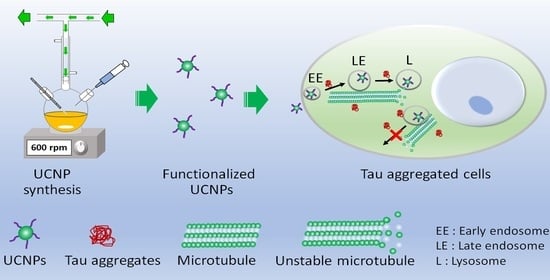
Uptake of Polyelectrolyte Functionalized Upconversion Nanoparticles by Tau-Aggregated Neuron Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of UCNPs
2.2.1. Core Synthesis
2.2.2. Epitaxial Growth of Shell on Core
2.3. Surface Functionalization of UCNPs by Polymer
2.3.1. Surface Modification of UCNP@OA
2.3.2. Characterization
2.4. Cellular Uptake and Exocytosis
2.4.1. Cell Culture
2.4.2. Cell Seeding and UCNP Treatment
2.4.3. Cell Staining and Imaging
3. Results and Discussion
3.1. Characterization of Synthesized Nanoparticles
3.2. Cellular Uptake and Exocytosis in Tau-Aggregated Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mandelkow, E.M.; Mandelkow, E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb. Perspect Med. 2012, 2, a006247. [Google Scholar] [CrossRef]
- Hernández, F.; Avila, J. Tauopathies. Cell Mol. Life Sci. 2007, 64, 2219–2233. [Google Scholar] [CrossRef]
- Morris, M.; Knudsen, G.M.; Maeda, S.; Trinidad, J.C.; Ioanoviciu, A.; Burlingame, A.L.; Mucke, L. Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat. Neurosci. 2015, 18, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, J.; Jung, E.S.; Kim, M.H.; Kim, I.B.; Son, H.; Kim, S.; Kim, S.; Park, Y.M.; Jung, I.M.; et al. Brain somatic mutations observed in Alzheimer’s disease associated with aging and dysregulation of tau phosphorylation. Nat. Commun. 2019, 10, 3090. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, S.; Grundke-Iqbal, I.; Iqbal, K. Levels of normal and abnormally phosphorylated tau in different cellular and regional compartments of Alzheimer disease and control brains. FEBS Lett. 1994, 29, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Köpke, E.; Tung, Y.C.; Shaikh, S.; Alonso, A.C.; Iqbal, K.; Grundke-Iqbal, I. Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J. Biol. Chem. 1993, 268, 24374–24384. [Google Scholar]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.-L.; Yardin, C.; Terro, F. Tau protein kinases: Involvement in Alzheimer’s disease. Ageing Res. Rev. 2013, 12, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Zhang, J.X.; Zhang, Y.; Wu, F.; Tang, Q.; Wang, C.; Shi, Z.Y.; Zhang, J.H.; Liu, S.; Wang, Y.; et al. Biphasic effects of forskolin on tau phosphorylation and spatial memory in rats. J. Alzheimers Dis. 2009, 17, 631–642. [Google Scholar] [CrossRef]
- Liu, S.J.; Zhang, J.Y.; Li, H.L.; Fang, Z.Y.; Wang, Q.; Deng, H.M.; Gong, C.X.; Grundke-Iqbal, I.; Iqbal, K.; Wang, J.Z. Tau becomes a more favorable substrate for GSK-3 when it is prephosphorylated by PKA in rat brain. J. Biol. Chem. 2004, 279, 50078–50088. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wu, Q.; Smith, A.; Grundke-Iqbal, I.; Iqbal, K. Tau is phosphorylated by GSK-3 at several sites found in Alzheimer disease and its biological activity markedly inhibited only after it is prephosphorylated by A-kinase. FEBS Lett. 1998, 436, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.-L.; Terro, F. Tau protein phosphatases in Alzheimer’s disease: The leading role of PP2A. Ageing Res. Rev. 2013, 12, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, F. Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog. Neurobiol. 2008, 85, 148–175. [Google Scholar] [CrossRef] [PubMed]
- Tiana, Q.; Lina, Z.-Q.; Wang, X.-C.; Chen, J.; Wang, Q.; Gong, C.-X.; Wang, J.-Z. Injection of okadaic acid into the meynert nucleus basalis of rat brain induces decreased acetylcholine level and spatial memory deficit. Neuroscie 2004, 126, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Blanchard, J.; Tung, Y.C.; Grundke-Iqbal, I.; Iqbal, K. Inhibition of protein phosphatase-2A (PP2A) by I1PP2A leads to hyperphosphorylation of tau, neurodegeneration, and cognitive impairment in rats. J. Alzheimers Dis. 2015, 45, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.-Y.; Liu, H.; Cong, X.-B.; Liu, Z.; Wang, Q.; Wang, J.-Z.; Zhu, L.-Q. Acetyl-L-carnitine attenuates okadaic acid induced tau hyperphosphorylation and spatial memory impairment in rats. J. Alzheimers Dis. 2010, 19, 735–746. [Google Scholar] [CrossRef]
