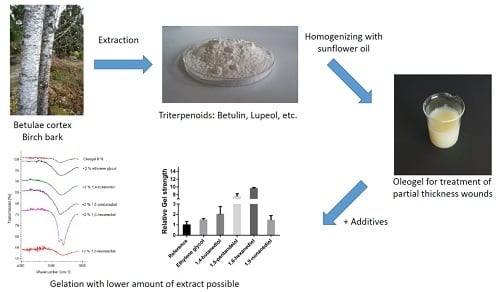
Oleogels with Birch Bark Dry Extract: Extract Saving Formulations through Gelation Enhancing Additives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gel Preparation
2.3. Rheology
2.4. ATR–Infrared Spectroscopy
2.5. Particle Size Measuring
3. Results and Discussion
3.1. Effect of Additives on Gel Strength
3.2. IR-Measurements
3.3. Influence of the Enhancer Concentration on the Gel Strength
3.4. Particles Size of Oleogels
3.5. Extract Saving Effect of Additives
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological Activities of Natural Triterpenoids and Their Therapeutic Implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.; Groundwater, P.; Li, G. The Pentacyclic Triterpenoids in Herbal Medicines and Their Pharmacological Activities in Diabetes and Diabetic Complications. Curr. Med. Chem. 2012, 20, 908–931. [Google Scholar]
- Armbruster, M.; Mönckedieck, M.; Scherließ, R.; Daniels, R.; Wahl, M. Birch Bark Dry Extract by Supercritical Fluid Technology: Extract Characterisation and Use for Stabilisation of Semisolid Systems. Appl. Sci. 2017, 7, 292. [Google Scholar] [CrossRef] [Green Version]
- Laszczyk, M.; Jäger, S.; Simon-Haarhaus, B.; Scheffler, A.; Schempp, C. Physical, Chemical and Pharmacological Characterization of a New Oleogel-Forming Triterpene Extract from the Outer Bark of Birch (Betulae Cortex). Planta Med. 2007, 72, 1389–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffler, A. Triterpene-Containing Oleogel-Forming Agent, Triterpene-Containing Oleogel and Method for Producing a Triterpene-Containing Oleogel. U.S. Patent 8,536,380, 17 September 2013. [Google Scholar]
- Kuznetsova, S.A.; Skvortsova, G.P.; Maliar, I.N.; Skurydina, E.S.; Veselova, O.F. Extraction of betulin from birch bark and study of its physico-chemical and pharmacological properties. Russ. J. Bioorganic Chem. 2014, 40, 742–747. [Google Scholar] [CrossRef]
- Xia, Y.-G.; Yang, B.-Y.; Liang, J.; Wang, D.; Yang, Q.; Kuang, H. Optimization of simultaneous ultrasonic-assisted extraction of water-soluble and fat-soluble characteristic constituents from Forsythiae Fructus Using response surface methodology and high-performance liquid chromatography. Pharmacogn. Mag. 2014, 10, 292–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidoin, M.-F.; Yang, J.; Pichette, A.; Roy, C. Betulin isolation from birch bark by vacuum and atmospheric sublimation. A thermogravimetric study. Thermochim. Acta 2003, 398, 153–166. [Google Scholar] [CrossRef]
- Suksamrarn, S.; Panseeta, P.; Kunchanawatta, S.; Distaporn, T.; Ruktasing, S.; Suksamrarn, A. Ceanothane- and Lupane-Type Triterpenes with Antiplasmodial and Antimycobacterial Activities from Ziziphus cambodiana. Chem. Pharm. Bull. 2006, 54, 535–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, T.; Arya, R.; Meena, S.; Joshi, P.; Pal, M.; Meena, B.; Upreti, D.; Rana, T.; Datta, D. Isolation, Characterization and Anticancer Potential of Cytotoxic Triterpenes from Betula utilis Bark. PLoS ONE 2006, 11, e0159430. [Google Scholar] [CrossRef] [PubMed]
- EMA: Marketing Authorisation Episalvan. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/episalvan (accessed on 20 February 2020).
- Grysko, M.; Daniels, R. Evaluation of the mechanism of gelation of an oleogel based on a triterpene extract from the outer bark of birch. Die Pharm. 2013, 68, 572–577. [Google Scholar]
- Bag, B.; Dash, D. Hierarchical Self-Assembly of a Renewable Nanosized Pentacyclic Dihydroxy-triterpenoid Betulin Yielding Flower-Like Architectures. ACS Langmuir 2015, 31, 13664–13672. [Google Scholar] [CrossRef] [PubMed]
- Bag, B.; Dash, D. First self-assembly study of betulinic acid, a renewable nano-sized, 6-6-6-6-5 pentacyclic monohydroxy triterpenic acid. Nanoscale 2011, 3, 4564–4566. [Google Scholar] [CrossRef] [PubMed]
- Pernetti, M.; Malssen, K.; Flöter, E.; Bot, A. Structuring edible oils by alternatives to crystalline fat. Curr. Opin. Colloid Interface Sci. 2007, 12, 221–231. [Google Scholar] [CrossRef]
- Daniel, J.; Rajasekharan, R. Organogelation of Plant Oils and Hydrocarbons by Long-Chain Saturated FA, Fatty Alcohols, Wax Esters, and Dicarboxylic Acids. J. Am. Oil Chem. Soc. 2003, 80, 417–421. [Google Scholar] [CrossRef]
- Patel, A. Edible Oil Structuring: Concepts, Methods and Applications; The Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Shchipunov, Y.; Shumilina, E. Lecithin bridging by hydrogen bonds in the organogel. Mater. Sci. Eng. C 1995, 3, 43–50. [Google Scholar] [CrossRef]
- Buerkle, L.; Rowan, S. Supramolecular gels formed from multi-component low molecular weight species. Chem. Soc. Rev. 2012, 41, 6089–6102. [Google Scholar] [CrossRef] [PubMed]
- Vahid, A.; Elliott, J. Transferable Intermolecular Potentials for Carboxylic Acids and Their Phase Behavior. Thermodynamics 2010, 56, 485–505. [Google Scholar] [CrossRef]
- Tsivintzelis, I.; Kontogeorgis, G.; Panayiotou, C. On the Dimerization of Carboxylic Acids: An Equation of State Approach. J. Phys. Chem. B 2017, 121, 2153–2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordström, J.; Matic, A.; Sun, J.; Forsyth, M.; MacFarlane, D. Aggregation, ageing and transport properties of surface modified fumed silica dispersions. Soft Matter 2010, 6, 2293–2299. [Google Scholar] [CrossRef]











| Constituent | Amount (w/w %) | Specific Surface Area (m2/g) | Particle Size (μm) |
|---|---|---|---|
| Betulin | 80.0 | 42 | 5.8 |
| Betulinic acid | 3.1 | ||
| Oleanolic acid | 0.5 | ||
| Lupeol | 4.3 | ||
| Erythrodiol | 0.8 | ||
| Undisclosed substances | 11.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaffar, K.A.; Daniels, R. Oleogels with Birch Bark Dry Extract: Extract Saving Formulations through Gelation Enhancing Additives. Pharmaceutics 2020, 12, 184. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12020184
Ghaffar KA, Daniels R. Oleogels with Birch Bark Dry Extract: Extract Saving Formulations through Gelation Enhancing Additives. Pharmaceutics. 2020; 12(2):184. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12020184
Chicago/Turabian StyleGhaffar, Kashif Ahmad, and Rolf Daniels. 2020. "Oleogels with Birch Bark Dry Extract: Extract Saving Formulations through Gelation Enhancing Additives" Pharmaceutics 12, no. 2: 184. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12020184