Quality-by-Design Approach for the Development of Nano-Sized Tea Tree Oil Formulation-Impregnated Biocompatible Gel with Antimicrobial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Method for Characterization of TTO
2.3. Preliminary Study for Selection of Critical Parameters and Limits for Mixture Experimental Design
2.4. Preparation of LNF
2.5. LNF Formulation Optimization Using Mixture Experimental Design
2.6. Characterization of LNF: Droplet Size and PDI Measurement
2.6.1. Zetasizer
2.6.2. Transmission Electron Microscopy (TEM)
2.7. Development of LNF Gel
2.8. Characterization of LNF Gel
2.8.1. Viscosity Measurements
2.8.2. In Vitro Release Study
2.8.3. Stability Study
2.9. In Vitro Antibacterial Evaluation of LNF Formulations
2.9.1. Bacterial Strains and Culture
2.9.2. Well Diffusion Assay
2.9.3. Time Killing Curve
3. Results and Discussion
3.1. Analytical Method for Characterisation of TTO
3.2. Preliminary Study for Selection of Ingredients, Critical Parameters, and Limits for Mixture Experimental Design
3.3. Mixture Experimental Design for LNF Formulation Optimization
3.3.1. Analysis of Variance
3.3.2. Response Surface Analysis
3.3.3. ANOVA for Cubic Model
3.3.4. Final Equation in Terms of Real Components Verification of Models
3.3.5. Verification of Models Optimization
3.3.6. Formulation Optimization
3.4. Characterization of LNF
3.5. Screening of Polymers for the Development of TTO-LNF Gel
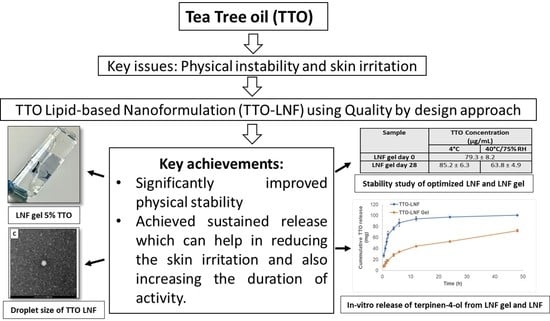
3.6. Characterization of TTO-LNF Gel
3.6.1. In Vitro Release Study
3.6.2. Stability Study
3.7. In Vitro Antibacterial Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kawakami:, M.; Sachs, R.M.; Shibamoto, T. Volatile Constituents of Essential Oils Obtained from Newly Developed Tea Tree (Melaleuca-Alternifolia) Clones. J. Agric. Food Chem. 1990, 38, 1657–1661. [Google Scholar] [CrossRef]
- De Groot, A.C.; Schmidt, E. Tea tree oil: Contact allergy and chemical composition. Contact Dermat. 2016, 75, 129–143. [Google Scholar] [CrossRef] [Green Version]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Effects of melaleuca alternifolia (tea tree) essential oil and the major monoterpene component terpinen-4-ol on the development of single- and multistep antibiotic resistance and antimicrobial susceptibility. Antimicrob. Agents Chemother. 2012, 56, 909–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dryden, M.S.; Dailly, S.; Crouch, M. A randomized, controlled trial of tea tree topical preparations versus a standard topical regimen for the clearance of mrsa colonization. J. Hosp. Infect. 2004, 56, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. Determining the antimicrobial actions of tea tree oil. Molecules 2001, 6, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.; Davey, R.; Peterson, G.M.; Carson, C.; Walton, S.F.; Spelman, T.; Calma, T.; Dettwiller, P.; Tobin, J.; McMillan, F.; et al. Treatment of scabies using a tea tree oil-based gel formulation in australian aboriginal children: Protocol for a randomised controlled trial. BMJ Open 2018, 8, e018507. [Google Scholar] [CrossRef]
