Synthesis and Characterization of pH-Responsive PEG-Poly(β-Amino Ester) Block Copolymer Micelles as Drug Carriers to Eliminate Cancer Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Block Copolymer
2.2. Characterization of PEG-PBAE
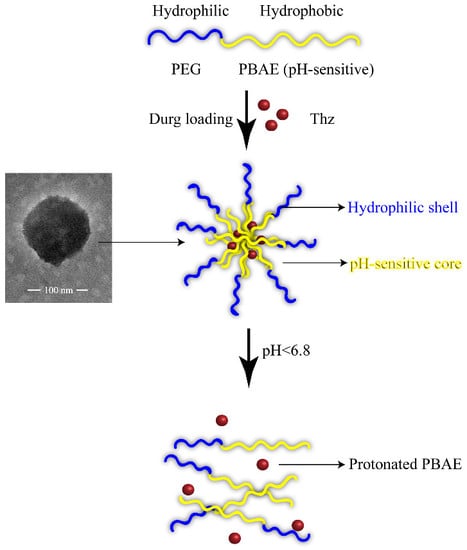
2.3. Preparation of Thz/PPM
2.4. Characterization of Thz/PPM
2.5. pH-Dependent Drug Release from Thz/PPM
2.6. In Vitro Cytotoxicity Assays
2.7. Experiment of MS Formation Rate In Vitro
2.8. In Vitro Cellular Uptake
2.9. Study of Cellular Uptake Mechanism
2.10. Endosomal Escape of Thz/PPM
2.11. Xenograft Tumor Model
2.12. In Vivo Antitumor Efficacy
2.13. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of PEG-PBAE Copolymers
3.2. Characterization of PEG-PBAE Copolymers
3.3. Characterization of Thz/PPM
3.4. pH-Dependent Release of the Drug
3.5. In Vitro Cytotoxicity Assays
3.6. Experiment of MS Formation Rate In Vitro
3.7. In Vitro Cellular Uptake
3.8. Study of Cellular Uptake Mechanism
3.9. Endosomal Escape of Thz/PPM
3.10. In Vivo Anti-Tumor Efficacy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Fernández, L.M. Use of statistics to assess the global burden of breast cancer. Breast J. 2006, 12 (Suppl. 1), S70–S80. [Google Scholar] [CrossRef]
- Gangopadhyay, S.; Nandy, A.; Hor, P.; Mukhopadhyay, A. Breast cancer stem cells: A novel therapeutic target. Clin. Breast Cancer 2013, 13, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.; de Sousae Melo, F.; Richel, D.J.; Medema, J.P. The developing cancer stem-cell model: Clinical challenges and opportunities. Lancet Oncol. 2012, 13, e83–e89. [Google Scholar] [CrossRef]
- Li, X.; Lewis, M.T.; Huang, J.; Gutierrez, C.; Osborne, C.K.; Wu, M.F.; Hilsenbeck, S.G.; Pavlick, A.; Zhang, X.; Chamness, G.C.; et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J. Natl. Cancer Inst. 2008, 100, 672–679. [Google Scholar] [CrossRef]
- Sachlos, E.; Risueño, R.M.; Laronde, S.; Shapovalova, Z.; Lee, J.H.; Russell, J.; Malig, M.; McNicol, J.D.; Fiebig-Comyn, A.; Graham, M.; et al. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell 2012, 149, 1284–1297. [Google Scholar] [CrossRef] [Green Version]
- Di Carlo, R.; Muccioli, G.; Bellussi, G.; Portaleone, P.; Ghi, P.; Racca, S.; Di Carlo, F. Steroid, Prolactin, and Dopamine Receptors in Normal and Pathologic Breast Tissue. Ann. N. Y. Acad. Sci. 1986, 464, 559–562. [Google Scholar] [CrossRef]
- Baker, P.B.; Merigian, K.S.; Roberts, J.R.; Pesce, A.J.; Kaplan, L.A.; Rashkin, M.C. Hyperthermia, hypertension, hypertonia, and coma in a massive thioridazine overdose. Am. J. Emerg. Med. 1988, 6, 346–349. [Google Scholar] [CrossRef]
- Buckley, N.A.; Whyte, I.M.; Dawson, A.H. Cardiotoxicity more common in thioridazine overdose than with other neuroleptics. J. Toxicol. Clin. Toxicol. 1995, 33, 199–204. [Google Scholar] [CrossRef]
- Kwon, G.S.; Okano, T. Polymeric micelles as new drug carriers. Adv. Drug Deliv. Rev. 1996, 21, 107–116. [Google Scholar] [CrossRef]
- Gong, J.; Chen, M.; Zheng, Y.; Wang, S.; Wang, Y. Polymeric micelles drug delivery system in oncology. J. Control. Release 2012, 159, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Toriyabe, N.; Hayashi, Y.; Harashima, H. The transfection activity of R8-modified nanoparticles and siRNA condensation using pH sensitive stearylated-octahistidine. Biomaterials 2013, 34, 1337–1343. [Google Scholar] [CrossRef]
