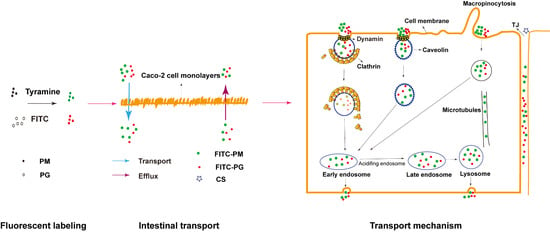
Transport Mechanisms of Polymannuronic Acid and Polyguluronic Acid Across Caco-2 Cell Monolayers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Cells, Antibodies, and Small Interfering RNAs
2.2. Fluorescent Labeling of PM and PG and Verification
2.3. Establishment of the Caco-2 Cell Monolayer Model
2.4. Cytotoxicity Tests
2.5. Transport Mechanisms of F-PM and F-PG
2.6. Determination of Cholesterol Content
2.7. Effect of CS on TJ
2.8. Western Blotting
2.9. siRNA Transfection and Drug Uptake
2.10. Statistical Analysis
3. Results
3.1. Fluorescent Labeling of PM and PG
3.2. Establishment and Verification of Caco-2 Cell Monolayers Model
3.3. Cytotoxicity of PM and PG
3.4. Effects of Time, Concentration, and Temperature on the Transport of F-PM and F-PG
3.5. Effects of Transport Inhibitors on the Transport of F-PM and F-PG
3.6. Effect of Intracellular Inhibitors on the Transport of F-PM and F-PG
3.7. Effect of Absorption Enhancer on the Transport of F-PM and F-PG
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fertah, M.; Belfkira, A.; Dahmane, E.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Wang, X.; Shi, H.J.; Wang, Y.M.; Xue, C.H.; Tang, Q.J. Polymannuronic acid ameliorated obesity and inflammation associated with a high-fat and high-sucrose diet by modulating the gut microbiome in a murine model. Brit. J. Nutr. 2017, 117, 1332–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, M.; Hiroki, T.; Takeshita, S.; Jiang, Z.D.; Kim, D.; Yamaguchi, K.; Oda, T. Comparative study on antioxidative and macrophage-stimulating activities of polyguluronic acid (PG) and polymannuronic acid (PM) prepared from alginate. Carbohyd. Res. 2012, 352, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujihara, M.; Nagumo, T. An Influence of the Structure of Alginate on the Chemotactic Activity of Macrophages and the Antitumor-Activity. Carbohyd. Res. 1993, 243, 211–216. [Google Scholar] [CrossRef]
- Fujihara, M.; Nagumo, T. The Effect of the Content of d-Mannuronic Acid and l-Guluronic Acid Blocks in Alginates on Antitumor-Activity. Carbohyd. Res. 1992, 224, 343–347. [Google Scholar] [CrossRef]
- Stabler, C.; Wilks, K.; Sambanis, A.; Constantinidis, I. The effects of alginate composition on encapsulated beta TC3 cells. Biomaterials 2001, 22, 1301–1310. [Google Scholar] [CrossRef]
- Chai, G.H.; Xu, Y.K.; Chen, S.Q.; Cheng, B.L.; Hu, F.Q.; You, J.A.; Du, Y.Z.; Yuan, H. Transport Mechanisms of Solid Lipid Nanoparticles across Caco-2 Cell Monolayers and their Related Cytotoxicology. Acs. Appl. Mater. Inter. 2016, 8, 5929–5940. [Google Scholar] [CrossRef] [PubMed]
- Salama, N.N.; Eddington, N.D.; Fasano, A. Tight junction modulation and its relationship to drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 15–28. [Google Scholar] [CrossRef]
- Wang, K.P.; Cheng, F.; Pan, X.L.; Zhou, T.; Liu, X.Q.; Zheng, Z.M.; Luo, L.; Zhang, Y. Investigation of the transport and absorption of Angelica sinensis polysaccharide through gastrointestinal tract both in vitro and in vivo. Drug Deliv. 2017, 24, 1360–1371. [Google Scholar] [CrossRef] [Green Version]