- Pazyar, N.; Yaghoobi, R.; Bagherani, N.; Kazerouni, A. A review of applications of tea tree oil in dermatology. Int. J. Derm. 2013, 52, 784–790. [Google Scholar] [CrossRef]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion components screening and selection: A technical note. AAPS PharmSciTech 2009, 10, 69–76. [Google Scholar] [CrossRef]
- Flores, F.C.; de Lima, J.A.; Ribeiro, R.F.; Alves, S.H.; Rolim, C.M.; Beck, R.C.; da Silva, C.B. Antifungal activity of nanocapsule suspensions containing tea tree oil on the growth of trichophyton rubrum. Mycopathologia 2013, 175, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Sonneville-Aubrun, O.; Simonnet, J.T.; L’Alloret, F. Nanoemulsions: A new vehicle for skincare products. Adv. Colloid Interface Sci. 2004, 108–109, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Srivastava, S.; Mishra, N.; Singh, D.K.; Luqman, S.; Chanda, D.; Yadav, N.P. Development, optimization, and characterization of a novel tea tree oil nanogel using response surface methodology. Drug Dev. Ind. Pharm. 2016, 42, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Najafi-Taher, R.; Ghaemi, B.; Amani, A. Delivery of adapalene using a novel topical gel based on tea tree oil nano-emulsion: Permeation, antibacterial and safety assessments. Eur. J. Pharm. Sci. 2018, 120, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Wulansari, A.; Jufri, M.; Budianti, A. Studies on the formulation, physical stability, and in vitro antibacterial activity of tea tree oil (melaleuca alternifolia) nanoemulsion gel. Int. J. Appl. Pharm. 2017, 9, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Chang, R.K.; Raw, A.; Lionberger, R.; Yu, L. Generic development of topical dermatologic products: Formulation development, process development, and testing of topical dermatologic products. AAPS J. 2013, 15, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Biju, S.S.; Ahuja, A.; Khar, R.K.; Chaudhry, R. Formulation and evaluation of an effective ph balanced topical antimicrobial product containing tea tree oil. Pharmazie 2005, 60, 208–211. [Google Scholar]
- Effendy, I.; Maibach, H.I. Surfactants and experimental irritant contact dermatitis. Contact Dermat. 1995, 33, 217–225. [Google Scholar] [CrossRef]
- Barot, B.S.; Parejiya, P.B.; Patel, H.K.; Gohel, M.C.; Shelat, P.K. Microemulsion-based gel of terbinafine for the treatment of onychomycosis: Optimization of formulation using d-optimal design. AAPS PharmSciTech 2012, 13, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug delivery. Nanomedicine 2010, 6, 9–24. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Marzuki, N.H.C.; Wahab, R.A.; Hamid, M.A. An overview of nanoemulsion: Concepts of development and cosmeceutical applications. Biotechnol. Biotechnol. Equip. 2019, 33, 779–797. [Google Scholar] [CrossRef] [Green Version]
- Reichling, J.; Landvatter, U.; Wagner, H.; Kostka, K.H.; Schaefer, U.F. In vitro studies on release and human skin permeation of australian tea tree oil (tto) from topical formulations. Eur. J. Pharm. Biopharm. 2006, 64, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Sonia, K.; Anupama, D. Microemulsion based transdermal drug delivery of tea tree oil. Int. J. Drug Dev. Res. 2011, 3, 191–198. [Google Scholar]
