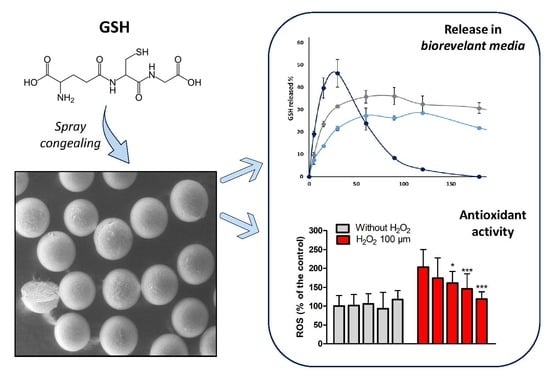
Glutathione-Loaded Solid Lipid Microparticles as Innovative Delivery System for Oral Antioxidant Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Solid Lipid Microparticles (SLMs)
2.3. HPLC Analysis of GSH
2.4. GSH-Loaded SLMs Characterization
2.4.1. Drug Content Determination
2.4.2. Morphological Analysis
2.4.3. Differential Scanning Calorimetry (DSC)
2.4.4. Hot Stage Microscopy (HSM) Analysis
2.4.5. Stability of GSH in Gastric and Intestinal pH
2.4.6. In Vitro Release Studies of SLMs in Simulated Intestinal Fluids
2.4.7. Measurement of DPPH Radical Scavenging Activity
2.5. Cell Culture
2.6. MTT Assay
2.7. Measurement of Intracellular ROS Levels
2.8. Statistical Analysis
3. Results and Discussion
3.1. GSH-Loaded SLMs Characterization
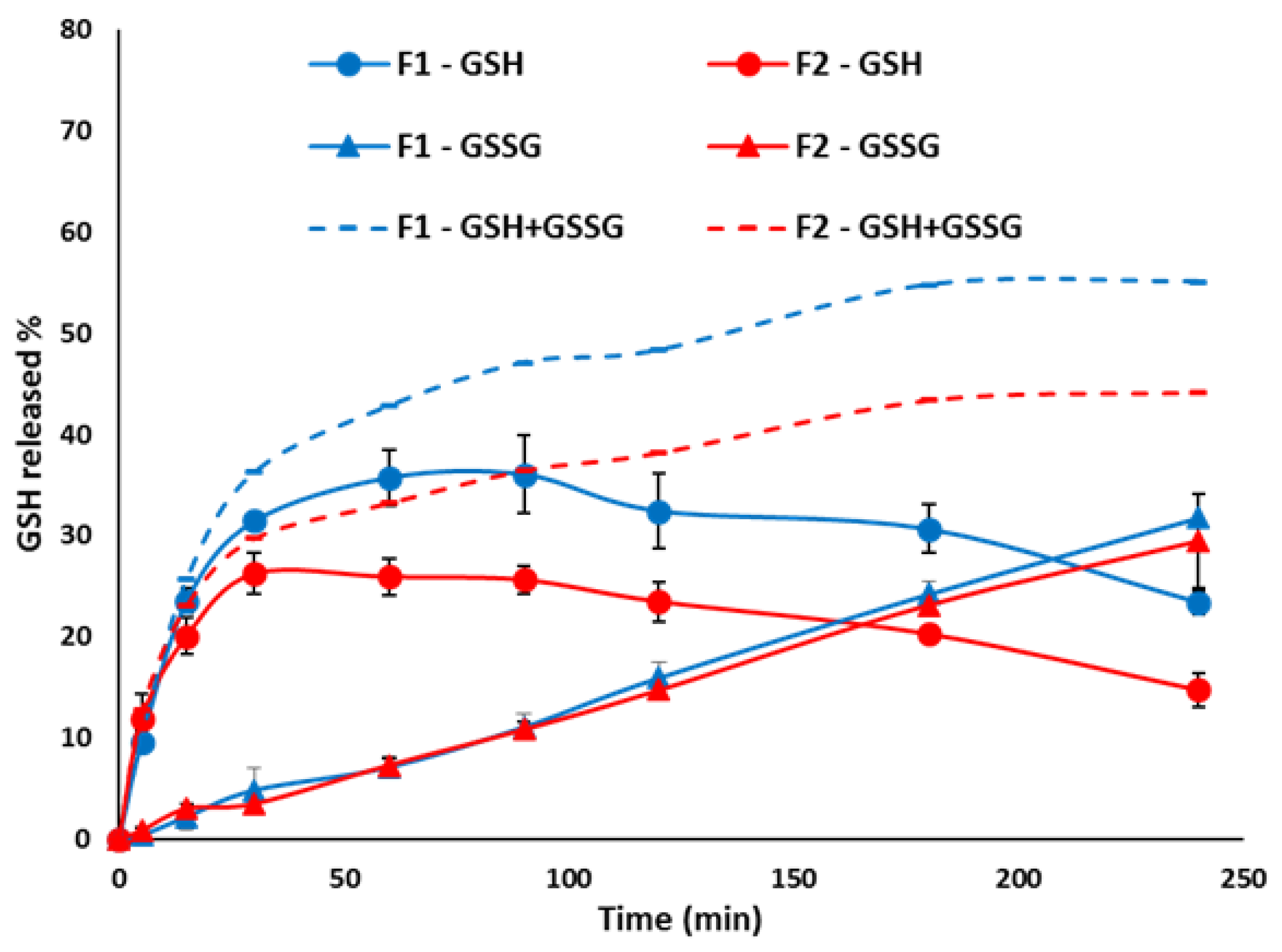
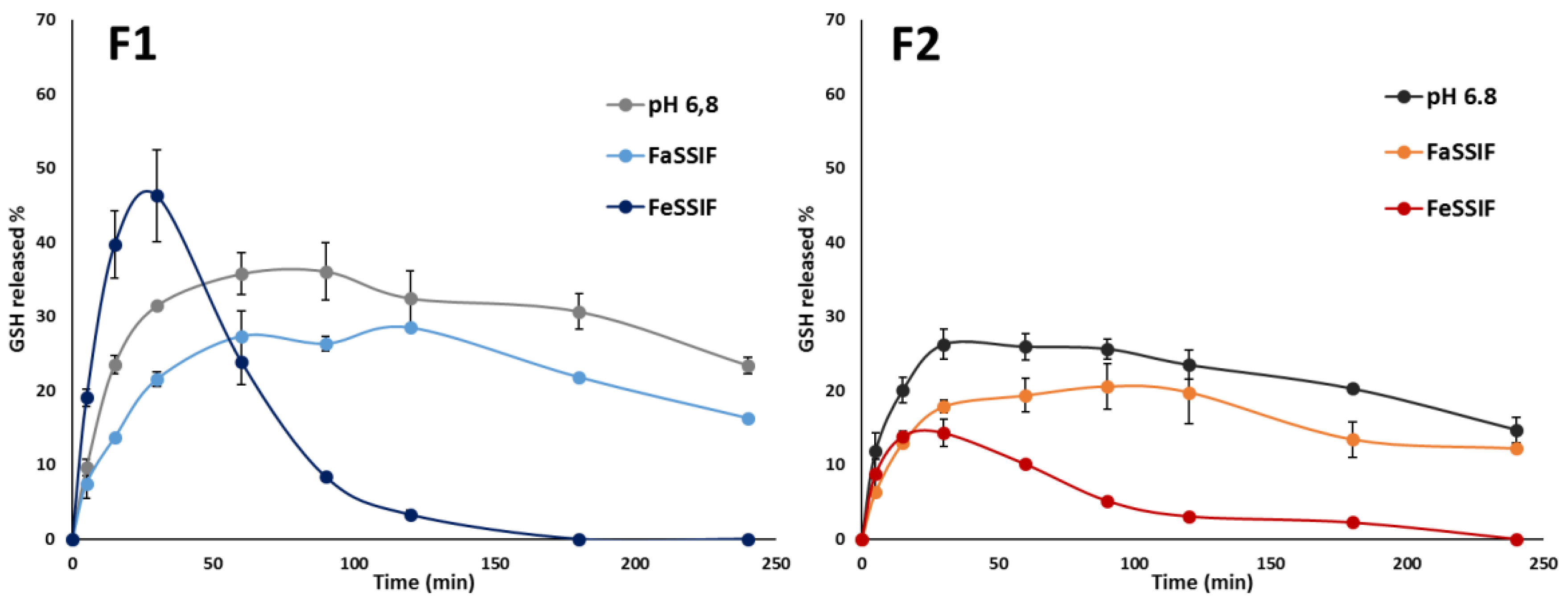
3.2. In Vitro Release Studies
3.3. Evaluation of Radical Scavenging Activity
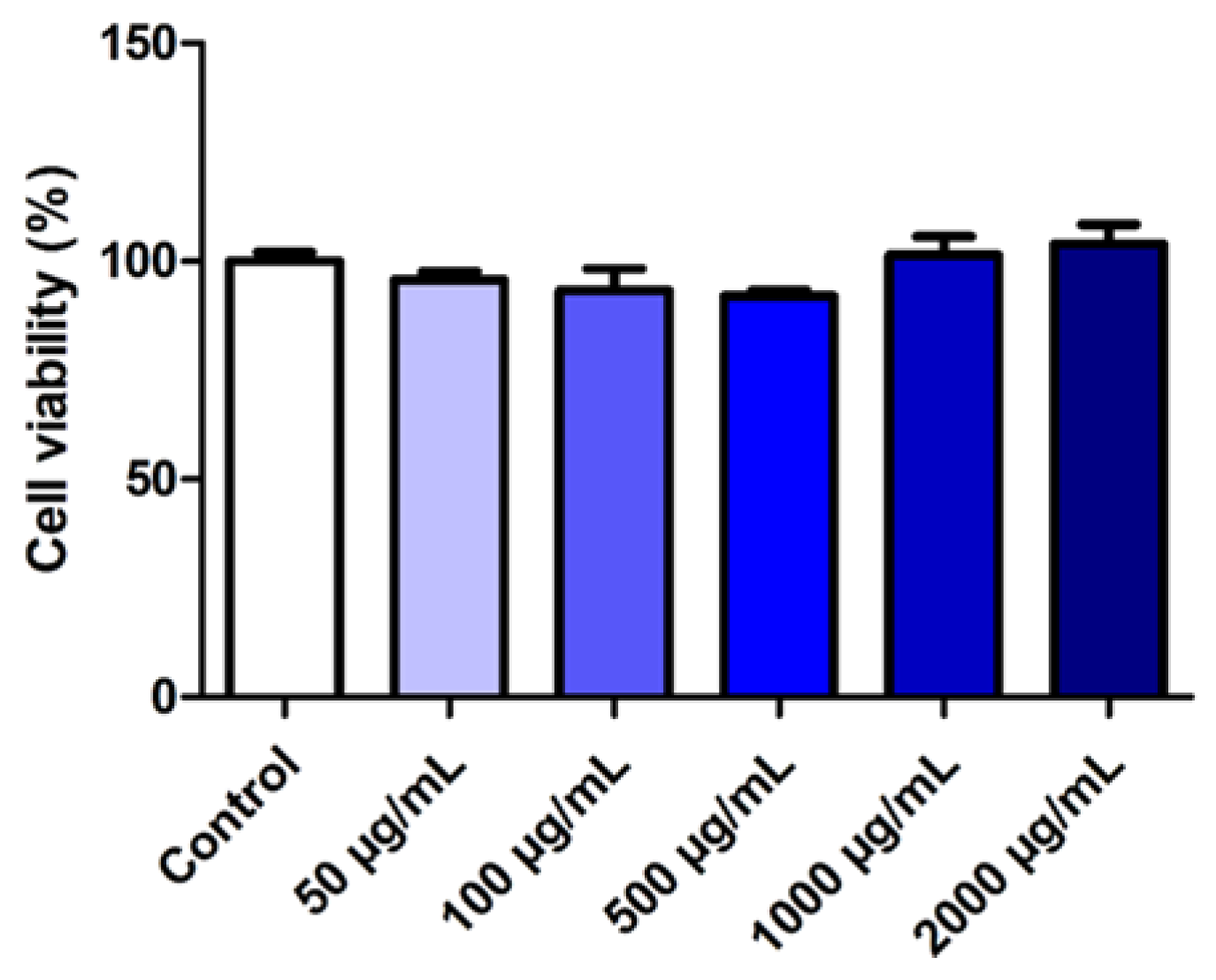
3.4. Evaluation of the Effect of SLMs on Cell Viability
3.5. Effect of GSH-Loaded SLMs on Intracellular ROS Levels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Kim, E.-H.; Hahm, K.B. Oxidative stress in inflammation-based gastrointestinal tract diseases: Challenges and opportunities. J. Gastroenterol. Hepatol. 2012, 27, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Moura, F.A.; de Andrade, K.Q.; dos Santos, J.C.F.; Araújo, O.R.P.; Goulart, M.O.F. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol. 2015, 6, 617–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Giustarini, D.; Galvagni, F.; Tesei, A.; Farolfi, A.; Zanoni, M.; Pignatta, S.; Milzani, A.; Marone, I.M.; Dalle-Donne, I.; Nassini, R.; et al. Glutathione, glutathione disulfide, and S-glutathionylated proteins in cell cultures. Free Radic. Biol. Med. 2015, 89, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Favilli, F.; Marraccini, P.; Iantomasi, T.; Vincenzini, M.T. Effect of orally administered glutathione on glutathione levels in some organs of rats: Role of specific transporters. Br. J. Nutr. 1997, 78, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Aw, T.Y.; Wierzbicka, G.; Jones, D.P. Oral glutathione increases tissue glutathione in vivo. Chem. Biol. Interact. 1991, 80, 89–97. [Google Scholar] [CrossRef]
- Sinha, R.; Sinha, I.; Calcagnotto, A.; Trushin, N.; Haley, J.S.; Schell, T.D.; Richie, J.P., Jr. Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function. Eur. J. Clin. Nutr. 2017, 72, 105–111. [Google Scholar] [CrossRef]
- Aw, T.Y.; Williams, M.W. Intestinal absorption and lymphatic transport of peroxidized lipids in rats: Effect of exogenous GSH. Am. J. Physiol. Liver Physiol. 1992, 263, G665–G672. [Google Scholar] [CrossRef]
- Kovacs-Nolan, J.; Rupa, P.; Matsui, T.; Tanaka, M.; Konishi, T.; Sauchi, Y.; Sato, K.; Ono, S.; Mine, Y. In vitro and ex vivo uptake of glutathione (GSH) across the intestinal epithelium and fate of oral GSH after in vivo supplementation. J. Agric. Food Chem. 2014, 62, 9499–9506. [Google Scholar] [CrossRef]
- Camera, E.; Picardo, M. Analytical methods to investigate glutathione and related compounds in biological and pathological processes. J. Chromatogr. B 2002, 781, 181–206. [Google Scholar] [CrossRef]
- Trapani, A.; Laquintana, V.; Denora, N.; Lopedota, A.; Cutrignelli, A.; Franco, M.; Trapani, G.; Liso, G. Eudragit RS 100 microparticles containing 2-hydroxypropyl-b-cyclodextrin and glutathione: Physicochemical characterization, drug release and transport studies. Eur. J. Pharm. Sci. 2007, 30, 64–74. [Google Scholar] [CrossRef]
- Denora, N.; Franco, Æ.M.; Trapani, Æ.G.; Liso, Æ.G. Effect of cyclodextrins on physico-chemical and release properties of Eudragit RS 100 microparticles containing glutathione. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 425–432. [Google Scholar]
