Efficacy of Local Minocycline Agents in Treating Peri-Implantitis: An Experimental In Vivo Study in Beagle Dogs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Sample Size Calculation
2.3. Experimental Procedures
2.3.1. Extraction, Implant Placement, and Peri-Implantitis Induction
2.3.2. Localized Drug Agent Delivery
- 0.5 g of minocycline hydrochloride with CA microsphere (MC; Minocline®, Dongkook Pharmaceutical, Seoul, Korea);
- Placebo CA microsphere ointment without minocycline hydrochloride (MP; Minocline Placebo, manufactured by Dongkook pharmaceuticals);
- 0.5 g of minocycline hydrochloride with PG microsphere—obtained from Sunstar (PG; Periocline®, Sunstar, Osaka, Japan);
- Mechanical debridement only (Control).
2.4. Outcome Variables
- Primary outcome: clinical, radiographical, and immuhohistochemical outcomes.
- Secondary outcome: LDA carrier sustainability, bacteriostatic effect durability.
2.5. Clinical Outcomes
2.6. Radiographical Outcomes
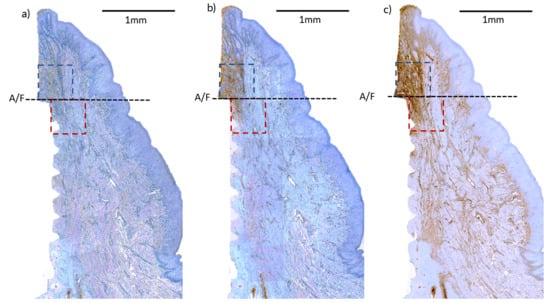
2.7. Histological Preparation and Immunohistochemical (IHC) Analysis
2.8. Drug Sustainability Evaluation
2.8.1. LDA Carrier Sustainability Evaluation
2.8.2. Bacteriostatic Effect Evaluation
2.9. Statistical Analysis
3. Results
3.1. Number of Animals and Implants Analyzed
3.2. Clinical Findings
3.3. Radiographic Analysis
3.4. Immunohistochemical (IHC) Analyses
3.5. Drug Sustainability Evaluation
3.5.1. Carrier Sustainability
3.5.2. Bacteriostatic Effect Sustainability
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Antibody | Specificity | Dilutions | Source |
|---|---|---|---|
| CD3 | T cells | 1:200 | Abcam |
| CD20 | B cells | 1:800 | Thermo-Fisher scientific |
| IgG | Plasma/B Cells | 1:800 | Cloud-Clone corp. |
| ΔSurgery − T1 | ΔT1 − T2 | ΔT2 − T3 | ΔT1 − T3 | |
|---|---|---|---|---|
| MC | −1.39 ± 0.69 | 0.17 ± 0.28 | −0.24 ± 0.25 | −0.07 ± 0.23 |
| MP | −1.61 ± 1.03 | 0.12 ± 0.29 | −0.06 ± 0.36 | −0.06 ± 0.53 |
| PG | −0.86 ± 0.24 | −0.15 ± 0.24 | −0.09 ± 0.46 | −0.25 ± 0.42 |
| Control | −1.09 ± 0.60 | 0.03 ± 0.18 | −0.16 ± 0.44 | −0.13 ± 0.56 |
| p-value | 0.445 | 0.357 | 0.880 | 0.843 |
References
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018, 89, S313–S318. [Google Scholar] [CrossRef]
- Renvert, S.; Lindahl, C.; Persson, G.R. Occurrence of cases with peri-implant mucositis or peri-implantitis in a 21–26 years follow-up study. J. Clin. Periodontol. 2018, 45, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Tallarico, M.; Radovanovic, S.; Delibasic, B.; Covani, U.; Rakic, M. Distinguishing predictive profiles for patient-based risk assessment and diagnostics of plaque induced, surgically and prosthetically triggered peri-implantitis. Clin. Oral Implant. Res. 2016, 27, 1243–1250. [Google Scholar] [CrossRef]
- Tallarico, M.; Canullo, L.; Wang, H.L.; Cochran, D.L.; Meloni, S.M. Classification systems for peri-implantitis: A narrative review with a proposal of a new evidence-based etiology codification. Int. J. Oral Maxillofac. Implant. 2018, 33, 871–879. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A. Peri-implant diseases: Diagnosis and risk indicators. J. Clin. Periodontol. 2008, 35, 292–304. [Google Scholar] [CrossRef]