- Clayton, K.N.; Salameh, J.W.; Wereley, S.T.; Kinzer-Ursem, T.L. Physical characterization of nanoparticle size and surface modification using particle scattering diffusometry. Biomicrofluidics 2016, 10, 054107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, G.H.; Park, J.H.; Shin, H.W.; Kim, J.E.; Park, Y.J. Quality-by-design approach for the development of telmisartan potassium tablets. Drug Dev. Ind. Pharm. 2018, 44, 837–848. [Google Scholar] [CrossRef]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for formulation of nanoemulsion drug delivery system: A review. Prev. Nutr. Food Sci. 2019, 24, 225–234. [Google Scholar] [CrossRef]
- Parikh, A.; Kathawala, K.; Tan, C.C.; Garg, S.; Zhou, X.F. Lipid-based nanosystem of edaravone: Development, optimization, characterization and in vitro/in vivo evaluation. Drug Deliv. 2017, 24, 962–978. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.; Carson, C.F.; Peterson, G.M.; Walton, S.F.; Hammer, K.A.; Naunton, M.; Davey, R.C.; Spelman, T.; Dettwiller, P.; Kyle, G.; et al. Therapeutic potential of tea tree oil for scabies. Am. J. Trop. Med. Hyg. 2016, 94, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Rutherford, T.; Nixon, R.; Tam, M.; Tate, B. Allergy to tea tree oil: Retrospective review of 41 cases with positive patch tests over 4.5 years. Australas J. Derm. 2007, 48, 83–87. [Google Scholar] [CrossRef]
- Tisserand, R. Challenges facing essential oil therapy: Proof of safety. In Proceedings of the Alliance of International Aromatherapists (AIA) Conference, Denver, CO, USA, 18–21 October 2007. [Google Scholar]
- Parikh, A.; Kathawala, K.; Song, Y.; Zhou, X.F.; Garg, S. Curcumin-loaded self-nanomicellizing solid dispersion system: Part i: Development, optimization, characterization, and oral bioavailability. Drug Deliv. Transl. Res. 2018, 8, 1389–1405. [Google Scholar] [CrossRef]
- Parikh, A.; Kathawala, K.; Tan, C.C.; Garg, S.; Zhou, X.F. Self-nanomicellizing solid dispersion of edaravone: Part i—Oral bioavailability improvement. Drug Des. Dev. Ther. 2018, 12, 2051–2069. [Google Scholar] [CrossRef] [Green Version]
- Parikh, A.; Kathawala, K.; Tan, C.C.; Garg, S.; Zhou, X.F. Development of a novel oral delivery system of edaravone for enhancing bioavailability. Int. J. Pharm. 2016, 515, 490–500. [Google Scholar] [CrossRef]
- FDA. Inactive Ingredient Search for Approved Drug Products; FDA: White Oak, MD, USA, 2019.
- Niazi, S.K. Handbook of pharmaceutical manufacturing formulations. In Liquid Products; Taylor & Francis: Boca Raton, FL, USA, 2004; Volume 3. [Google Scholar]
- Masoumi, H.R.; Basri, M.; Samiun, W.S.; Izadiyan, Z.; Lim, C.J. Enhancement of encapsulation efficiency of nanoemulsion-containing aripiprazole for the treatment of schizophrenia using mixture experimental design. Int. J. Nanomed. 2015, 10, 6469–6476. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.; Song, Y.; Peddie, F.; Evans, A.M. Particle size reduction to the nanometer range: A promising approach to improve buccal absorption of poorly water-soluble drugs. Int. J. Nanomed. 2011, 6, 1245–1251. [Google Scholar]
- Franz, T.J.; Lehman, P.A.; Franz, S.F.; North-Root, H.; Demetrulias, J.L.; Kelling, C.K.; Moloney, S.J.; Gettings, S.D. Percutaneous penetration of n-nitrosodiethanolamine through human skin (in vitro): Comparison of finite and infinite dose applications from cosmetic vehicles. Fundam. Appl. Toxicol. 1993, 21, 213–221. [Google Scholar] [CrossRef]