- Trapani, A.; Lopedota, A.; Franco, M.; Cioffi, N.; Ieva, E.; Garcia-Fuentes, M.; Alonso, M.J. A comparative study of chitosan and chitosan/cyclodextrin nanoparticles as potential carriers for the oral delivery of small peptides. Eur. J. Pharm. Biopharm. 2010, 75, 26–32. [Google Scholar] [CrossRef]
- Bertoni, S.; Albertini, B.; Dolci, L.S.; Passerini, N. Spray congealed lipid microparticles for the local delivery of â-galactosidase to the small intestine. Eur. J. Pharm. Biopharm. 2018, 132, 1–10. [Google Scholar] [CrossRef]
- Christophersen, P.C.; Zhang, L.; Yang, M.; Nielsen, H.M.; Müllertz, A.; Mu, H. Solid lipid particles for oral delivery of peptide and protein drugs I—Elucidating the release mechanism of lysozyme during lipolysis. Eur. J. Pharm. Biopharm. 2013, 85, 473–480. [Google Scholar] [CrossRef]
- Bertoni, S.; Dolci, L.S.; Albertini, B.; Passerini, N. Spray congealing: A versatile technology for advanced drug delivery systems. Ther. Deliv. 2018, 9, 833–845. [Google Scholar] [CrossRef]
- Klein, S. The Use of Biorelevant Dissolution Media to Forecast the In Vivo Performance of a Drug. AAPS J. 2010, 12, 397–405. [Google Scholar] [CrossRef]
- Passerini, N.; Albertini, B.; Di, M.; Corace, G.; Luppi, B.; Canistro, D.; Vivarelli, F.; Cirillo, S.; Soleti, A.; Merizzi, G.; et al. Development of microparticles for oral administration of the non-conventional radical scavenger IAC and testing in an in flammatory rat model. Int. J. Pharm. 2016, 512, 126–136. [Google Scholar] [CrossRef]
- Themelis, T.; Gotti, R.; Gatti, R.J. A novel hydrophilic interaction liquid chromatography method for the determination of underivatized amino acids in alimentary supplements. J. Pharm. Biomed. Anal. 2017, 145, 751–757. [Google Scholar] [CrossRef]
