Nanoemulsion Structural Design in Co-Encapsulation of Hybrid Multifunctional Agents: Influence of the Smart PLGA Polymers on the Nanosystem-Enhanced Delivery and Electro-Photodynamic Treatment
Abstract
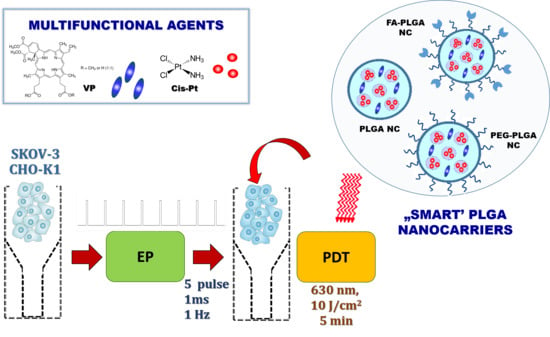
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Co-Encapsulation of Hybrid Agents in Polymeric Nanocarriers by Double Emulsion (w/o/w) Solvent Evaporation Method
2.3. Nanocarrier Size, Polydispersity, and Particle Charge
2.4. Shape and Morphology
2.5. Encapsulation Efficiency
2.6. Colloidal Stability
2.7. Cell Lines
2.8. Uptake of Encapsulated Hybrid Cargo—Flow Cytometry Analysis
2.9. Electroporation Protocol
2.10. Intracellular Internalization Studies by Confocal Microscopy
2.11. Photodynamic Activity Protocol
2.12. Statistical Analysis
3. Results and Discussion
3.1. Characteristic of “Smart” PLGA Nanocarriers Obtained by Nanoemulsion Structural Design
3.2. Evaluation of Colloidal Stability
3.3. Cellular Internalization—Flow Cytometry and Confocal Microscopy Evaluation
3.4. Evaluation of PDT and EP-PDT
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nanoemulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Bazylińska, U.; Kulbacka, J.; Wilk, K.A. Dicephalic ionic surfactants in fabrication of biocompatiblenanoemulsions: Factors influencing droplet size and stability. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 312–320. [Google Scholar] [CrossRef]
- Fornaguera, C.; Feiner-Gracia, N.; Calderó, N.G.; García-Celma, M.J.; Solans, C. PLGA nanoparticles from nano-emulsion templating as imaging agents: Versatile technology to obtain nanoparticles loaded with fluorescent dyes. Colloids Surf. B Biointerfaces 2016, 147, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Fornaguera, C.; Dols-Perez, A.; Calderó, N.G.; García-Celma, M.J.; Camarasa, J.; Solans, C. PLGA nanoparticles prepared by nano-emulsion templating using low-energy methods as efficient nanocarriers for drug delivery across the blood–brain barrier. J. Control. Release 2015, 211, 134–143. [Google Scholar] [CrossRef]
- Bazylińska, U.; Saczko, J. Nanoemulsion-templated polylelectrolyte multifunctional nanocapsules for DNA entrapment and bioimaging. Colloids Surf. B Biointerfaces 2016, 137, 191–202. [Google Scholar] [CrossRef]
- Bazylińska, U. Rationally designed double emulsion process for co-encapsulation of hybrid cargo in stealth nanocarriers. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 476–482. [Google Scholar] [CrossRef]
- McClements, J.D. Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr. Opin. Colloid Interface Sci. 2012, 17, 235–245. [Google Scholar] [CrossRef]
- Ahmed, N.; Michelin-Jamois, M.; Fessi, H.; Elaissari, A. Modified double emulsion process as a new route to prepare submicron biodegradable magnetic/polycaprolactone particles for in vivo theranostics. Soft Matter 2012, 8, 2554–2564. [Google Scholar] [CrossRef]
- Salvadori, C.; Svara, T.; Rocchigiani, G.; Millanta, F.; Pavlin, D.; Cemazar, M.; Lampreht, U.; Sersa, T.G.; Tozon, N.; Poli, A. Effects of electrochemotherapy with cisplatin and peritumoral IL-12 gene electrotransfer on canine mast cell tumors: A histopathologic and immunohistochemical study. Radiol. Oncol. 2017, 51, 286–294. [Google Scholar] [CrossRef]
- Labanauskiene, J.; Gehl, J.; Didziapetriene, J. Evaluation of cytotoxic effect of photodynamic therapy in combination with electroporation in vitro. Bioelectrochemistry 2007, 70, 78–82. [Google Scholar] [CrossRef]
- Kulbacka, J.; Kotulska, M.; Rembiałkowska, N.; Choromańska, A.; Kamińska, I.; Garbiec, A.; Rossowska, J.; Daczewska, M.; Jachimska, B.; Saczko, J. Cellular stress induced by photodynamic reaction with CoTPPS and MnTMPyPCl5 in combination with electroporation in human colon adenocarcinoma cell lines (LoVo and LoVoDX). Cell Stress Chaperones 2013, 18, 719–731. [Google Scholar] [CrossRef] [Green Version]
