1. Introduction
Vitamin A derivatives are a group of lipid-soluble compounds including retinol, retinal, retinyl acetate, retinyl linoleate and retinyl palmitate (RP). Retinoids have important effects on skin cells. Retinoid levels in the skin are involved in the correct cellular maturation of keratinocytes, and when the skin is damaged, they induce keratinocyte proliferation and modulate epidermal differentiation [
1]. Moreover, retinoids stimulate the production of extracellular matrix proteins such as collagen I by dermal fibroblasts. Alterations in these levels produce a de-structuring of corneocytes’ layers and, consequently, an increase in transepidermal water loss. Retinoids can also lighten hyperpigmented skin by decreasing melanocyte tyrosinase activity, inhibit the sebocyte proliferation and lipid synthesis, and alter their keratin expression. Alterations in retinoids skin levels can cause skin dehydration, lack of elasticity, sebum overproduction and hyperpigmentation, among other effects [
2,
3].
As retinoids cannot be synthesized by the body, they must be supplied through other sources [
4]. Since these molecules are easily degraded by oxidation or photodegradation, and they are very hydrophobic, their topical bioavailability when applied on the skin surface remains quite low. In addition, retinoids have adverse effects such as hepatotoxicity, changes in lipid metabolism and bone density, teratogenicity, and they can cause significant skin irritation. Most of these effects occur after oral administration. Regarding topical administration, the main adverse effects are phototoxicity and skin irritation. One plausible mechanism of phototoxicity may be related to the formation of free radicals after the exposure of retinoids to UV light that damages the DNA. Relevant clinical studies or studies in animal models are therefore needed to establish whether the pro-oxidant activity of photoexcited vitamin A is observed in vivo, and to assess the related risks [
5].
Skin irritation is linked to retinoids due to its pharmacological effects through retinoic acid receptor signaling [
6]. Cytokines such as IL-1, TNF-α, IL-6, and IL-8 are thought to be more important in retinoid-induced dermatitis [
7]. Retinyl Palmitate was irritating to rabbits’ skin, and a slight irritant to rabbit eyes. [
4]. Thus, although retinoids have been classically incorporated into emulsions to overcome some of these limitations, skin irritation and photodegradability issues are still a problem [
8]. The inclusion of retinol derivatives such as retinyl palmitate into nanocarriers for topical delivery is an interesting strategy that can lead to higher stability and enhance skin penetration [
9]. Liposomes are a suitable choice for retinoids’ encapsulation, as the active ingredients can be incorporated into the membrane of the particles, ending in skin penetration and enhanced stability.
Liposomes are spherical vesicles formed of a lipid bilayer and an aqueous cavity. They consist of phospholipids or synthetic amphiphilic molecules, usually combined with sterols to reduce their membrane permeability. Phospholipids tend to self-assemble in the presence of water due to their amphiphilic nature. The hydrophilic head is oriented towards the water, while the apolar tails are located in the inner part of the bilayer. The nature of the lipids will determine the liposomes’ properties. Saturated phospholipids can obtain liposomes with a lower permeability and greater stability than unsaturated phospholipids [
10].
Classic liposomes usually accumulate in the stratum corneum and skin annexes. Therefore, they are not a good means to reach deeper layers of the skin or for transdermal absorption [
11]. Therefore, different liposomal approaches have appeared. One example of these are ethosomes, which have ethanol in the vesicle cavity that behaves as a penetration promoter [
12]. Another example are transfersomes, which are ultra-deformable liposomes [
13]. This type of liposome has an “edge activator” (i.e., sodium cholate, sodium deoxycholate, span 80, Tween 80 or dipotassium glycyrrhizinate, among others) in its lipid membrane that allows it to increase its elasticity. There are several theories that explain the high penetration ability of these vesicles. The most accepted theory is that the high deformability of the transfersomes allows them to cross the intercellular channels of the stratum corneum [
14,
15]. Several researchers have demonstrated the improvement in topical penetration, for example, with retinol in dermatomized human skin and the keratinocites 3D model [
9], and with lidocaine-loaded transfersomes, in order to avoid a painful local anesthetic injection [
16].