- Riezman, H.; Woodman, P.G.; van Meer, G.; Marsh, M. Molecular mechanisms of endocytosis. Cell 1997, 91, 731–738. [Google Scholar] [CrossRef] [Green Version]
- Zhang, E.; Chu, F.L.; Xu, L.X.; Liang, H.; Song, S.L.; Ji, A.G. Use of fluorescein isothiocyanate isomer I to study the mechanism of intestinal absorption of fucoidan sulfate in vivo and in vitro. Biopharm. Drug Dispos. 2018, 39, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Sevin, E.; Dehouck, L.; Fabulas-da Costa, A.; Cecchelli, R.; Dehouck, M.P.; Lundquist, S.; Culot, M. Accelerated Caco-2 cell permeability model for drug discovery. J. Pharm. Tox. Met. 2013, 68, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.B.; Calpena, A.C.; Mallandrich, M.; Clares, B. Validation of an Ex Vivo Permeation Method for the Intestinal Permeability of Different BCS Drugs and Its Correlation with Caco-2 In Vitro Experiments. Pharmaceutics 2019, 11, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabria, A.R.; Weidenfeller, C.; Jones, A.R.; de Vries, H.E.; Shusta, E.V. Puromycin-purified rat brain microvascular endothelial cell cultures exhibit improved barrier properties in response to glucocorticoid induction. J. Neurochem. 2006, 97, 922–933. [Google Scholar] [CrossRef]
- Demeuse, P.; Fragner, P.; Leroy-Noury, C.; Mercier, C.; Payen, L.; Fardel, O.; Couraud, P.O.; Roux, F. Puromycin selectively increases mdr1a expression in immortalized rat brain endothelial cell lines. J. Neurochem. 2004, 88, 23–31. [Google Scholar] [CrossRef]
- Duizer, E.; Gilde, A.J.; Versantvoort, C.H.M.; Groten, J.P. Effects of cadmium chloride on the paracellular barrier function of intestinal epithelial cell lines. Toxicol. Appl. Pharm. 1999, 155, 117–126. [Google Scholar] [CrossRef]
- Martirosyan, A.; Grintzalis, K.; Polet, M.; Laloux, L.; Schneider, Y.J. Tuning the inflammatory response to silver nanoparticles via quercetin in Caco-2 (co-)cultures as model of the human intestinal mucosa. Toxicol. Lett. 2016, 253, 36–45. [Google Scholar] [CrossRef]
- Kowapradit, J.; Opanasopit, P.; Ngawhirunpat, T.; Rojanarata, T.; Ruktanonchai, U.; Sajomsang, W. Methylated N-(4-N,N-dimethylaminocinnamyl) chitosan enhances paracellular permeability across Caco-2 cells. Drug Deliv. 2010, 17, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv. Rev. 2012, 64, 280–289. [Google Scholar] [CrossRef]
- Billat, P.A.; Roger, E.; Faure, S.; Lagarce, F. Models for drug absorption from the small intestine: Where are we and where are we going? Drug Discov. Today 2017, 22, 761–775. [Google Scholar] [CrossRef] [Green Version]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, W.W.; Fan, Y.C.; Zheng, N.; He, B.; Yuan, L.; Zhang, H.; Wang, X.Q.; Wang, J.C.; Zhang, X.; Zhang, Q. Transferrin receptor specific nanocarriers conjugated with functional 7peptide for oral drug delivery. Biomaterials 2013, 34, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Kadlecova, Z.; Spielman, S.J.; Loerke, D.; Mohanakrishnan, A.; Reed, D.K.; Schmid, S.L. Regulation of clathrin-mediated endocytosis by hierarchical allosteric activation of AP2. J. Cell Biol. 2017, 216, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Macia, E.; Ehrlich, M.; Massol, R.; Boucrot, E.; Brunner, C.; Kirchhausen, T. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 2006, 10, 839–850. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.S.; Dai, W.B.; He, B.; Wang, J.C.; He, Z.G.; Zhang, X.; Zhang, Q. Monitoring the transport of polymeric micelles across MDCK cell monolayer and exploring related mechanisms. J. Control Release 2012, 158, 413–423. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Xu, Q.Q.; Wu, D.G.; Ren, H.; Zhao, P.; Lao, W.G.; Wang, Y.; Tao, Q.Y.; Qian, X.J.; Wei, Y.H.; et al. Japanese Encephalitis Virus Enters Rat Neuroblastoma Cells via a pH-Dependent, Dynamin and Caveola-Mediated Endocytosis Pathway. J. Virol. 2012, 86, 13407–13422. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.L.; Liu, Y.G.; Zhou, Y.T.; Zhao, P.; Ren, H.; Xiao, M.; Zhu, Y.Z.; Qi, Z.T. Endophilin-A2-mediated endocytic pathway is critical for enterovirus 71 entry into caco-2 cells. Emerg. Microbes. Infec. 2019, 8, 773–786. [Google Scholar] [CrossRef]
- Jiang, R.L.; Lopez, V.; Kelleher, S.L.; Lonnerdal, B. Apo- and Holo-Lactoferrin Are Both Internalized by Lactoferrin Receptor via Clathrin-Mediated Endocytosis but Differentially Affect ERK-Signaling and Cell Proliferation in Caco-2 Cells. J. Cell Physiol. 2011, 226, 3022–3031. [Google Scholar] [CrossRef] [Green Version]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control Release 2014, 190, 485–499. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, S.; Lu, X.N.; Zhang, H.R.; Fu, Y.; Luo, Y.Z. Cholesterol sequestration by nystatin enhances the uptake and activity of endostatin in endothelium via regulating distinct endocytic pathways. Blood 2011, 117, 6392–6403. [Google Scholar] [CrossRef] [Green Version]
- Weimbs, T.; Low, S.H.; Chapin, S.J.; Mostov, K.E. Apical targeting in polarized epithelial cells: there’s more afloat than rafts. Trends Cell Biol. 1997, 7, 393–399. [Google Scholar] [CrossRef]
- Hagiwara, K.; Nakata, M.; Koyama, Y.; Sato, T. The effects of coating pDNA/chitosan complexes with chondroitin sulfate on physicochemical characteristics and cell transfection. Biomaterials 2012, 33, 7251–7260. [Google Scholar] [CrossRef] [PubMed]
- Steed, E.; Balda, M.S.; Matter, K. Dynamics and functions of tight junctions. Trends Cell Biol. 2010, 20, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Banan, A.; Zhang, L.J.; Shaikh, M.; Fields, J.Z.; Choudhary, S.; Forsyth, C.B.; Farhadi, A.; Keshavarzian, A. theta isoform of protein kinase C alters barrier function in intestinal epithelium through modulation of distinct claudin isotypes: A novel mechanism for regulation of permeability. J. Pharm. Exp. 2005, 313, 962–982. [Google Scholar] [CrossRef]