- Zielichowska, A.; Saczko, J.; Garbiec, A.; Dubińska-Magiera, M.; Rossowska, J.; Surowiak, P.; Choromańska, A.; Daczewska, M.; Kulbacka, J.; Lage, H. The photodynamic effect of far-red range phthalocyanines (AlPc and Pc green) supported by electropermeabilization in human gastric adenocarcinoma cells of sensitive and resistant type. Biomed. Pharmacother. 2015, 69, 145–152. [Google Scholar] [CrossRef]
- Kulbacka, J.; Pucek, A.; Wilk, K.A.; Dubińska-Magiera, M.; Rossowska, J.; Kulbacki, M.; Kotulska, M. The Effect of millisecond pulsed electric fields (msPEF) on intracellular drug transport with negatively charged large nanocarriers made of solid lipid nanoparticles (SLN): In vitro study. J. Membr. Biol. 2016, 249, 645–661. [Google Scholar] [CrossRef]
- Kulbacka, J.; Pucek, A.; Kotulska, M.; Dubińska-Magiera, M.; Rossowska, J.; Rols, M.P.; Wilk, K.A. Electroporation and lipid nanoparticles with cyanine IR-780 and flavonoids as efficient vectors to enhanced drug delivery in colon cancer. Bioelectrochemistry 2016, 110, 19–31. [Google Scholar] [CrossRef]
- Mutaliyeva, B.; Grigoriev, D.; Madybekova, G.; Sharipova, A.; Aidarova, S.; Saparbekova, S.; Miller, R. Microencapsulation of insulin and its release using w/o/w double emulsion method. Colloids Surf. A Physicochem. Eng. Asp. 2017, 521, 147–152. [Google Scholar] [CrossRef]
- Thompson, K.L.; Mable, C.J.; Lane, J.A.; Derry, M.J.; Fielding, L.A.; Armes, S.P. Preparation of pickering double emulsions using block copolymer worms. Langmuir 2015, 31, 4137–4144. [Google Scholar] [CrossRef]
- Chen, S.; Yang, K.; Tuguntaev, R.G.; Mozhi, A.; Zhang, J.; Wang, P.C.; Liang, X.J. Targeting tumor microenvironment with PEG-based amphiphilic nanoparticles to overcome chemoresistance. Nanomedicine 2016, 12, 269–286. [Google Scholar] [CrossRef]
- Kommineni, N.; Mahira, S.; Domb, A.D.; Khan, W. Cabazitaxel-loaded nanocarriers for cancer therapy with reduced side effects. Pharmaceutics 2019, 11, 141. [Google Scholar] [CrossRef]
- Jang, C.; Lee, J.H.; Sahu, A.; Tae, G. The synergistic effect of folate and RGD dual ligand of nanographene oxide on tumor targeting and photothermal therapy in vivo. Nanoscale 2015, 7, 18584–18594. [Google Scholar] [CrossRef]
- Bazylińska, U.; Wawrzyńczyk, D. Encapsulation of TOPO stabilized NaYF4:Er3+,Yb3+ nanoparticles in biocompatible nanocarriers: Synthesis, optical properties and colloidal stability. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 556–563. [Google Scholar] [CrossRef]
- Bazylińska, U.; Zielińska, K.; Saczko, J.; Wilk, K.A. Novel multilayer IR-786-loaded nanocarriers for intracellular delivering: Characterization, imaging, and internalization in human cancer cell lines. Chem. Lett. 2012, 41, 1354–1356. [Google Scholar] [CrossRef]
- Gamper, N.; Stockand, J.D.; Shapiro, M.S. The use of chinese hamster ovary (CHO) cells in the study of ion channels. J. Pharmacol. Toxicol. Methods 2005, 51, 177–185. [Google Scholar] [CrossRef]
- Méndez-Vilas, A.; Solano, A. (Eds.) State of the art of smart polymers: From fundamentals to final applications. In Polymer Science: Research Advances, Practical Applications and Educational Aspects, 1th ed.; Formatex Reserach Center: Extremadura, Spain, 2016; pp. 476–487. [Google Scholar]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Poly (lactic-co-glycolide) fiber: An Overview. J. Eng. Fibers Fabr. 2014, 47, 47–66. [Google Scholar]
- Callaghan, R.; Luk, F.; Bebawy, M. Inhibition of the multidrug resistance P-glycoprotein: Time for a change of strategy? Drug Metab. Dispos. 2014, 42, 623–631. [Google Scholar] [CrossRef]