- Ulbrich, K.; Subr, V. Polymeric anticancer drugs with pH-controlled activation. Adv. Drug Deliv. Rev. 2004, 56, 1023–1050. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.J. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zeng, F.; Wu, S.; Wang, X. Polymer micelle with pH-triggered hydrophobic-hydrophilic transition and de-cross-linking process in the core and its application for targeted anticancer drug delivery. Biomacromolecules 2012, 13, 4126–4137. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Zhao, B.; Li, Z.D.; Lin, W.J.; Zhang, C.Y.; Guo, X.D.; Wang, J.F.; Zhang, L.J. pH-sensitive micelles self-assembled from multi-arm star triblock co-polymers poly(ε-caprolactone)-b-poly(2-(diethylamino)ethyl methacrylate)-b-poly(poly(ethylene glycol) methyl ether methacrylate) for controlled anticancer drug delivery. Acta Biomater. 2013, 9, 7679–7690. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yang, Y.Q.; Huang, T.X.; Zhao, B.; Guo, X.D.; Wang, J.F.; Zhang, L.J. Self-assembled pH-responsive MPEG-b-(PLA-co-PAE) block copolymer micelles for anticancer drug delivery. Biomaterials 2012, 33, 6273–6283. [Google Scholar] [CrossRef]
- Kim, J.H.; Li, Y.; Kim, M.S.; Kang, S.W.; Jeong, J.H.; Lee, D.S. Synthesis and evaluation of biotin-conjugated pH-responsive polymeric micelles as drug carriers. Int. J. Pharm. 2012, 427, 435–442. [Google Scholar] [CrossRef]
- Fields, R.J.; Cheng, C.J.; Quijano, E.; Weller, C.; Kristofik, N.; Duong, N.; Hoimes, C.; Egan, M.E.; Saltzman, W.M. Surface modified poly(β amino ester)-containing nanoparticles for plasmid DNA delivery. J. Control. Release 2012, 164, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Tieu, D.D.; Ghodke, B.V.; Vo, N.J.; Perkins, J.A. Single-stage excision of localized head and neck venous malformations using preoperative glue embolization. Otolaryngol. Head Neck Surg. 2013, 148, 678–684. [Google Scholar] [CrossRef]
- Gao, G.H.; Park, M.J.; Li, Y.; Im, G.H.; Kim, J.H.; Kim, H.N.; Lee, J.W.; Jeon, P.; Bang, O.Y.; Lee, J.H.; et al. The use of pH-sensitive positively charged polymeric micelles for protein delivery. Biomaterials 2012, 33, 9157–9164. [Google Scholar] [CrossRef] [PubMed]
- Bailly, N.; Thomas, M.; Klumperman, B. Poly(N-vinylpyrrolidone)-block-poly(vinyl acetate) as a drug delivery vehicle for hydrophobic drugs. Biomacromolecules 2012, 13, 4109–4117. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Guo, S.; Liu, C.; Yang, C.; Dou, J.; Li, L.; Zhai, G. Preparation and in vitro evaluation of apigenin-loaded polymeric micelles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 429, 24–30. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, Y.; Zhao, L.; Zhang, C.; Li, Y.; Li, J.; Li, X.; Liu, Y. Enhanced antitumor efficacy by cyclic RGDyK-conjugated and paclitaxel-loaded pH-responsive polymeric micelles. Acta Biomater. 2015, 23, 127–135. [Google Scholar] [CrossRef]
- Devalapally, H.; Shenoy, D.; Little, S.; Langer, R.; Amiji, M. Poly(ethylene oxide)-modified poly(β-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: Part 3. Therapeutic efficacy and safety studies in ovarian cancer xenograft model. Cancer Chemother. Pharmacol. 2007, 59, 477–484. [Google Scholar] [CrossRef]
- Ko, J.; Park, K.; Kim, Y.S.; Kim, M.S.; Han, J.K.; Kim, K.; Park, R.W.; Kim, I.S.; Song, H.K.; Lee, D.S.; et al. Tumoral acidic extracellular pH targeting of pH-responsive MPEG-poly(β-amino ester) block copolymer micelles for cancer therapy. J. Control. Release 2007, 123, 109–115. [Google Scholar] [CrossRef]
- Na, K.; Lee, K.H.; Bae, Y.H. pH-sensitivity and pH-dependent interior structural change of self-assembled hydrogel nanoparticles of pullulan acetate/oligo-sulfonamide conjugate. J. Control. Release 2004, 97, 513–525. [Google Scholar] [CrossRef]