The bilayer lipid matrix of cell membranes is composed of a mixture of different lipids. Among them, there is a growing interest in sphingolipids due their effects on skin cellular processes. Ceramides are a structurally heterogeneous and complex group of sphingolipids. It is well known that ceramides play an essential role in structuring and maintaining the water permeability barrier function of the skin. Ceramides maintain the dense crystalline structure of the lamellar lipids that are arranged between the corneocytes. They represent the 50% of lipids in the stratum corneum [
17]. The rest of the lipids of the stratum corneum are cholesterol and free fatty acids. Together, they keep the lamellar structure of the stratum corneum and the barrier function of healthy skin in good condition. However, most skin disorders that have a diminished barrier function present a decrease in total ceramide content, with some differences in the ceramide pattern. Alterations in ceramide III levels are related to different skin diseases. In psoriatic skin, ceramides III and VII show a significant decrease versus normal stratum corneum [
18]. In patients with atopic dermatitis, a decrease in the amount of ceramide III has been demonstrated to be correlated with an increase in transepidermal water loss [
19]. Formulations containing lipids identical to those in skin and, in particular, ceramide supplementation, could improve disturbed skin conditions. Several authors have introduced ceramides in lipid-based vesicles to deliver them into the skin to restore lipid composition and to improve altered skin permeability [
20,
21]. Exogenously supplied, short-chain ceramides, such as ceramide III, induced keratinocyte differentiation in vitro and reinforced the pro-differentiation effects of other drugs [
17].
Based on the effects of ceramide III and retinyl palmitate, as previously described, the formulations in this study were designed to improve and maintain the skin’s barrier properties. The aims of this work were the development and characterization of a ceramide III-based transfersome cream formulation encapsulating retinyl palmitate, and the study of RP biodistribution through the different skin layers.
2. Materials and Methods
2.1. Materials
Ceramide III (Evonik Nutrition & Care, Essen, Germany), α-Tocopherol (Merck Chemicals and life, Barcelona, Spain), phosphatidylcholine (Lipoid, Ludwigshafen, Germany), Tween 80 (Croda Iberica S.A., Barcelona, Spain), Retynil Palmitate and Ethanol Absolute (Scharlab S.L. Barcelona, Spain) and purified water (Inhouse) were used to formulate the transfersomes. Dissodium EDTA (Sucesores de Jose Escuder, S.L., Barcelona, Spain), PEG-6 stearate (and) Ceteth-20 (and) Steareth-20 (Gattefosse España, Madrid, Spain), Cetyl Sterayl alcohol (Basf, Barcelona, Spain), medium chain triglicerides (Oximed expres S.A., Barcelona, Spain) and Xanthan gum (Azelis españa S.A., Barcelona, Spain) were used to formulate the emulsion. Metanol (Scharlab S.L., Barcelona, Spain), Nile Red, Hoeschst, phosphate buffer saline, paraformaldehyde (Sigma Aldrich, Madrid, Spain), uranyl acetate, optimal cutting temperature compound (IESMAT S.A., Barcelona, Spain), were used to perform the different analyses.
2.2. Production of Retinyl Palmitate-Loaded Transfersomes
Transfersomes were manufactured by the sonication method [
22,
23]. α-Tocopherol (0.02%
w/
w), ceramide III (0.10%
w/
w), phosphatydilcholine (1.78%
w/
w), tween 80 (0.10%
w/
w) and RP (1.10%
w/
w) were dissolved in ethanol (10%
w/
w) (organic phase). Then, milliQ water (qs 100%
w/
w) was added to the organic phase, and the system was vortexed for 1 min. Afterwards, the mixture was sonicated with a probe sonicator (amplitude of 80%, 5 min, Energy 7000 Ws, Frequency 23.88 kHz). The transfersomes’ suspension was left to settle at room temperature, protected from the light. Additionally, empty transfersomes (without RP) were fabricated, to study the effect of RP on the physio-chemical characteristics of the nanosystems.