- Fu, Q.X.; Wang, H.Z.; Xia, M.X.; Deng, B.; Shen, H.Y.; Ji, G.; Li, G.W.; Xie, Y. The effect of phytic acid on tight junctions in the human intestinal Caco-2 cell line and its mechanism. Eur. J. Pharm. Sci. 2015, 80, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.W.; Chan, J.M.; Farokhzad, O.C. pH-Responsive Nanoparticles for Drug Delivery. Mol. Pharm. 2010, 7, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Mi, F.L.; Liao, Z.X.; Hsiao, C.W.; Sonaje, K.; Chung, M.F.; Hsu, L.W.; Sung, H.W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Wey, S.P.; Juang, J.H.; Sonaje, K.; Ho, Y.C.; Chuang, E.Y.; Hsu, C.W.; Yen, T.C.; Lin, K.J.; Sung, H.W. The glucose-lowering potential of exendin-4 orally delivered via a pH-sensitive nanoparticle vehicle and effects on subsequent insulin secretion in vivo. Biomaterials 2011, 32, 2673–2682. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Yeh, T.H.; Hsu, L.W.; Tseng, M.T.; Lee, P.L.; Sonjae, K.; Ho, Y.C.; Sung, H.W. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials 2011, 32, 6164–6173. [Google Scholar] [CrossRef]
- Smith, J.; Wood, E.; Dornish, M. Effect of chitosan on epithelial cell tight junctions. Pharm Res-Dordr. 2004, 21, 43–49. [Google Scholar] [CrossRef] [PubMed]







| Factors | Apical Side (0.4 mL) | Basal Side (1.0 mL) | Temperature (°C) | Transport Time (h) |
|---|---|---|---|---|
| Transport | F-PM/F-PG 1 (50 μg/mL) | HBSS | 37 | 0.5, 1, 2, 4 |
| Efflux | HBSS | F-PM/F-PG (50 μg/mL) | 37 | 0.5, 1, 2, 4 |
| Concentration | F-PM/F-PG (50,100,200,400,800 μg/mL) | HBSS | 37 | 2 |
| Temperature | F-PM/F-PG (50 μg/mL) | HBSS | 4/37 | 2 |
| Inhibitors 2 | inhibitors for 1 h (remove) F-PM/F-PG (50 μg/mL) | HBSS | 37 | 2 |
| Transferrin | chlorpromazine or dynasore for 1 h FITC-transferrin (10 μg/mL) | HBSS | 37 | 2 |
| Dextran | EIPA for 1 h (remove) FITC-dextran (10 μg/mL) | HBSS | 37 | 2 |
| Absorption enhancer | 0.25% CS (pH = 6.5) and F-PM/F-PG (50μg/mL) 3 | HBSS | 37 | 2 |
| Permeability | sodium fluorescein (10 μg/mL) | HBSS | 37 | 0.5, 1, 2, 3, 4 |
| Transport Inhibitors | Functions | Concentrations |
|---|---|---|
| Endocytosis Process | ||
| chlorpromazine | inhibitor of clathrin-related route | 20 μg/mL |
| dynasore | block the formation of a pinched-off vesicle | 30 μg/mL |
| MβCD | caveolae (or lipid raft)-mediated route | 2.5 mg/mL |
| EIPA | inhibit the macropinocytosis pathway | 20 μg/mL |
| Endocellular Transport Process | ||
| monensin | inhibit acidification of endosomes | 30 μg/mL |
| nocodazole | inhibitor of microtubules | 10 μg/mL |
| bafilomycin A1 | inhibit the maturation process of lysosomes | 100 μM |
| Conditions | Ex Max (nm) | Em Max (nm) | ||
|---|---|---|---|---|
| Wavelength | Intensity (lnt) | Wavelength | Intensity (lnt) | |
| PM | 329.0 | 7.823 | - | - |
| PG | 330.6 | 9.263 | - | - |
| FITC | 495.5 | 130.555 | 525.9 | 340.020 |
| FITC and PM | 512.5 | 140.311 | 529.0 | 399.075 |
| FITC and PG | 511.8 | 139.330 | 528.6 | 400.653 |
| F-PM | 494.0 | 63.748 | 514.6 | 385.438 |
| F-PG | 491.2 | 49.981 | 513.5 | 304.158 |
| Sample | C0(μg/mL) | The Fluorescence Intensity | CF(μg/mL) | RL (%) | The Mean of RL (%) |