- Ittner, L.M.; Fath, T.; Ke, Y.D.; Bi, M.; Eersel, J.; Li, K.M.; Gunning, P.; Götz, J. Parkinsonism and impaired axonal transport in a mouse model of frontotemporal dementia. Proc. Natl. Acad. Sci. USA 2008, 105, 15997–19002. [Google Scholar] [CrossRef] [Green Version]
- Rhein, V.; Song, X.; Wiesner, A.; Ittner, L.M.; Baysang, G.; Meier, F.; Ozmen, L.; Bluethmann, H.; Dröse, S.; Brandt, U.; et al. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc. Natl. Acad. Sci. USA 2009, 106, 20057–20062. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Li, B.; Zhang, F. Molecular fluorophores for deep-tissue bioimaging. ACS Cent. Sci. 2020, 6, 1302–1316. [Google Scholar] [CrossRef]
- Nakata, M.; Nagasaka, K.; Shimoda, M.; Takashima, I.; Yamamoto, S. Focal brain lesions induced with ultraviolet irradiation. Sci. Rep. 2018, 8, 7968. [Google Scholar] [CrossRef] [Green Version]
- Martínez, R.; Polo, E.; Barbosa, S.; Taboada, P.; Del Pino, P.; Pelaz, B. 808 nm-activable core@multishell upconverting nanoparticles with enhanced stability for efficient photodynamic therapy. J. Nanobiotechnol. 2020, 18, 85. [Google Scholar]
- Ostrowski, A.D.; Chan, E.M.; Gargas, D.J.; Katz, E.M.; Han, G.; Schuck, P.J.; Milliron, D.J.; Cohen, B.E. Controlled synthesis and single-particle imaging of bright, sub-10 nm lanthanide-doped upconverting nanocrystals. ACS Nano. 2012, 6, 2686–2692. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Vijayaraghavan, P.; Chiang, W.-H.; Chen, H.-H.; Liu, T.-I.; Shen, M.-Y.; Omoto, A.; Kamimura, M.; Soga, K.; Chiu, H.-C. Targeted delivery of functionalized upconversion nanoparticles for externally triggered photothermal/photodynamic therapies of brain glioblastoma. Theranostics 2018, 8, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Abel, K.A.; Boyer, J.-C.; Andrei, C.M.; Van Veggel, F.C.J.M. Analysis of the shell thickness distribution on NaYF4/NaGdF4 core/shell nanocrystals by EELS and EDS. J. Phys. Chem. Lett. 2011, 2, 185–189. [Google Scholar] [CrossRef]
- Himmelstoß, S.F.; Hirsch, T. Long-term colloidal and chemical stability in aqueous media OF NaYF4-type upconversion nanoparticles modified by ligand-exchange. Part. Part. Syst. Charact. 2019, 36, 1900235. [Google Scholar] [CrossRef] [Green Version]
- Tak, H.; Haque, M.M.; Kim, M.J.; Lee, J.H.; Baik, J.-H.; Kim, Y.S.; Kim, D.J.; Grailhe, R.; Kim, Y.K. Bimolecular fluorescence Complementation; Lighting-up tau-tau interaction in living cells. PLoS ONE 2013, 8, e81682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, S.; Wegmann, S.; Cho, H.; DeVos, S.L.; Commins, C.; Roe, A.D.; Nicholls, S.B.; Carlson, G.A.; Pitstick, R.; Nobuhara, C.K. Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer’s disease brain. Nat. Commun. 2015, 6, 8490. [Google Scholar] [CrossRef]
- Nobuhara, C.K.; DeVos, S.L.; Commins, C.; Wegmann, S.; Moore, B.D.; Roe, A.D.; Costantino, I.; Frosch, M.P.; Pitstick, R.; Carlson, G.A. Tau antibody targeting pathological species blocks neuronal uptake and interneuron propagation of tau in vitro. Am. J. Pathol. 2017, 187, 1399–1412. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.H.; De, R.; Lee, K.T. Uptake of Polyelectrolyte Functionalized Upconversion Nanoparticles by Tau-Aggregated Neuron Cells. Pharmaceutics 2021, 13, 102. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13010102
Song YH, De R, Lee KT. Uptake of Polyelectrolyte Functionalized Upconversion Nanoparticles by Tau-Aggregated Neuron Cells. Pharmaceutics. 2021; 13(1):102. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13010102
Chicago/Turabian StyleSong, Yo Han, Ranjit De, and Kang Taek Lee. 2021. "Uptake of Polyelectrolyte Functionalized Upconversion Nanoparticles by Tau-Aggregated Neuron Cells" Pharmaceutics 13, no. 1: 102. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13010102