Rational Design of a Multipurpose Bioadhesive Vaginal Film for Co-Delivery of Dapivirine and Levonorgestrel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis and Characterization of Thiomer
2.2.2. Mucin and Polymer Interaction
2.2.3. Tissue Toxicity of Thiomer
2.2.4. High-Performance Liquid Chromatography (HPLC) for Simultaneous Detection of DPV and LNG
2.2.5. Liquid Chromatography-Mass Spectrometry/Mass Spectrometry (LC-MS/MS) for the Detection of Bioanalytical Samples
2.2.6. Film Formulation Development and Physicochemical Characterization
2.2.7. In Vitro Dissolution Test
2.2.8. Ex Vivo Tissue Mucoadhesion
2.2.9. In Vivo Evaluations in Macaques
Film Retention and In Vivo Drug Release
Film Distribution in Genital Tract
Assessment of DPV/LNG Combination Film in Macaques
2.2.10. Statistical Analysis
3. Results
3.1. Thiolation Degree Determination in Thiomers
3.2. Mechanism of Polymer and Mucin Interaction
3.3. Tissue Toxicity of Thiomers
3.4. Film Physicochemical Characterizations
3.5. In Vitro Drug Dissolution
3.6. Ex Vivo Tissue Mucoadhesiveness
3.7. In Vivo Retention and Safety of Bioadhesive Films in Macaque Genital Tract
3.8. Distribution of Bioadhesive Films in Macaque Genital Tract
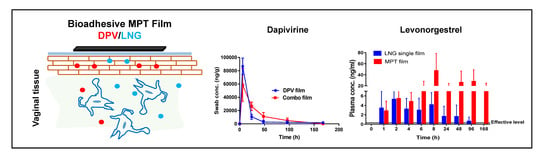
3.9. Local and Systemic PK of DPV/LNG Bioadhesive Film
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Unintended Pregnancy Rates Declined Globally from 1990 to 2014. Available online: https://www.guttmacher.org/news-release/2018/unintended-pregnancy-rates-declined-globally-1990-2014 (accessed on 5 March 2018).
- Bearak, J.; Popinchalk, A.; Alkema, L.; Sedgh, G. Global, regional, and subregional trends in unintended pregnancy and its outcomes from 1990 to 2014: Estimates from a Bayesian hierarchical model. Lancet Glob. Health 2018, 6, e380–e389. [Google Scholar] [CrossRef] [Green Version]
- UNAIDS DATA 2017. Available online: http://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf (accessed on 30 October 2019).
- Kikuchi, K.; Wakasugi, N.; Poudel, K.C.; Sakisaka, K.; Jimba, M. High rate of unintended pregnancies after knowing of HIV infection among HIV positive women under antiretroviral treatment in Kigali, Rwanda. Biosci. Trends 2011, 5, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodsong, C.; Musara, P.; Chandipwisa, A.; Montgomery, E.; Alleman, P.; Chirenje, M.; Chipato, T.; Martinson, F.; Hoffman, I. Interest in multipurpose prevention of HIV and pregnancy: Perspectives of women, men, health professionals and community stakeholders in two vaginal gel studies in southern Africa. BJOG Int. J. Obstet. Gynaecol. 2014, 121 (Suppl. S5), 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, G.S. Conventional methods of contraception: Condom, diaphragm, and vaginal foam. Clin. Obs. Gynecol. 1974, 17, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Bajos, N.; Warszawski, J.; Ducot, B.; Spira, A. Should condom use be promoted for contraception to prevent transmission of sexual transmitted diseases and AIDS? ACSF Group. French National Survey of Sexual behavior. Rev. Epidemiol. Sante Publique 1998, 46, 391–397. [Google Scholar]
- Van de Perre, P.; Jacobs, D.; Sprecher-Goldberger, S. The latex condom, an efficient barrier against sexual transmission of AIDS-related viruses. AIDS 1987, 1, 49–52. [Google Scholar]
- Njoroge, B.; Gallo, M.F.; Sharma, A.; Bukusi, E.A.; Nguti, R.; Bell, A.J.; Jamieson, D.J.; Williams, D.; Eschenbach, D.A. Diaphragm for STI and HIV prevention: Is it a safe method for women at high risk? Sex. Transm. Dis. 2010, 37, 382–385. [Google Scholar] [CrossRef] [Green Version]
- Bird, S.T.; Harvey, S.M.; Maher, J.E.; Beckman, L.J. Acceptability of an existing, female-controlled contraceptive method that could potentially protect against HIV: A comparison of diaphragm users and other method users. Women’s Health Issues 2004, 14, 85–93. [Google Scholar] [CrossRef]
- Fitch, J.T.; Stine, C.; Hager, W.D.; Mann, J.; Adam, M.B.; McIlhaney, J. Condom effectiveness: Factors that influence risk reduction. Sex. Transm. Dis. 2002, 29, 811–817. [Google Scholar] [CrossRef]
- A Multipurpose Prevention Ring for Women’s Sexual and Reproductive Health; International Partnership for Microbicides: Silver Spring, MD, USA, 2018; Available online: https://www.ipmglobal.org/our-work/our-products/dapivirine-contraceptive-ring. (accessed on 16 December 2019).