- Jantratid, E.; Janssen, N.; Reppas, C.; Dressman, J.B. Dissolution media simulating conditions in the proximal human gastrointestinal tract: An update. Pharm. Res. 2008, 25, 1663–1676. [Google Scholar] [CrossRef]
- Newland, B.; Wolff, P.; Zhou, D.; Wang, W.; Zhang, H.; Rosser, A.; Wang, W.; Werner, C. Synthesis of ROS scavenging microspheres from a dopamine containing poly (â-amino ester) for applications for neurodegenerative disorders. Biomater. Sci. 2016, 4, 400–404. [Google Scholar] [CrossRef]
- Cole, S.P. Rapid chemosensitivity testing of human lung tumor cells using the MTT assay. Cancer Chemother. Pharmacol. 1986, 17, 259–263. [Google Scholar] [CrossRef]
- Shi, J.-J.; Gong, L.; Zhang, Y.-H.; Yang, P.; He, J. Microwave-assisted sonochemical synthesis of Cu and Mn doped GSH–ZnS polypeptide quantum dots and their enhanced photoelectrochemical properties. RSC Adv. 2016, 6, 109386–109393. [Google Scholar] [CrossRef]
- Sivasankaran, U.; Jesny, S.; Jose, A.R.; Girish Kumar, K. Fluorescence Determination of Glutathione Using Tissue Paper-derived Carbon Dots as Fluorophores. Anal. Sci. 2017, 33, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Hu, J.; Wu, D.; Yang, B.; Tao, Y.; Qin, Y.; Kong, Y. Smart construction of an efficient enantioselective sensing device based on bioactive tripeptide. Anal. Methods 2019, 11, 1951–1957. [Google Scholar] [CrossRef]
- Prajapati, J.B.; Katariya, H.; Patel, R. Peyer’e patch targeting of Isradipine loaded Solid Lipid Nanoparticles: It’s cellular uptake study. J. Drug Deliv. Sci. Technol. 2018, 43, 318–326. [Google Scholar] [CrossRef]
- Da Silva, E.; Bresson, S.; Rousseau, D. Characterization of the three major polymorphic forms and liquid state of tristearin by Raman spectroscopy. Chem. Phys. Lipids 2009, 157, 113–119. [Google Scholar] [CrossRef]
- Bunjes, H.; Westesen, K.; Koch, M.H.J. Crystallization tendency and polymorphic transitions in triglyceride nanoparticles. Int. J. Pharm. 1996, 129, 159–173. [Google Scholar] [CrossRef]