- Matys, J.; Botzenhart, U.; Gedrange, T.; Dominiak, M. Thermodynamic effects after Diode and Er:YAG laser irradiation of grade IV and V titanium implants placed in bone—An ex vivo study. Preliminary report. Biomed. Eng. Biomed. Tech. 2016, 61, 499. [Google Scholar] [CrossRef]
- Matys, J.; Romeo, U.; Mroczka, K.; Grzech-Leśniak, K.; Dominiak, M. Temperature changes and SEM effects of three different implants-abutment connection during debridement with Er:YAG laser: An Ex vivo study. Materials 2019, 12, 3748. [Google Scholar] [CrossRef] [Green Version]
- Lindhe, J.; Meyle, J.; Group D of the European Workshop on Periodontology. Peri-implant diseases: Consensus report of the sixth European workshop on periodontology. J. Clin. Periodontol. 2008, 35, 282–285. [Google Scholar] [CrossRef] [Green Version]
- Cha, J.K.; Paeng, K.; Jung, U.W.; Choi, S.H.; Sanz, M.; Sanz-Martin, I. The effect of five mechanical instrumentation protocols on implant surface topography and roughness: A scanning electron microscope and confocal laser scanning microscope analysis. Clin. Oral Implant. Res. 2019, 30, 578–587. [Google Scholar] [CrossRef]
- Sirinirund, B.; Garaicoa-Pazmino, C.; Wang, H.L. Effects of mechanical instrumentation with commercially available instruments used in supportive peri-implant therapy: An in vitro study. Int. J. Oral. Maxillofac. Implant. 2019, 34, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Świder, K.; Dominiak, M.; Grzech-Leśniak, K.; Matys, J. Effect of different laser wavelengths on periodontopathogens in peri-implantitis: A review of in vivo studies. Microorganisms 2019, 7, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahrmann, P.; Ronay, V.; Sener, B.; Jung, R.E.; Attin, T.; Schmidlin, P.R. Cleaning potential of glycine air-flow application in an in vitro peri-implantitis model. Clin. Oral Implant. Res. 2013, 24, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Slots, J.; Rosling, B.G. Suppression of the periodontopathic microflora in localized juvenile periodontitis by systemic tetracycline. J. Clin. Periodontol. 1983, 10, 465–486. [Google Scholar] [CrossRef] [PubMed]
- Yeom, H.R.; Park, Y.J.; Lee, S.J.; Rhyu, I.C.; Chung, C.P.; Nisengard, R.J. Clinical and microbiological effects of minocycline-loaded microcapsules in adult periodontitis. J. Periodontol. 1997, 68, 1102–1109. [Google Scholar] [CrossRef]
- Mombelli, A.; Feloutzis, A.; Brägger, U.; Lang, N.P. Treatment of peri-implantitis by local delivery of tetracycline. Clin. Oral Implant. Res. 2001, 12, 287–294. [Google Scholar] [CrossRef]
- Hardy, J.H.; Newman, H.N.; Strahan, J.D. Direct irrigation and subgingival plaque. J. Clin. Periodontol. 1982, 9, 57–65. [Google Scholar] [CrossRef]
- Cha, J.K.; Lee, J.S.; Kim, C.S. Surgical therapy of peri-implantitis with local minocycline: A 6-month randomized controlled clinical trial. J. Dent. Res. 2019, 98, 288–295. [Google Scholar] [CrossRef]