- Baeten, J.M.; Palanee-Phillips, T.; Brown, E.R.; Schwartz, K.; Soto-Torres, L.E.; Govender, V.; Mgodi, N.M.; Matovu Kiweewa, F.; Nair, G.; Mhlanga, F.; et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. New Engl. J. Med. 2016, 375, 2121–2132. [Google Scholar] [CrossRef]
- Brache, V.; Faundes, A. Contraceptive vaginal rings: A review. Contraception 2010, 82, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, E.; Fraser, I.S.; Lacarra, M.; Mishell, D.R., Jr.; Jackanicz, T. Effect of different insertion regimens on side effects with a combination contraceptive vaginal ring. Contraception 1997, 56, 233–239. [Google Scholar] [CrossRef]
- Novak, A.; de la Loge, C.; Abetz, L.; van der Meulen, E.A. The combined contraceptive vaginal ring, NuvaRing: An international study of user acceptability. Contraception 2003, 67, 187–194. [Google Scholar] [CrossRef]
- Roumen, F.J.; Dieben, T.O. Clinical acceptability of an ethylene-vinyl-acetate nonmedicated vaginal ring. Contraception 1999, 59, 59–62. [Google Scholar] [CrossRef]
- Gong, T.; Zhang, W.; Parniak, M.A.; Graebing, P.W.; Moncla, B.; Gupta, P.; Empey, K.M.; Rohan, L.C. Preformulation and Vaginal Film Formulation Development of Microbicide Drug Candidate CSIC for HIV prevention. J. Pharm. Innov. 2017, 12, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, M.; Shi, Y.; Gong, T.; Dezzutti, C.S.; Moncla, B.; Sarafianos, S.G.; Parniak, M.A.; Rohan, L.C. Vaginal Microbicide Film Combinations of Two Reverse Transcriptase Inhibitors, EFdA and CSIC, for the Prevention of HIV-1 Sexual Transmission. Pharm. Res. 2015, 32, 2960–2972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Parniak, M.A.; Sarafianos, S.G.; Cost, M.R.; Rohan, L.C. Development of a vaginal delivery film containing EFdA, a novel anti-HIV nucleoside reverse transcriptase inhibitor. Int. J. Pharm. 2014, 461, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Akil, A.; Parniak, M.A.; Dezzuitti, C.S.; Moncla, B.J.; Cost, M.R.; Li, M.; Rohan, L.C. Development and Characterization of a Vaginal Film Containing Dapivirine, a Non- nucleoside Reverse Transcriptase Inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv. Transl. Res. 2011, 1, 209–222. [Google Scholar] [CrossRef] [Green Version]
- Regev, G.; Patel, S.K.; Moncla, B.J.; Twist, J.; Devlin, B.; Rohan, L.C. Novel Application of Hot Melt Extrusion for the Manufacturing of Vaginal Films Containing Microbicide Candidate Dapivirine. AAPS PharmSciTech 2019, 20, 239. [Google Scholar] [CrossRef]
- Bunge, K.E.; Dezzutti, C.S.; Rohan, L.C.; Hendrix, C.W.; Marzinke, M.A.; Richardson-Harman, N.; Moncla, B.J.; Devlin, B.; Meyn, L.A.; Spiegel, H.M.; et al. A Phase 1 Trial to Assess the Safety, Acceptability, Pharmacokinetics, and Pharmacodynamics of a Novel Dapivirine Vaginal Film. J. Acquir. Immune Defic. Syndr. 2016, 71, 498–505. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.A.; Marzinke, M.A.; Bakshi, R.P.; Fuchs, E.J.; Radebaugh, C.L.; Aung, W.; Spiegel, H.M.; Coleman, J.S.; Rohan, L.C.; Hendrix, C.W. Comparison of Dapivirine Vaginal Gel and Film Formulation Pharmacokinetics and Pharmacodynamics (FAME 02B). AIDS Res. Hum. Retrovir. 2017, 33, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Leitner, V.M.; Walker, G.F.; Bernkop-Schnurch, A. Thiolated polymers: Evidence for the formation of disulphide bonds with mucus glycoproteins. Eur. J. Pharm. Biopharm. 2003, 56, 207–214. [Google Scholar] [CrossRef]