- Mirzahosseini, A.; Noszál, B. Species-Specific Standard Redox Potential of Thiol-Disulfide Systems: A Key Parameter to Develop Agents against Oxidative Stress. Sci. Rep. 2016, 6, 37596. [Google Scholar] [CrossRef] [Green Version]
- Kutscher, H.; Stein, S.; Sinko, P. Nonenzymatic, Self-Elimination Degradation Mechanism of Glutathione. Chem. Biodivers. 2009, 6, 527–539. [Google Scholar]
- Dalle–Donne, I.; Milzani, A.; Gagliano, N.; Colombo, R.; Giustarini, D.; Rossi, R. Molecular Mechanisms and Potential Clinical Significance of S-Glutathionylation. Antioxid. Redox Signal. 2007, 10, 445–474. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Jantratid, E.; Janssen, N.; Chokshi, H.; Tang, K.; Dressman, J.B. Designing biorelevant dissolution tests for lipid formulations: Case example—Lipid suspension of RZ-50. Eur. J. Pharm. Biopharm. 2008, 69, 776–785. [Google Scholar] [CrossRef]
- Albertini, B.; Bertoni, S.; Perissutti, B.; Passerini, N. An investigation into the release behavior of solid lipid microparticles in different simulated gastrointestinal fluids. Colloids Surf. B Biointerfaces 2019, 173, 276–285. [Google Scholar] [CrossRef]
- Pompella, A.; Visvikis, A.; Paolicchi, A.; De Tata, V.; Casini, A.F. The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol. 2003, 66, 1499–1503. [Google Scholar] [CrossRef]
- Simmonds, N.J.; Allen, R.E.; Stevens, T.R.J.; Niall, R.; Van Someren, M.; Blake, D.R.; Rampton, D.S. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology 1992, 103, 186–196. [Google Scholar] [CrossRef]
- Lih-Brody, L.; Powell, S.R.; Collier, K.P.; Reddy, G.M.; Cerchia, R.; Kahn, E.; Weissman, G.S.; Katz, S.; Floyd, R.A.; McKinley, M.J.; et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig. Dis. Sci. 1996, 41, 2078–2086. [Google Scholar] [CrossRef]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I. HT29 