- Haidari, H.; Kopecki, Z.; Bright, R.; Cowin, A.J.; Garg, S.; Goswami, N.; Vasilev, K. Ultrasmall agnp-impregnated biocompatible hydrogel with highly effective biofilm elimination properties. ACS Appl. Mater. Interfaces 2020, 12, 41011–41025. [Google Scholar] [CrossRef]
- Haidari, H.; Goswami, N.; Bright, R.; Kopecki, Z.; Cowin, A.J.; Garg, S.; Vasilev, K. The interplay between size and valence state on the antibacterial activity of sub-10 nm silver nanoparticles. Nanoscale Adv. 2019, 1, 2365–2371. [Google Scholar] [CrossRef] [Green Version]
- Osterberg, R.E.; See, N.A. Toxicity of excipients--a Food and Drug Administration perspective. Int. J. Toxicol. 2003, 22, 377–380. [Google Scholar] [CrossRef]
- National Toxicology Program. Ntp toxicology and carcinogenesis studies of polysorbate 80 (cas no. 9005-65-6) in f344/n rats and b6c3f1 mice (feed studies). Natl. Toxicol. Program Tech. Rep. Ser. 1992, 415, 1–225. [Google Scholar]
- Lee, S.G.; Kang, J.B.; Kim, S.R.; Kim, C.J.; Yeom, D.W.; Yoon, H.Y.; Kwak, S.S.; Choi, Y.W. Enhanced topical delivery of tacrolimus by a carbomer hydrogel formulation with transcutol p. Drug Dev. Ind. Pharm. 2016, 42, 1636–1642. [Google Scholar] [CrossRef]
- Hebert, A.; Friedlander, S.; Allen, D.B. Topical fluticasone propionate lotion does not cause hpa axis suppression. J. Pediatr. 2006, 149, 378–382. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Miller, B.H.; Cutivate Lotion Study Group. Two randomized, double-blind, placebo-controlled studies of fluticasone propionate lotion 0.05% for the treatment of atopic dermatitis in subjects from 3 months of age. J. Am. Acad. Derm. 2006, 54, 715–717. [Google Scholar] [CrossRef]
- Contri, R.V.; Frank, L.A.; Kaiser, M.; Pohlmann, A.R.; Guterres, S.S. The use of nanoencapsulation to decrease human skin irritation caused by capsaicinoids. Int. J. Nanomed. 2014, 9, 951–962. [Google Scholar]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Flores, F.C.; Ribeiro, R.; Ourique, A.; Rolim, C.; Silva, C.; Pohlmann, A.; Beck, R.; Stanisçuaski Guterres, S. Nanostructured systems containing an essential oil: Protection against volatilization. Química Nova 2010, 34, 968–972. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Toutain-Kidd, C.M.; Kadivar, S.C.; Bramante, C.T.; Bobin, S.A.; Zegans, M.E. Polysorbate 80 inhibition of pseudomonas aeruginosa biofilm formation and its cleavage by the secreted lipase lipa. Antimicrob. Agents Chemother. 2009, 53, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, C.K.; Kjems, J.; Mygind, T.; Snabe, T.; Meyer, R.L. Effects of tween 80 on growth and biofilm formation in laboratory media. Front. Microbiol. 2016, 7, 1878. [Google Scholar] [CrossRef]




| Symbol | Variables | Units | Range (%) | |
| Lower | Higher | |||
| A | Surfactant (Kolliphor™ RH40: Tween 80, 50:50) | %, w/w | 5 | 20 |
| B | Co-surfactant (Transcutol P) | %, w/w | 5 | 50 |
| C | Water | %, w/w | 25 | 80 |
| Symbol | Response | Units | Range (%) | |
| Lower | Higher | |||
| PDI | Polydispersity index | - | 0.01 | 0.2 |
| DS | Droplet size | nm | 1 | 200 |
| Batch | Surfactant (%) | Co-Surfactant (%) | Water (%) | Droplet Size (nm) | PDI |
|---|---|---|---|---|---|
| TTO003QbD1 | 20 | 5 | 70 | 14.42 | 0.03 |