- Cho, H.J.; Yoon, H.Y.; Koo, H.; Ko, S.H.; Shim, J.S.; Lee, J.H.; Kim, K.; Kwon, I.C.; Kim, D.D. Self-assembled nanoparticles based on hyaluronic acid-ceramide (HA-CE) and Pluronic® for tumor-targeted delivery of docetaxel. Biomaterials 2011, 32, 7181–7190. [Google Scholar] [CrossRef]
- Zhao, L.; Du, J.; Duan, Y.; Zang, Y.; Zhang, H.; Yang, C.; Cao, F.; Zhai, G. Curcumin loaded mixed micelles composed of Pluronic P123 and F68: Preparation, optimization and in vitro characterization. Colloids Surf. B Biointerfaces 2012, 97, 101–108. [Google Scholar] [CrossRef]
- Dabholkar, R.D.; Sawant, R.M.; Mongayt, D.A.; Devarajan, P.V.; Torchilin, V.P. Polyethylene glycol-phosphatidylethanolamine conjugate (PEG-PE)-based mixed micelles: Some properties, loading with paclitaxel, and modulation of P-glycoprotein-mediated efflux. Int. J. Pharm. 2006, 315, 148–157. [Google Scholar] [CrossRef]
- Hong, W.; Chen, D.; Jia, L.; Gu, J.; Hu, H.; Zhao, X.; Qiao, M. Thermo- and pH-responsive copolymers based on PLGA-PEG-PLGA and poly(l-histidine): Synthesis and in vitro characterization of copolymer micelles. Acta Biomater. 2014, 10, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Paull, K.; Alvarez, M.; Hose, C.; Monks, A.; Grever, M.; Fojo, A.T.; Bates, S.E. Rhodamine efflux patterns predict p-glycoprotein substrates in the national cancer institute drug screen. Mol. Pharm. 1994, 46, 627–638. [Google Scholar]
- Wang, L.H.; Rothberg, K.G.; Anderson, R.G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 1993, 123, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Jallouli, Y.; Kroubi, M.; Yuan, X.B.; Feng, W.; Kang, C.S.; Pu, P.Y.; Betbeder, D. Characterization of endocytosis of transferrin-coated PLGA nanoparticles by the blood-brain barrier. Int. J. Pharm. 2009, 379, 285–292. [Google Scholar] [CrossRef]
- Ramírez-García, P.D.; Retamal, J.S.; Shenoy, P.; Imlach, W.; Sykes, M.; Truong, N.; Constandil, L.; Pelissier, T.; Nowell, C.J.; Khor, S.Y.; et al. A pH-responsive nanoparticle targets the neurokinin 1 receptor in endosomes to prevent chronic pain. Nat. Nanotechnol. 2019, 14, 1150–1159. [Google Scholar] [CrossRef]
- Truong, N.P.; Gu, W.; Prasadam, I.; Jia, Z.; Crawford, R.; Xiao, Y.; Monteiro, M.J. An influenza virus-inspired polymer system for the timed release of siRNA. Nat. Commun. 2013, 4, 1902. [Google Scholar] [CrossRef] [Green Version]












| Copolymer | Mw(Da) a | Mn(Da) a | Mw/Mn a | Mn(Da) b | Mp | pKa |
|---|---|---|---|---|---|---|
| PBAE | 5646 | 4773 | 1.18 | 5024 | 6129 | 6.6 |
| PEG-PBAE | 7325 | 6534 | 1.13 | 6915 | 8040 | 6.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Sun, J.; Zhang, X.; Jia, L.; Qiao, M.; Zhao, X.; Hu, H.; Chen, D.; Wang, Y. Synthesis and Characterization of pH-Responsive PEG-Poly(β-Amino Ester) Block Copolymer Micelles as Drug Carriers to Eliminate Cancer Stem Cells. Pharmaceutics 2020, 12, 111. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12020111
Li W, Sun J, Zhang X, Jia L, Qiao M, Zhao X, Hu H, Chen D, Wang Y. Synthesis and Characterization of pH-Responsive PEG-Poly(β-Amino Ester) Block Copolymer Micelles as Drug Carriers to Eliminate Cancer Stem Cells. Pharmaceutics. 2020; 12(2):111. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12020111
Chicago/Turabian StyleLi, Weinan, Jialin Sun, Xiaoyu Zhang, Li Jia, Mingxi Qiao, Xiuli Zhao, Haiyang Hu, Dawei Chen, and Yanhong Wang. 2020. "Synthesis and Characterization of pH-Responsive PEG-Poly(β-Amino Ester) Block Copolymer Micelles as Drug Carriers to Eliminate Cancer Stem Cells" Pharmaceutics 12, no. 2: 111. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12020111