2.3. Transfersome Incorporation in a Cream Formulation
A mixture of surfactants (PEG-6 stearate (and) Ceteth-20 (and) Steareth-20 4%
w/
w and Cetyl Stearyl Alcohol 0.5%
w/
w), oils (Medium Chain Triglycerides or MCT 3%
w/
w) and an aqueous phase (12.6%
w/
w) with Xanthan gum (0.1%
w/
w) and disodium EDTA (0.1%
w/
w) were warmed up separately at 70–80 °C in a thermostatic bath. Once both phases were heated, the oil phase was added to the aqueous phase under mechanical stirring at 15,000 rpm for 2 min (Ultra-Turrax IKA T25, disperser unit S25KD 25F), and the mixture was allowed to cool down at 30 °C. Then, the transfersomes aqueous suspension, with RP at a concentration of 1.1%
w/
w, was added to the cream at a ratio of 1:1, so that the final RP concentration was 0.55%
w/
w (chosen taking the recommended concentrations for Retinol and Retinyl palmitate into account [
5]). For the manufacture of the non-transfersomes emulsion, water was added up to 100%.
2.4. Transfersomes Physic-Chemical Characterization
RP-loaded transfersomes were subjected to a stability study in 25 ± 2 °C/60% ± 5% RH and 40 ± 2 °C/75% ± 5% RH chambers for 18 and 6 months, respectively, and conditioned in hermetically sealed glass vials.
Hydrodynamic size, polydispersion index (PDI) and zeta potential were studied through dynamic light scattering (DLS) using a Zetasizer Nano ZS (Malvern, UK). Dilutions of 1:10 in water were used for the measurements.
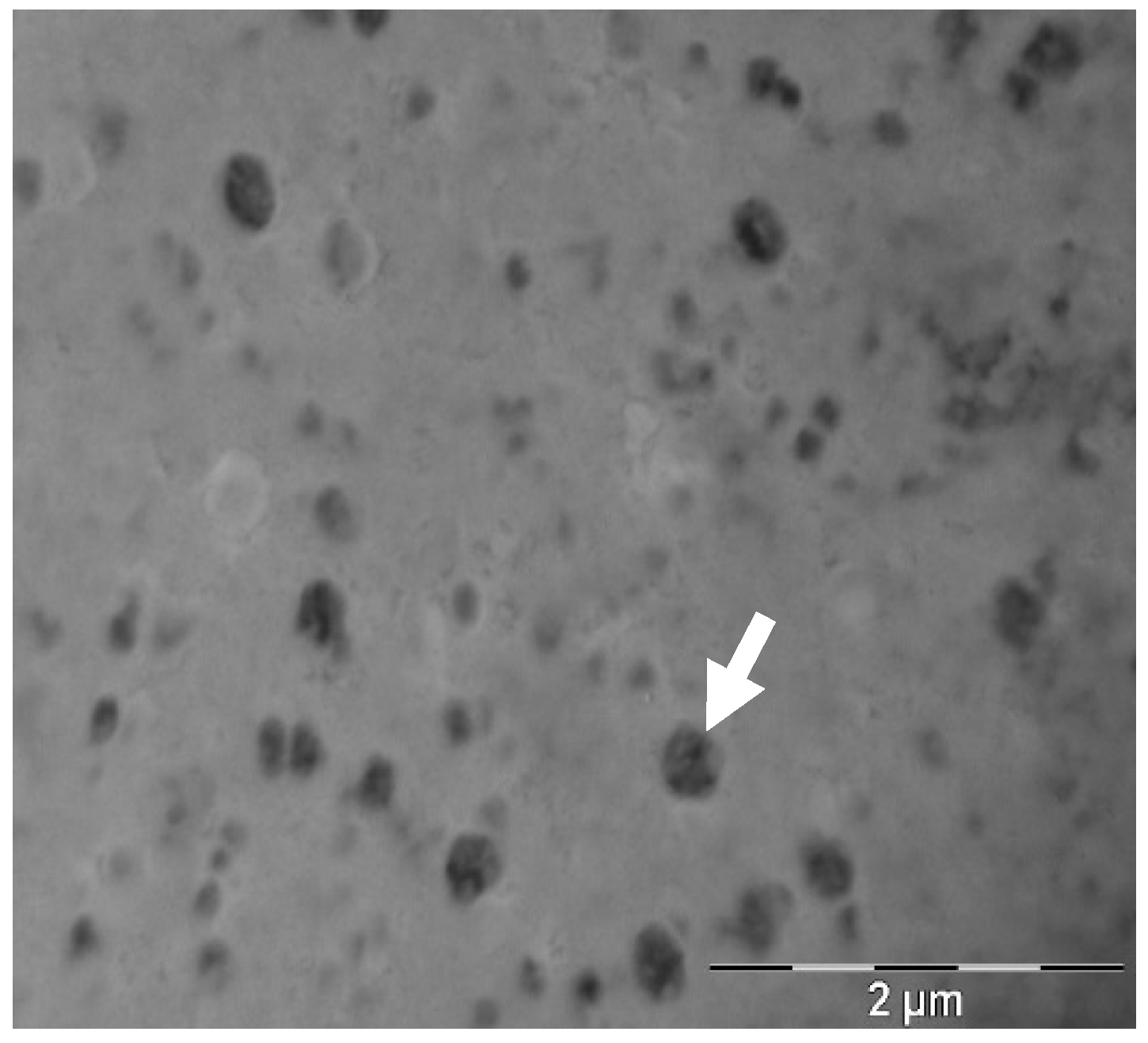
Transfersome morphology was studied through transmission electron microscopy (TEM) using a Jeol JEM 1010 100 kv (Jeol, Tokyo, Japan). TEM grids were coated with formvar of a 1:10 transfersome dilution in milliQ water and incubated for 1 min at room temperature. Grids were then washed with water and stained with a 2% w/w uranyl acetate solution for 1 min at room temperature. Afterwards, they were dried in overnight and analyzed within two weeks of staining.
The flexibility of the transfersomes was analyzed by extruding the transfersomes solution in an Avanti Mini Extruder with a 100 nm pore size polycarbonate membrane, at 1 mL of volume capacity. Pressure was applied by hand. The ability of the transfersomes to recover their initial size after extrusion was analyzed though DLS. The deformability index (DI) was defined as Equation (1),
