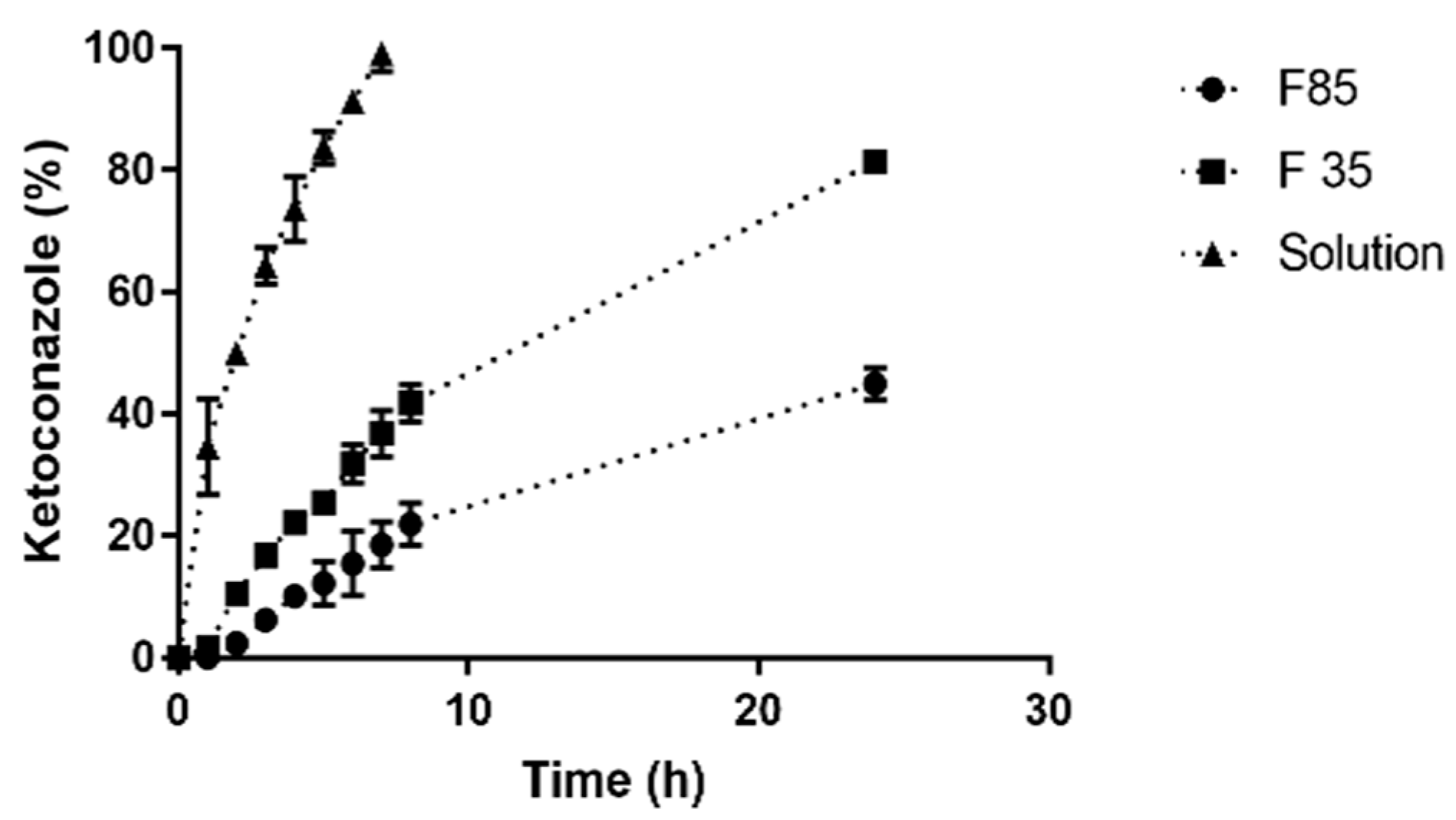
Drug release studies are routinely conducted to examine the ability of nanoparticles to modulate drug delivery. Ketoconazole release profile from F 30 and F 85, evidenced a faster release for the former formulation, suggesting that the size of the nanoparticle influences the rate of drug release by NLC. Small particles develop a larger surface area allowing for faster drug release [
30,
60]. Another factor at play probably was the lipid matrix crystallinity of the nanoparticles, since the higher fat content of Ucuùba fat in F 85 formulation together with low surfactant content determined a higher lipid matrix crystallinity index value for F 85 which may have contributed to the slower release of ketoconazole.
In general for NLC, the release of the drug is attributed to the components degradation, lipid matrix erosion or drug diffusion from nanoparticles [
60]. Sometimes NLCs present a release characterized by two phases: An immediate release (or burst release) followed by a sustained release [
60,
61]. In this study, it was observed that the release profile of the formulation F 85 does not present an immediate release, while for F 30 the situation was different. Using KinetDS software the drug release data were fitted using several kinetic models in order to better understand the mechanism of ketoconazole release for both F 30 and F 85 formulations. The release profile obtained for F 85 NLC was best fitted by Higuchi equation (
R2 = 0.9801), that usually describes the diffusion of the drug from homogenous and granular matrix systems [
62]. This suggests that ketoconazole release is mainly due to drug diffusion from the lipid matrix and partitioning between the lipid and the aqueous phase [
63]. Release data for F 30 NLC were fitted instead using the Weibull equation with lag time (
R2 = 0.9959). The release mechanism for F 30 NLC is then described best by an initial burst release phase, probably due to the released drug being adsorbed on the surface of nanoparticles, followed by a typical sustained drug-release behavior.
The measurements of lipid crystallinity and its modification are strongly connected with drug release and drug incorporation in lipid-based drug delivery systems. In order to study the lipid matrix crystalline organization, XRD is a widely used technique also for the investigation of SLN and NLC [
64]. The lipid polymorphism is a property that allows the triglycerides to exist in the form of several crystalline structures characterized by different molecular packing [
65]. The three most common types of structure for triglycerides are hexagonal, orthorhombic and triclinic, which are usually referred to as polymorphic forms α, β’and β, respectively. The type of crystalline form depends on the composition of the lipid mixture and on the molecular structure of each lipid, as well as on the environmental conditions during processing and storage (cooling rate, for example). For the Ucuùba fat-based NLC, the sharp peaks identified at 18.9°, 22.6° and 23.2°, correspond to short molecular spacings at 0.46, 0.393 and 0.382 nm, indicative of β and β’ structures, respectively [
65,
66].
Zeta potential values are often used to predict the stability of nanoparticles. In fact, this parameter reflects the charge of the surface of nanoparticles in suspensions and high zeta potential values indicate high repulsive forces between particles, preventing them from forming large particle size agglomerates [
10,
57,
67,
68]. In general, nanomaterials with zeta potential above 30 mV are considered physically stable [
34,
69]. However, in our formulations the zeta potential absolute values were found to be smaller than 30 mV. However, these systems contain a steric stabilizer, i.e., the non-ionic and PEGylated surfactant TPGS, and as a consequence, the absorption of the surfactant on nanoparticle surface decreases the zeta potential, due to the shift of the shear plane of the electrical double layer existing around each particle [
10]. However, the surfactant layer provides a steric contribution to particle stability, due to the presence of the hydrophilic PEG polymer chains [
47].