- Guggi, D.; Kast, C.E.; Bernkop-Schnurch, A. In vivo evaluation of an oral salmon calcitonin-delivery system based on a thiolated chitosan carrier matrix. Pharm. Res. 2003, 20, 1989–1994. [Google Scholar] [CrossRef] [PubMed]
- Krauland, A.H.; Guggi, D.; Bernkop-Schnurch, A. Oral insulin delivery: The potential of thiolated chitosan-insulin tablets on non-diabetic rats. J. Control. Release 2004, 95, 547–555. [Google Scholar] [CrossRef]
- Hornof, M.; Weyenberg, W.; Ludwig, A.; Bernkop-Schnurch, A. Mucoadhesive ocular insert based on thiolated poly(acrylic acid): Development and in vivo evaluation in humans. J. Control. Release 2003, 89, 419–428. [Google Scholar] [CrossRef]
- Cevher, E.; Sensoy, D.; Taha, M.A.; Araman, A. Effect of thiolated polymers to textural and mucoadhesive properties of vaginal gel formulations prepared with polycarbophil and chitosan. AAPS PharmSciTech 2008, 9, 953–965. [Google Scholar] [CrossRef]
- Valenta, C.; Marschutz, M.; Egyed, C.; Bernkop-Schnurch, A. Evaluation of the inhibition effect of thiolated poly(acrylates) on vaginal membrane bound aminopeptidase N and release of the model drug LH-RH. J. Pharm. Pharmacol. 2002, 54, 603–610. [Google Scholar] [CrossRef]
- Baloglu, E.; Ay Senyigit, Z.; Karavana, S.Y.; Vetter, A.; Metin, D.Y.; Hilmioglu Polat, S.; Guneri, T.; Bernkop-Schnurch, A. In vitro evaluation of mucoadhesive vaginal tablets of antifungal drugs prepared with thiolated polymer and development of a new dissolution technique for vaginal formulations. Chem. Pharm. Bull. (Tokyo) 2011, 59, 952–958. [Google Scholar] [CrossRef] [Green Version]
- Sakloetsakun, D.; Iqbal, J.; Millotti, G.; Vetter, A.; Bernkop-Schnurch, A. Thiolated chitosans: Influence of various sulfhydryl ligands on permeation-enhancing and P-gp inhibitory properties. Drug Dev. Ind. Pharm. 2011, 37, 648–655. [Google Scholar] [CrossRef]
- Hombach, J.; Palmberger, T.F.; Bernkop-Schnurch, A. Development and in vitro evaluation of a mucoadhesive vaginal delivery system for nystatin. J. Pharm. Sci. 2009, 98, 555–564. [Google Scholar] [CrossRef]
- Nel, A.; van Niekerk, N.; Kapiga, S.; Bekker, L.G.; Gama, C.; Gill, K.; Kamali, A.; Kotze, P.; Louw, C.; Mabude, Z.; et al. Safety and Efficacy of a Dapivirine Vaginal Ring for HIV Prevention in Women. New Engl. J. Med. 2016, 375, 2133–2143. [Google Scholar] [CrossRef] [PubMed]
- Backman, T. Benefit-risk assessment of the levonorgestrel intrauterine system in contraception. Drug Saf. 2004, 27, 1185–1204. [Google Scholar] [CrossRef]
- Tittle, V.; Bull, L.; Boffito, M.; Nwokolo, N. Pharmacokinetic and Pharmacodynamic Drug Interactions Between Antiretrovirals and Oral Contraceptives. Clin. Pharmacokinet. 2015, 54, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Balkus, J.E.; Palanee-Phillips, T.; Reddy, K.; Siva, S.; Harkoo, I.; Nakabiito, C.; Kintu, K.; Nair, G.; Chappell, C.; Kiweewa, F.M.; et al. Brief Report: Dapivirine Vaginal Ring Use Does Not Diminish the Effectiveness of Hormonal Contraception. J. Acquir. Immune Defic. Syndr. 2017, 76, e47–e51. [Google Scholar] [CrossRef]