where
rp is the radius of a the extruded transfersomes and
rm is the radius of the membrane pores [
24].
2.5. Cream Physic-Chemical Characterization
Appearance (visual observation), pH (pHmeter Crison Instruments S.A. Alella, Spain) and viscosity (Brookfield RDV-III Ultra, Spain. Spindler: SC4-21, Speed: 200 rpm, Temperature: 25 °C, Spain) were studied for the transfersome- and non-transfersome-loaded emulsions.
2.6. Diffusion Assay of RP-Loaded Transfersomes
In vitro diffusion of free RP and RP from the transfersomes (n = 6) was studied using vertical Franz Cells (VidraFoc, Spain, receptor compartment of 12 mL, diffusional area of 2.54 cm2). MCT was used as a receptor medium (RM) to keep sink conditions along the experiment. The dose of each formulation tested in the donor compartment was 240 mg (1.04 mg/cm2). The temperature of the experiment was maintained at 32 °C, and the stirring speed of the RM was 500 rpm. The membrane used was Polyvinylidne Fluoride (PVDF, Millipore, Spain) of a pore diameter 0.22 µm.
Aliquots of 300 µL were taken at times 1, 2, 3, 4, 6, 24 and 30 h and injected into HPLC to quantify the amount of diffused RP.
2.7. RP HPLC Analysis and Encapsulation Efficiency
The encapsulation efficiency (% EE) of RP in the liposomes was determined indirectly (Equation (2)). Briefly, transfersomes were centrifuged in 30 KDa Amicon ultracentrifugal filter (Merck Millipore) at 4500 rpm for 30 min. The amount of RP in the filtrate and in transfersomes were analyzed using a HPLC (Waters 2695, Spain), with a photodiode array detector (Waters 2996, Spain) with the corresponding calibration curve (Range 3.40 to 280 µg/mL with an r
2 > 0.999). The column was a C18 (12.5 × 4.6 mm) with particle size of 5 µm. The mobile phase was an isocratic mixture of Metanol:water (98:2). The flow rate was 1.8 mL/min and the injection volume was 20 µL. the samples’ temperature was set at 5 °C and column temperature at 40 °C. % EE was determined using Equation (2),
where
WNE is the amount of RP quantified in the filtrate (RP not encapsulated) and
WT is the RP quantified in the total amount of RP used for the preparation of transfersomes.