| TTO003QbD2 | 5 | 40.0 | 50 | 203.5 | 0.32 |
| TTO003QbD3 | 5 | 17.8 | 72.2 | 39.08 | 0.30 |
| TTO003QbD4 | 15.5 | 36.5 | 43 | 16.74 | 0.11 |
| TTO003QbD5 | 20 | 26.6 | 48.4 | 15.25 | 0.05 |
| TTO003QbD6 | 19 | 16.3 | 59.7 | 14.85 | 0.05 |
| TTO003QbD7 | 20 | 50 | 25 | 16.10 | 0.05 |
| TTO003QbD8 | 5 | 50 | 40 | 211.66 | 0.41 |
| TTO003QbD9 | 10 | 5 | 80 | 18.51 | 0.05 |
| TTO003QbD10 | 12.6 | 28.9 | 53.5 | 17.41 | 0.07 |
| TTO003QbD11 | 20 | 41.8 | 33.2 | 16.05 | 0.04 |
| TTO003QbD12 | 5 | 27.4 | 62.6 | 94.45 | 0.19 |
| TTO003QbD13 | 13.10 | 28.8 | 53.1 | 17.26 | 0.08 |
| TTO003QbD14 | 12.1 | 50 | 32.9 | 21.01 | 0.26 |
| ANOVA | |||||
| Droplet Size | |||||
| Fit Statistics | Linear | Quadratic | Special Cubic | Cubic | Special Quartic |
| SD | 48.6 | 28.7 | 30.7 | 14.3 | 33 |
| R2 | 0.587 | 0.895 | 0.895 | 0.987 | 0.913 |
| Adjusted R2 | 0.511 | 0.829 | 0.805 | 0.957 | 0.775 |
| Predicted R2 | 0.270 | 0.470 | 0.142 | 0.627 | −1.59 |
| Adeq. Precision | 7.87 | 11.7 | 10.2 | 17.75 | 7.73 |
| F-value | 7.81 | 13.63 | 9.95 | 33.5 | 6.59 |
| p-value | 0.007 | 0.000964 | 0.00391 | 0.00206 | 0.0264 |
| PDI | |||||
| SD | 0.0622 | 0.0494 | 0.0456 | 0.0319 | 0.0506 |
| R2 | 0.802 | 0.909 | 0.932 | 0.981 | 0.940 |
| Adjusted R2 | 0.766 | 0.852 | 0.874 | 0.939 | 0.845 |
| Predicted R2 | 0.654 | 0.667 | 0.484 | 0.744 | −0.558 |
| Adeq. Precision | 13.3 | 12 | 13.3 | 14.6 | 10.6 |
| F-value | 22.3 | 16.0 | 16.0 | 23.0 | 9.87 |
| p-value | 0.000135 | 0.000550 | 0.000904 | 0.00424 | 0.0110 |
| Batch | Formulation | % Polymer | Physical Appearance | Mean Viscosity (Pa.s) at 21.441 Shear Rate (1/s) |
|---|---|---|---|---|
| 1 | Hydroxyethylcellulose + LNF | 1–3 | Lotion/clear/2 layers | NM 1 |
| 2 | Carbopol 974P + LNF | 0.6–1 | Gel/cloudy | NM 1 |
| 3 | Silky nourishment body cream (Unilever) | - | Cream | 5.72 |
| 4 | LNF gel 5% TTO | 1.25 | Lotion/clear | 5.76 |
| Sample | Droplet Size (nm) | PDI | TTO Concentration (µg/mL) | |||
|---|---|---|---|---|---|---|
| 4 °C | 40 °C | 4 °C | 40 °C | 4 °C | 40 °C | |
| LNF gel day 0 | 33.4 ± 4.4 | 0.47 ± 0.05 | 79.3 ± 8.2 | |||
| LNF gel day 28 | 31.2 ± 4.8 | 25.2 ± 5.2 | 0.44 ± 0.03 | 0.44 ± 0.05 | 85.2 ± 6.3 | 63.8 ± 4.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muta, T.; Parikh, A.; Kathawala, K.; Haidari, H.; Song, Y.; Thomas, J.; Garg, S. Quality-by-Design Approach for the Development of Nano-Sized Tea Tree Oil Formulation-Impregnated Biocompatible Gel with Antimicrobial Properties. Pharmaceutics 2020, 12, 1091. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12111091
Muta T, Parikh A, Kathawala K, Haidari H, Song Y, Thomas J, Garg S. Quality-by-Design Approach for the Development of Nano-Sized Tea Tree Oil Formulation-Impregnated Biocompatible Gel with Antimicrobial Properties. Pharmaceutics. 2020; 12(11):1091. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12111091
Chicago/Turabian StyleMuta, Thabata, Ankit Parikh, Krishna Kathawala, Hanif Haidari, Yunmei Song, Jackson Thomas, and Sanjay Garg. 2020. "Quality-by-Design Approach for the Development of Nano-Sized Tea Tree Oil Formulation-Impregnated Biocompatible Gel with Antimicrobial Properties" Pharmaceutics 12, no. 11: 1091. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12111091