- Chen, B.; Le, W.; Wang, Y.; Li, Z.; Wang, D.; Ren, L.; Lin, L.; Cui, S.; Hu, J.J.; Hu, Y.; et al. Targeting negative surface charges of cancer cells by multifunctional nanoprobes. Theranostics 2016, 6, 1887–1898. [Google Scholar] [CrossRef]
- Gutiérrez-Valenzuela, C.A.; Guerrero-Germán, P.; Tejeda-Mansir, A.; Esquivel, R.; Guzmán-Z, R.; Lucero-Acuña, A. Folate functionalized PLGA nanoparticles loaded with plasmid pVAX1-NH36: Mathematical analysis of release. Appl. Sci. 2016, 6, 364. [Google Scholar] [CrossRef]
- Lin, P.C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef]
- Zyuzin, M.V.; Honold, T.; Carregal-Romero, S.; Kantner, K.; Karg, M. Influence of temperature on the colloidal stability of polymer-coated gold nanoparticles in cell culture media. Small 2016, 12, 1723–1731. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Trzcinska, R.; Trzebicka, B.; Müller, A.H.E.; Dworak, A. Loading of polymer nanocarriers: Factors, mechanisms and applications. Prog. Polym. Sci. 2014, 39, 43–86. [Google Scholar] [CrossRef]
- Van der Steen, S.C.; Raavé, R.; Langerak, S.; Van Houdt, L.; Van Duijnhoven, S.M.; Van Lith, S.A.; Massuger, L.F.; Daamen, W.F.; Leenders, W.P.; Van Kuppevelt, T.H. Targeting the extracellular matrix of ovarian cancer using functionalized, drug loaded lyophilisomes. Eur. J. Pharm. Biopharm. 2017, 113, 229–239. [Google Scholar] [CrossRef]
- Siwowska, K.; Schmid, R.M.; Cohrs, S.; Schibli, R.; Müller, C. Folate receptor-positive gynecological cancer cells: In vitro and in vivo characterization. Pharmaceuticals 2017, 10, 72. [Google Scholar] [CrossRef]
- Quarta, A.; Bernareggi, D.; Benigni, F.; Luison, E.; Nano, G.; Nitti, S.; Cesta, M.C.; Di Ciccio, L.; Canevari, S.; Pellegrino, T.; et al. Targeting FR-expressing cells in ovarian cancer with Fab-functionalized nanoparticles: A full study to provide the proof of principle from in vitro to in vivo. Nanoscale 2015, 7, 2336–2351. [Google Scholar] [CrossRef]
- Lange, C.; Bednarski, P.J. Evaluation for synergistic effects by combinations of photodynamic therapy (PDT) with temoporfin (mTHPC) and Pt(II) complexes carboplatin, cisplatin or oxaliplatin in a set of five human cancer cell lines. Int. J. Mol. Sci. 2018, 19, 3183. [Google Scholar] [CrossRef]







| System | Composition | DH [nm] | PDI | ζ [mV] | EECisPt | EEVP |
|---|---|---|---|---|---|---|
| V1 | NCs-PLGA + VP + CisPt | 193 ± 6 | 0.16 ± 0.01 | −9 ± 1 | 92 ± 1 | 97 ± 3 |
| V2 | NCs-PLGA-PEG + VP + CisPt | 187 ± 5 | 0.12 ± 0.01 | −4 ± 1 | 88 ± 1 | 92 ± 1 |
| V3 | NCs-PLGA-FA + VP + CisPt | 200 ± 7 | 0.20 ± 0.02 | −15 ± 2 | 90 ± 2 | 95 ± 3 |
| V4 | NCs-PLGA-FA + VP | 197 ± 7 | 0.22 ± 0.02 | −16 ± 2 | - | 96 ± 3 |
| V5 | NCs-PLGA-FA + CisPt | 194 ± 6 | 0.25 ± 0.02 | −16 ± 2 | 92 ± 2 | - |
| V6 | NCs-PLGA-FA empty | 189 ± 5 | 0.10 ± 0.01 | −17 ± 3 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazylińska, U.; Kulbacka, J.; Chodaczek, G. Nanoemulsion Structural Design in Co-Encapsulation of Hybrid Multifunctional Agents: Influence of the Smart PLGA Polymers on the Nanosystem-Enhanced Delivery and Electro-Photodynamic Treatment. Pharmaceutics 2019, 11, 405. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11080405
Bazylińska U, Kulbacka J, Chodaczek G. Nanoemulsion Structural Design in Co-Encapsulation of Hybrid Multifunctional Agents: Influence of the Smart PLGA Polymers on the Nanosystem-Enhanced Delivery and Electro-Photodynamic Treatment. Pharmaceutics. 2019; 11(8):405. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11080405
Chicago/Turabian StyleBazylińska, Urszula, Julita Kulbacka, and Grzegorz Chodaczek. 2019. "Nanoemulsion Structural Design in Co-Encapsulation of Hybrid Multifunctional Agents: Influence of the Smart PLGA Polymers on the Nanosystem-Enhanced Delivery and Electro-Photodynamic Treatment" Pharmaceutics 11, no. 8: 405. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11080405