- Vanderkerckhove, B.; Quirynen, M.; Van Steenberghe, D. The use of locally-delivered minocycline in the treatment of chronic periodontitis. A review of the literature. J. Clin. Periodontol. 1998, 25, 964–968. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, J.Y.; Yeom, H.R.; Kim, K.H.; Lee, S.C.; Shim, I.K.; Chung, C.P.; Lee, S.J. Injectable polysaccharide microcapsules for prolonged release of minocycline for the treatment of periodontitis. Biotechnol. Lett. 2005, 27, 1761–1766. [Google Scholar] [CrossRef]
- Yoon, S.W.; Kim, M.J.; Paeng, K.W.; Yu, K.A.; Lee, C.K.; Song, Y.W.; Cha, J.K.; Sanz, M.; Jung, U.W. Locally applied slow-release of minocycline microspheres in the treatment of peri-implant mucositis: An experimental in vivo study. Pharmaceutics 2020, 12, 668. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Xu, P.; Pang, Z.; Zhao, J.; Chai, Z.; Li, X.; Li, H.; Jiang, M.; Cheng, H.; Zhang, B.; et al. Local delivery of minocycline-loaded PEG-PLA nanoparticles for the enhanced treatment of periodontitis in dogs. Int. J. Nanomed. 2014, 9, 3963–3970. [Google Scholar] [CrossRef] [Green Version]
- Fickl, S.; Kebschull, M.; Calvo-Guirado, J.L.; Hurzeler, M.; Zuhr, O. Experimental peri-implantitis around different types of implants—A clinical and radiographic study in dogs. Clin. Implant Dent. Relat. Res. 2015, 17 (Suppl. 2), e661–e669. [Google Scholar] [CrossRef] [PubMed]
- Loe, H.; Silness, J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef]
- Silness, J.; Löe, H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar]
- Carcuac, O.; Abrahamsson, I.; Albouy, J.P.; Linder, E.; Larsson, L.; Berglundh, T. Experimental periodontitis and peri-implantitis in dogs. Clin. Oral Implant. Res. 2013, 24, 363–371. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J.; Jonsson, K.; Ericsson, I. The topography of the vascular systems in the periodontal and peri-implant tissues in the dog. J. Clin. Periodontol. 1994, 21, 189–193. [Google Scholar] [CrossRef]
- Syrbu, S.I.; Cohen, M.B. An enhanced antigen-retrieval protocol for immunohistochemical staining of formalin-fixed, paraffin-embedded tissues. Methods Mol. Biol. 2011, 717, 101–110. [Google Scholar] [CrossRef]
- Sanz-Martín, I.; Rojo, E.; Maldonado, E.; Stroppa, G.; Nart, J.; Sanz, M. Structural and histological differences between connective tissue grafts harvested from the lateral palatal mucosa or from the tuberosity area. Clin. Oral Investig. 2019, 23, 957–964. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Thaya, R.; Vaseeharan, B.; Sivakamavalli, J.; Iswarya, A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Al-Anbr, M.N.; Khaled, J.M.; Benelli, G. Synthesis of chitosan-alginate microspheres with high antimicrobial and antibiofilm activity against multi-drug resistant microbial pathogens. Microb. Pathog. 2018, 114, 17–24. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Lee, J.-B.; Kweon, H.H.-I.; Cho, H.-J.; Kim, C.-S.; Kim, Y.-T. Characteristics of local delivery agents for treating peri-implantitis on dental implant surfaces: A preclinical study. J. Oral Implantol. 2019, 45, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Albouy, J.P.; Abrahamsson, I.; Berglundh, T. Spontaneous progression of experimental peri-implantitis at implants with different surface characteristics: An experimental study in dogs. J. Clin. Periodontol. 2012, 39, 182–187. [Google Scholar] [CrossRef] [PubMed]