2.8. Pig Skin Penetration Assays
Three to four month old male and female pigs were obtained from the Animal Facility at Bellvitge Campus of Barcelona University (Barcelona, Spain). Immediately after the animals (n = 3) were sacrificed, using an overdose of sodium thiopental anesthesia, the ears were surgically removed and frozen. On the day of experiment, ears were defrosted and the skin was excised.
2.8.1. Skin Penetration Assay: Full Thickness Pig Ear Skin
Skin samples were mounted on Franz-cells (
n = 6) according to the description in
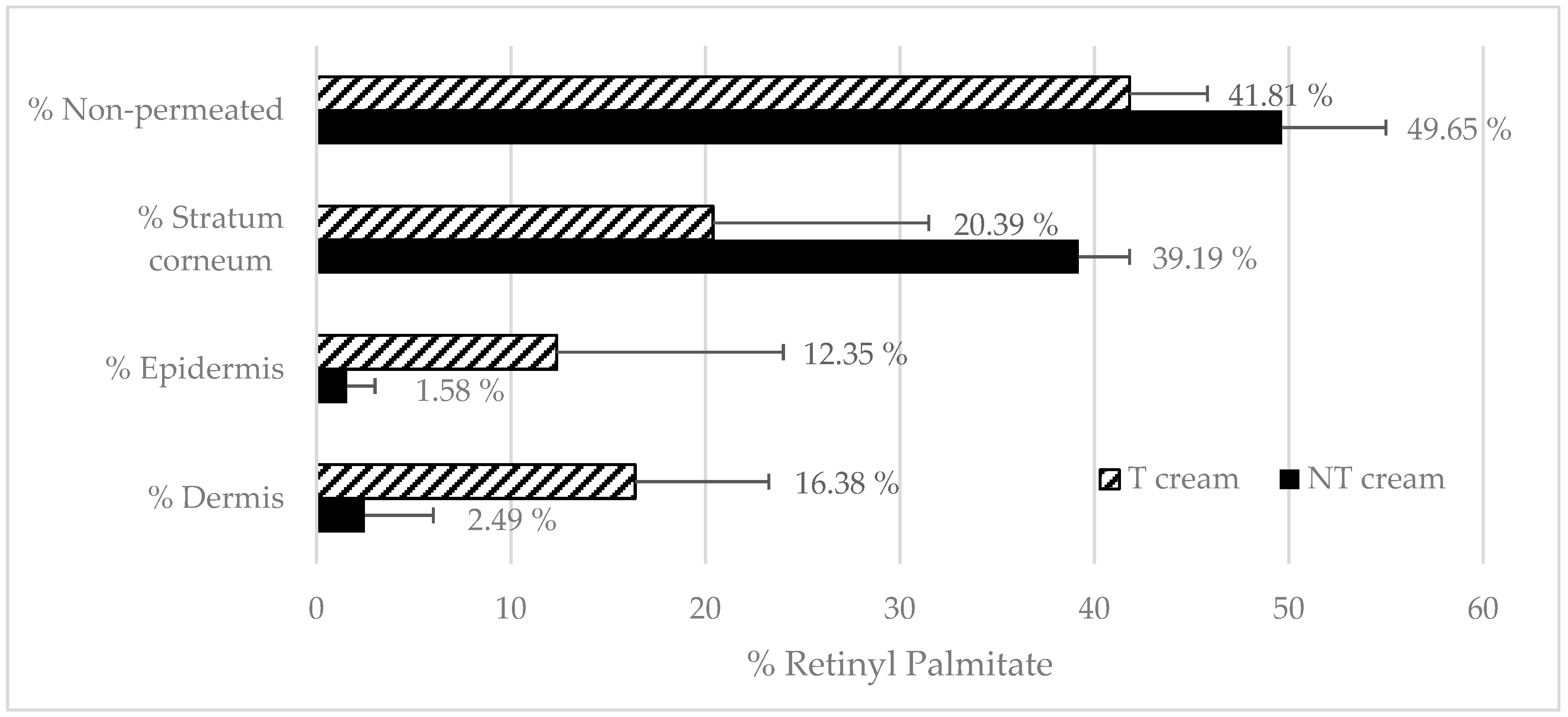
Section 2.6. The following formulations were tested: RP-loaded transfersomes, a free RP solution in MCT, transfersome-loaded emulsion (T emulsion) and a non-encapsulated RP emulsion. An amount of 240 mg of formula was administered in infinite doses in non-occluded conditions.