- Bernkop-Schnurch, A.; Hornof, M.; Zoidl, T. Thiolated polymers--thiomers: Synthesis and in vitro evaluation of chitosan-2-iminothiolane conjugates. Int. J. Pharm. 2003, 260, 229–237. [Google Scholar] [CrossRef]
- Iqbal, J.; Shahnaz, G.; Dünnhaupt, S.; Müller, C.; Hintzen, F.; Bernkop-Schnürch, A. Preactivated thiomers as mucoadhesive polymers for drug delivery. Biomaterials 2012, 33, 1528–1535. [Google Scholar] [CrossRef] [Green Version]
- Grab, S.; Sweeney, Y.C.; Dorothy, L.P.; Lisa, C.R. A quantitative disintegration method to evaluate polymeric films. J. Clin. Transl. Sci. 2018, 1. [Google Scholar] [CrossRef] [Green Version]
- Grab, S.; Rohan, L.C. A Quantitative Disintegration Method for Polymeric Films. J. Pharm. Innov. 2018, 13, 321–329. [Google Scholar] [CrossRef]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Cherala, G.; Edelman, A.; Dorflinger, L.; Stanczyk, F.Z. The elusive minimum threshold concentration of levonorgestrel for contraceptive efficacy. Contraception 2016, 94, 104–108. [Google Scholar] [CrossRef]
- Friend, D.R.; Doncel, G.F. Combining prevention of HIV-1, other sexually transmitted infections and unintended pregnancies: Development of dual-protection technologies. Antivir. Res. 2010, 88 (Suppl. S1), S47–S54. [Google Scholar] [CrossRef] [PubMed]
- Akil, A.; Devlin, B.; Cost, M.; Rohan, L.C. Increased Dapivirine tissue accumulation through vaginal film codelivery of dapivirine and Tenofovir. Mol. Pharm. 2014, 11, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Archer, D.F.; Bigrigg, A.; Smallwood, G.H.; Shangold, G.A.; Creasy, G.W.; Fisher, A.C. Assessment of compliance with a weekly contraceptive patch (Ortho Evra/Evra) among North American women. Fertil. Steril. 2002, 77, S27–S31. [Google Scholar] [CrossRef]
- Gradauer, K.; Dunnhaupt, S.; Vonach, C.; Szollosi, H.; Pali-Scholl, I.; Mangge, H.; Jensen-Jarolim, E.; Bernkop-Schnurch, A.; Prassl, R. Thiomer-coated liposomes harbor permeation enhancing and efflux pump inhibitory properties. J. Control. Release 2013, 165, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernkop-Schnurch, A.; Kast, C.E.; Guggi, D. Permeation enhancing polymers in oral delivery of hydrophilic macromolecules: Thiomer/GSH systems. J. Control. Release 2003, 93, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnurch, A.; Steininger, S. Synthesis and characterisation of mucoadhesive thiolated polymers. Int. J. Pharm. 2000, 194, 239–247. [Google Scholar] [CrossRef]
- Dunnhaupt, S.; Barthelmes, J.; Thurner, C.C.; Waldner, C.; Sakloetsakun, D.; Bernkop-Schnurch, A. S-protected thiolated chitosan: Synthesis and in vitro characterization. Carbohydr. Polym. 2012, 90, 765–772. [Google Scholar] [CrossRef] [Green Version]
- Polis, C.B.; Phillips, S.J.; Hillier, S.L.; Achilles, S.L. Levonorgestrel in contraceptives and multipurpose prevention technologies: Does this progestin increase HIV risk or interact with antiretrovirals? AIDS 2016, 30, 2571–2576. [Google Scholar] [CrossRef] [Green Version]
- To, E.E.; Hendrix, C.W.; Bumpus, N.N. Dissimilarities in the metabolism of antiretroviral drugs used in HIV pre-exposure prophylaxis in colon and vagina tissues. Biochem. Pharmacol. 2013, 86, 979–990. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.R.; Astley, S.; Adams, D.J.; Lacey, C.J.; Ali, S.W.; Wells, M. Expression of cytochrome P450 enzymes in the cervix. An immunohistochemical study. Int. J. Gynecol. Cancer 1993, 3, 159–163. [Google Scholar] [CrossRef]