| Clinical Parameter | Group | Baseline (T1) | 4 Weeks (T2) | 8 Weeks (T3) |
|---|---|---|---|---|
| Mean PPD (mm) | MC | 3.81 ± 0.99 | 3.06 ± 0.59 ‡ | 2.00 ± 0.62 ‡,* |
| MP | 3.81 ± 0.91 | 3.67 ± 0.83 | 2.17 ± 0.58 ‡,* | |
| PG | 3.44 ± 0.30 | 3.19 ± 0.35 | 2.42 ± 0.60 ‡,* | |
| Control | 3.47 ± 0.41 | 3.11 ± 0.62 | 2.17 ± 0.65 ‡,* | |
| p-value | 0.980 | 0.506 | 0.782 | |
| Mean GI | MC | 1.92 ± 0.13 | 1.78 ± 0.12 | 1.78 ± 0.18 |
| MP | 1.92 ± 0.13 | 1.72 ± 0.16 | 1.53 ± 0.35 | |
| PG | 1.89 ± 0.12 | 1.69 ± 0.18 ‡ | 1.44 ± 0.34 ‡ | |
| Control | 1.81 ± 0.22 | 1.58 ± 0.08 | 1.61 ± 0.31 | |
| p-value | 0.720 | 0.154 | 0.491 | |
| Mean BOP (%) | MC | 91.7 ± 12.7 | 77.8 ± 12.2 | 77.8 ± 18.3 |
| MP | 91.7 ± 12.7 | 69.7 ± 14.9 | 69.5 ± 29.4 | |
| PG | 88.8 ± 12.4 | 69.5 ± 17.7 ‡ | 44.5 ± 34.1 ‡ | |
| Control | 91.5 ± 8.5 | 64.0 ± 11.4 ‡ | 50.2 ± 33.3 ‡ | |
| p-value | 0.955 | 0.433 | 0.414 | |
| Mean PLI | MC | 2.70 ± 0.34 | 2.33 ± 0.62 | 1.45 ± 0.29 ‡,* |
| MP | 2.75 ± 0.25 | 2.39 ± 0.57 | 1.31 ±0.35 ‡,* | |
| PG | 2.75 ± 0.25 | 2.50 ± 0.53 | 1.25 ± 0.48 ‡,* | |
| Control | 2.67 ± 0.26 | 1.91 ± 0.98 | 1.17 ± 0.37 ‡ | |
| p-value | 0.890 | 0.383 | 0.585 |
| MC | MP | PG | Control | p-Value | ||
|---|---|---|---|---|---|---|
| CD 3 (%) | A/F—Top | 7.14 ± 2.84 | 9.24 ± 6.09 | 9.49 ± 6.84 | 5.99 ± 3.65 | 0.516 |
| A/F—Bottom | 8.20 ± 3.78 | 9.34 ± 5.89 | 7.69 ± 6.28 | 6.73 ± 4.34 | 0.824 | |
| Total mean | 7.67 ± 3.39 | 9.29 ± 6.00 | 8.59 ± 6.63 | 6.34 ± 4.01 | ||
| CD 20 (%) | A/F—Top | 9.21 ± 4.67 | 8.28 ± 4.58 | 8.76 ± 9.07 | 9.93 ± 4.67 | 0.388 |
| A/F—Bottom | 8.02 ± 4.14 | 7.48 ± 4.50 | 8.06 ± 4.15 | 7.17 ± 5.32 | 0.887 | |
| Total mean | 8.48 ± 4.39 | 7.85 ± 4.56 | 8.43 ± 7.19 | 8.55 ± 5.19 | ||
| Ig G (%) | A/F—Top | 8.41 ± 4.99 | 9.75 ± 6.29 | 11.72 ± 10.99 | 13.10 ± 9.90 | 0.898 |
| A/F—Bottom | 4.97 ± 3.25 | 7.93 ± 8.23 | 8.45 ± 5.26 | 9.57 ± 8.80 | 0.467 | |
| Total mean | 6.59 ± 4.50 | 8.84 ± 7.38 | 10.00 ± 8.63 | 11.33 ± 9.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.-W.; Kim, M.-J.; Paeng, K.-W.; Yu, K.A.; Lee, C.-K.; Song, Y.W.; Cha, J.-K.; Jung, U.-W. Efficacy of Local Minocycline Agents in Treating Peri-Implantitis: An Experimental In Vivo Study in Beagle Dogs. Pharmaceutics 2020, 12, 1016. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12111016
Yoon S-W, Kim M-J, Paeng K-W, Yu KA, Lee C-K, Song YW, Cha J-K, Jung U-W. Efficacy of Local Minocycline Agents in Treating Peri-Implantitis: An Experimental In Vivo Study in Beagle Dogs. Pharmaceutics. 2020; 12(11):1016. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12111016
Chicago/Turabian StyleYoon, Sung-Wook, Myong-Ji Kim, Kyeong-Won Paeng, Kyeong Ae Yu, Chong-Kil Lee, Young Woo Song, Jae-Kook Cha, and Ui-Won Jung. 2020. "Efficacy of Local Minocycline Agents in Treating Peri-Implantitis: An Experimental In Vivo Study in Beagle Dogs" Pharmaceutics 12, no. 11: 1016. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12111016