|---|---|---|---|---|---|
| F-PM 1 | 50 | 6,052,844 | 6.16 | 12.32 | 12.66 |
| 25 | 3,138,633 | 3.15 | 12.62 | ||
| 12.5 | 1,660,217 | 1.63 | 13.05 | ||
| F-PG | 50 | 4,699,162 | 4.77 | 9.53 | 9.52 |
| 25 | 2,405,221 | 2.40 | 9.60 | ||
| 12.5 | 1,220,862 | 1.14 | 9.43 | ||
| FITC-transferrin | 6.25 | 6,049,258 | 6.15 | 98.47 | 98.49 |
| 3.12 | 3,026,260 | 3.04 | 97.25 | ||
| 1.56 | 1,589,678 | 1.56 | 99.76 | ||
| FITC-dextran | 6.25 | 3,215,504 | 3.26 | 51.75 | 52.51 |
| 3.12 | 1,648,774 | 1.60 | 51.83 | ||
| 1.56 | 895,269 | 0.84 | 53.96 |
| Time (min) | 30 | 60 | 120 | 180 | 240 |
|---|---|---|---|---|---|
| Papp 1 (×10−7 cm/s) | 4.05 ± 0.742 | 1.83 ± 0.170 | 1.07 ± 0.268 | 0.79 ± 0.113 | 0.70 ± 0.028 |
| RT (%) | 0.02 ± 0.004 | 0.05 ± 0.003 | 0.07 ± 0.009 | 0.10 ± 0.012 | 0.13 ± 0.013 |
| Time (min) | Transshipment at Different Times (ug/cm2) | Papp (×10−6 cm/s) | ||
|---|---|---|---|---|
| F-PM1 | F-PG2 | F-PM | F-PG | |
| 30 | 0.18 ± 0.050 | 0.12 ± 0.042 | 2.05 ± 0.559 | 1.32 ± 0.468 |
| 60 | 0.35 ± 0.004 | 0.20 ± 0.027 | 1.97 ± 0.022 | 1.22 ± 0.149 |
| 120 | 0.71 ± 0.047 | 0.44 ± 0.014 | 1.95 ± 0.153 | 1.21 ± 0.038 |
| 240 | 0.72 ± 0.039 | 0.53 ± 0.051 | 0.99 ± 0.054 | 0.74 ± 0.071 |
| Time (min) | Transshipment at Different Times (μg/cm2) | Papp (×10−6 cm/s) | ||
|---|---|---|---|---|
| F-PM | F-PG | F-PM | F-PG | |
| 0–120 | - | - | - | - |
| 120 | 0.60 ± 0.002 | 0.33 ± 0.007 | 1.67 ± 0.005 | 0.91 ± 0.019 |
| 240 | 0.94 ± 0.251 | 0.59 ± 0.067 | 1.30 ± 0.035 | 0.83 ± 0.093 |
| Concentration (μg/mL) | Transport Concentration (μg/mL) | Papp (×10−6 cm/s) | RT (%) | |||
|---|---|---|---|---|---|---|
| F-PM | F-PG | F-PM | F-PG | F-PM | F-PG | |
| 50 | 0.26 ± 0.031 | 0.16 ± 0.033 | 2.12 ± 0.260 | 1.31 ± 0.276 | 1.71 ± 0.210 | 1.14 ± 0.111 |
| 100 | 0.29 ± 0.061 | 0.15 ± 0.044 | 1.21 ± 0.253 | 0.63 ± 0.184 | 1.04 ± 0.101 | 0.56 ± 0.074 |
| 200 | 0.25 ± 0.042 | 0.12 ± 0.006 | 0.52 ± 0.086 | 0.24 ± 0.013 | 0.44 ± 0.035 | 0.20 ± 0.005 |
| 400 | 0.23 ± 0.019 | 0.14 ± 0.031 | 0.24 ± 0.020 | 0.15 ± 0.032 | 0.20 ± 0.008 | 0.13 ± 0.013 |
| 800 | 0.26 ± 0.065 | 0.13 ± 0.014 | 0.13 ± 0.033 | 0.07 ± 0.007 | 0.12 ± 0.014 | 0.06 ± 0.003 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Bai, X.; Hu, B.; Xing, M.; Cao, Q.; Ji, A.; Song, S. Transport Mechanisms of Polymannuronic Acid and Polyguluronic Acid Across Caco-2 Cell Monolayers. Pharmaceutics 2020, 12, 167. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12020167
Wang Y, Bai X, Hu B, Xing M, Cao Q, Ji A, Song S. Transport Mechanisms of Polymannuronic Acid and Polyguluronic Acid Across Caco-2 Cell Monolayers. Pharmaceutics. 2020; 12(2):167. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12020167
Chicago/Turabian StyleWang, Yu, Xu Bai, Bo Hu, Maochen Xing, Qi Cao, Aiguo Ji, and Shuliang Song. 2020. "Transport Mechanisms of Polymannuronic Acid and Polyguluronic Acid Across Caco-2 Cell Monolayers" Pharmaceutics 12, no. 2: 167. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12020167