- Farin, F.M.; Bigler, L.G.; Oda, D.; McDougall, J.K.; Omiecinski, C.J. Expression of cytochrome P450 and microsomal exposide hydrolase in cervical and oral epithelial cells immortalized by human papillomavirus type 16 E6/E7 genes. Carcinogenesis 1995, 16, 1670. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.; Hills-Nieminen, C. Interactions between Antiretrovirals (ARVs) and Hormonal Contraceptives. Expert Opin. Drug Metab. Toxicol. 2013, 9, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Back, D.J.; Houlgrave, R.; Tjia, J.F.; Ward, S.; Orme, M.L. Effect of the progestogens, gestodene, 3-keto desogestrel, levonorgestrel, norethisterone and norgestimate on the oxidation of ethinyloestradiol and other substrates by human liver microsomes. J. Steroid Biochem. Mol. Biol. 1991, 38, 219–225. [Google Scholar] [CrossRef]









| Component | Bioadhesive Film (w/w %) | Quick-Dissolving Film (w/w %) |
|---|---|---|
| Milli-Q water | 86.10 | 83.6 |
| Polyvinyl alcohol 40-88 | 7.02 | 7.02 |
| Polyethylene glycol 8000 | 2.34 | 2.34 |
| Methocel E5 | 1.75 | 1.75 |
| Sodium starch glycolate | 0 | 3.5 |
| Thiomer | 1 | 0 |
| Glycerin | 0.73 | 0.73 |
| Propylene glycol | 0.73 | 0.73 |
| Drug (DPV/LNG) | 0.34 | 0.34 |
| Total | 100.00 | 100.00 |
| Chitosan (MWR, kDa) | Synthesis pH | Thiol Degree (µmol/g) | Rate of Solubility in Water |
|---|---|---|---|
| 50–700 | 5 | 183.45 ± 130.29 | +++ |
| 190–310 | 5 | 182.94 ± 31.44 | +++ |
| 190–310 | 6 | 61.83 ± 13.31 | + |
| 190–310 | 7 | 71.26 ± 27.11 | + |
| Characterizations | DPV Quick-Dissolving Film | DPV Bioadhesive Film | LNG Bioadhesive Film | DPV/LNG MPT Bioadhesive Film |
|---|---|---|---|---|
| Appearance | White, transparent, smooth, and soft | White, transparent, smooth, and soft | White, transparent, smooth, and soft | White, transparent, smooth, and soft |
| Weight (mg) | 69.20 ± 1.76 | 69.35 ± 5.9 | 79.14 ± 8.8 | 65.85 ± 3.93 |
| Thickness (µm) | 155.90 ± 9.75 | 110.75 ± 12.06 | 127.5 ± 11.8 | 105.28 ± 6.96 |
| Water Content % (w/w) | 4.87 ± 0.20 | 6.68 ± 0.43 | 4.95 ± 0.17 | 6.36 ± 0.50 |
| Drug content (mg/film) | 1.41 ± 0.13 | 1.68 ± 0.15 | 1.80 ± 0.09 | 1.71 ± 0.15/DPV 1.50 ± 0.13/LNG |
| Drug loading % (w/w) | 2.04 ± 0.19 | 2.42 ± 0.22 | 2.27 ± 0.11 | 2.60 ± 0.23/DPV 2.28 ± 0.20/LNG |
| Puncture Strength (Kg/mm) | 3.77 ± 0.58 | 12.41 ± 0.44 | 14.18 ± 2.28 | 13.03 ± 0.50 |
| Disintegration (sec) | 56.36 ± 6.49 | 153.12 ± 33.81 | 211.33 ± 70.52 | 228.44 ± 77.46 |
| Bioadhesive Film | Quick-Dissolving Film | |||||
|---|---|---|---|---|---|---|
| Days of Detection | Number of Macaques | % above LLOQ | Median (Range) | Number of Macaques | % above LLOQ | Median (Range) |
| Day 1 | 5 | 100 | 12.3 (2.87–36.71) | 5 | 100 | 18.56 (10.18–69.92) |
| Day 2 | 5 | 100 | 2.62 (0.91–24.63) | 5 | 100 | 1.68 (1.51–2.85) |
| Day 3 | 5 | 100 | 0.49 (0.21–11.32) | 5 | 100 | 0.49 (0.4–1.62) |
| Day 4 | 5 | 100 | 0.40 (0.11–20.03) | 5 | 100 | 0.21 (0.11–0.52) |
| Day 7 | 5 | 40 | 33.01 (0.1–64.41) | ND | ND | ND |
| DPV | 6 h | Day 1 | Day 4 | Day 7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Macaques | % above LLOQ | Median (Range) | Number of Macaques | % above LLOQ | Median (Range) | Number of Macaques | % above LLOQ | Median (Range) | Number of Macaques | % above LLOQ | Median (Range) | |