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Calinescu, C.; Mondovi, B.; Federico, R.; Ispas-Szabo, P.; Mateescu, M.A. Carboxymethyl starch: Chitosan monolithic matrices containing diamine oxidase and catalase for intestinal delivery. Int. J. Pharm. 2012, 428, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Tamma, G.; Valenti, G.; Grossini, E.; Donnini, S.; Marino, A.; Marinelli, R.A.; Calamita, G. Aquaporin Membrane Channels in Oxidative Stress, Cell Signaling, and Aging: Recent Advances and Research Trends. Oxid. Med. Cell. Longev. 2018, 2018, 1501847. [Google Scholar] [CrossRef]
- Prata, C.; Hrelia, S.; Fiorentini, D. Peroxiporins in Cancer. Int. J. Mol. Sci. 2019, 20, 1371. [Google Scholar] [CrossRef]








| SLMs | Constituents (%, w/w) | Drug Loading (%, w/w) | ||
|---|---|---|---|---|
| Dynasan 114 | Dynasan 118 | API | GSH | |
| F1 | 95.0 | - | 5.0 | 5.09 ± 0.24 |
| F2 | 48.5 | 48.5 | 5.18 ± 0.12 | |
| F3 | 80.0 | - | 20.0 | 21.66 ± 0.29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertoni, S.; Albertini, B.; Facchini, C.; Prata, C.; Passerini, N. Glutathione-Loaded Solid Lipid Microparticles as Innovative Delivery System for Oral Antioxidant Therapy. Pharmaceutics 2019, 11, 364. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11080364
Bertoni S, Albertini B, Facchini C, Prata C, Passerini N. Glutathione-Loaded Solid Lipid Microparticles as Innovative Delivery System for Oral Antioxidant Therapy. Pharmaceutics. 2019; 11(8):364. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11080364
Chicago/Turabian StyleBertoni, Serena, Beatrice Albertini, Carlotta Facchini, Cecilia Prata, and Nadia Passerini. 2019. "Glutathione-Loaded Solid Lipid Microparticles as Innovative Delivery System for Oral Antioxidant Therapy" Pharmaceutics 11, no. 8: 364. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11080364