After 24 h, RP mass-balance was performed: RM was analyzed directly by mean HPLC, then the non-penetrated formulation in the donor compartment (non-penetrated) was recovered and RP was extracted from the emulsion according to the method described in
Section 2.7. Skin pieces were taken and washed with distillated water (wash), and stratum corneum, epidermis and dermis were obtained, and RP extracted according to the methodology described in
Section 2.8.2 and analyzed by HPLC [
25,
26].
2.8.2. Skin Layers Recovery
An incubation solution of RP in receptor medium at a concentration of 0.22 mg/mL was prepared, then skin layers were separated and incubated for 24 h with the incubation solution. After incubation, an extraction process was performed. Skin samples were subjected to 20 min of bath sonication in Metanol:water (98:2) and RP from the different skin layers was quantified by means of HPLC (
Section 2.7). Stratum corneum was removed by applying 30 tape strips (pressure 225 g/cm
2 for three seconds [
27]). To separate epidermis and dermis, samples (after stripping) were immersed in 60 °C PBS for 2 min and excised with the help of forceps and a scalpel. The recovery percentage was applied to the results obtained in the penetration assays.
The percentage of recovery was calculated from Equation (3), where “Qextracted” is the amount of RP extracted from the sample after solvent incubation (Metanol:Water 98:2), “Q0h” is the initial amount of RP in the incubation solution at time 0, “Q24h” is the amount of RP in the incubation formula after 24 h of experiment and “Sample mass” is the mass of each skin layer sample. RP analyses were performed according to
Section 2.7.
2.9. Fluorescence Biodistribution Assay
To study the biodistribution of the transfersomes in the skin, fluorescent-marked transfersomes were incubated on top of full-thickness ear pig skin samples using vertical Franz Cells (according to
Section 2.8.1). Non-loaded transfersomes (autofluorescence control), Nile red-loaded transfersomes and free Nile red solution were added to the experiment (all at concentrations of 0.312 µg/mL).
After 24 h of incubation, skin samples were taken and cut into 0.25 cm2 pieces. They were then fixed with 4% paraformaldehyde (PF) for 30 min. Then, samples were washed with a phosphate buffer solution (PBS), and incubated in increasing concentrations of sucrose (up to 30% w/w) as a cryoprotectant. Samples were mounted in an optimal cutting temperature compound (OCT, from Fischer Scientific) and cut in the cryostat (Leica Biosystems) with a thickness between 30 and 50 µm, and placed on the superfrost slides with poly-lysine coating.
The slides were washed with PBS to remove the remaining OCT and incubated with Hoeschst (2 µg/mL) for 30 min, and then washed with PBS. Samples were visualized by a Leica DMIRB Wide Field Fluorescence and Transmitted Light Microscope [
28].
2.10. TEWL after In Vivo Topical Administration
An in vivo test was carried out in humans with T and NT emulsion. The study was conducted according to the Declaration of Helsinki. Volunteers gave their written consent. Transepidermal water loss (TEWL) was measured by the Vapometer (Delfin Technologies, Kuopio, Finland) before and after the application of the different creams to monitor SC removal [
29]. A template with three application zones (NT emulsion, T emulsion and negative control: no emulsion applied) with an area of 2.54 cm
2 for the forearm was used.
Six male and female individuals (
n = 6) with ages ranging from 23 to 44 years old participated. An amount of 0.025 g of each emulsion was applied on the skin by the same operator in the same conditions. After 2 h, skin was gently cleaned [
30].