| Vaginal swab | ||||||||||||
| Single film | 3 | 100 | 86744.53 (64936.83–108126.9) | 5 | 100 | 11327.68 (664.89–19245.81) | 5 | 80 | 82.80 (0.1–8656.03) | 5 | 60 | 1.53 (0.1–5339.14) |
| Combo film | 3 | 100 | 49154.43 (21220.13–105228.6) | 5 | 100 | 27022.57 (4573.604–41360.52) | 5 | 100 | 2039.18 (81.76–20154.96) | 5 | 80 | 26.34 (0.1–34.133) |
| Vaginal tissue | ||||||||||||
| Single film | 3 | 100 | 6425 (2571–13071) | 2 | 100 | 925.5 (107–1744) | 2 | 100 | 1768 (361–3175) | 3 | 100 | 215 (101–735) |
| Combo film | 3 | 66.7 | 21238 (0.1–213096) | 2 | 100 | 10331.5 (7452–13211) | 2 | 100 | 2851.5 (1759–3944) | 3 | 66.7 | 495 (0.1–605) |
| Cervical tissue | ||||||||||||
| Single film | 3 | 100 | 2424 (359–4138) | 2 | 100 | 297.5 (264–331) | 2 | 100 | 478 (141–815) | 3 | 100 | 342 (201–626) |
| Combo film | 3 | 100 | 5390 (2959–10194) | 2 | 100 | 6698 (2206–11190) | 2 | 100 | 5581 (3258–7904) | 3 | 100 | 93.9 (32.7–1943) |
| LNG | 6 h | Day 1 | Day 4 | Day 7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Macaques | % above LLOQ | Median (Range) | Number of Macaques | % above LLOQ | Median (Range) | Number of Macaques | % above LLOQ | Median (Range) | Number of Macaques | % above LLOQ | Median (Range) | |
| Vaginal swab | ||||||||||||
| Single film | 3 | 100 | 16819.47 (10601.74–45273.93) | 5 | 100 | 12623.8 (43223.21–411886.5) | 5 | 100 | 2888.28 (50.26–29221.25) | 5 | 100 | 4663.56 (84.98–97194.82) |
| Combo film | 3 | 100 | 6533.28 (4390.29–14106.52) | 5 | 100 | 11317.51 (210.43–16382.36) | 5 | 100 | 12.52 (3.11–3703.99) | 5 | 20 | 0.1 (0.1–2.81) |
| Vaginal tissue | ||||||||||||
| Single film | 3 | 100 | 1631 (444–19086) | 2 | 100 | 21344 (13300–29388) | 2 | 100 | 3901.5 (1062–6741) | 3 | 100 | 188 (103–226) |
| Combo film | 3 | 100 | 5548 (473–132699) | 2 | 100 | 1785 (1168–2402) | 2 | 100 | 303 (102–504) | 3 | 66.7 | 264 (0.1–679) |
| Cervical tissue | ||||||||||||
| Single film | 3 | 100 | 7258.5 (3059–11458) | 2 | 100 | 7582.5 (6759–8406) | 2 | 100 | 9335.5 (4620–14051) | 3 | 100 | 154 (118–166) |
| Combo film | 3 | 100 | 2771.85 (2273–3450) | 2 | 100 | 1987.5 (873–3102) | 2 | 100 | 901.42 (554–1248.84) | 3 | 66.7 | 13.9 (0.1–222) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Regev, G.; Patel, S.K.; Patton, D.; Sweeney, Y.; Graebing, P.; Grab, S.; Wang, L.; Sant, V.; Rohan, L.C. Rational Design of a Multipurpose Bioadhesive Vaginal Film for Co-Delivery of Dapivirine and Levonorgestrel. Pharmaceutics 2020, 12, 1. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12010001
Li J, Regev G, Patel SK, Patton D, Sweeney Y, Graebing P, Grab S, Wang L, Sant V, Rohan LC. Rational Design of a Multipurpose Bioadhesive Vaginal Film for Co-Delivery of Dapivirine and Levonorgestrel. Pharmaceutics. 2020; 12(1):1. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12010001
Chicago/Turabian StyleLi, Jing, Galit Regev, Sravan Kumar Patel, Dorothy Patton, Yvonne Sweeney, Philip Graebing, Sheila Grab, Lin Wang, Vinayak Sant, and Lisa C. Rohan. 2020. "Rational Design of a Multipurpose Bioadhesive Vaginal Film for Co-Delivery of Dapivirine and Levonorgestrel" Pharmaceutics 12, no. 1: 1. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12010001