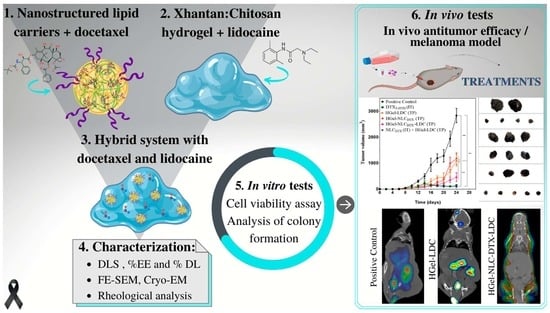
Docetaxel and Lidocaine Co-Loaded (NLC-in-Hydrogel) Hybrid System Designed for the Treatment of Melanoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. NLC Preparation
2.3. NLC Characterization
2.3.1. Determination of Particle Size, Polydispersity and Zeta Potential
2.3.2. DTX Quantification and Encapsulation in NLC
2.4. Hydrogel Preparation
2.5. Hydrogel Characterization
2.5.1. Rheological Analyses
2.5.2. Field Emission Scanning (FE-SEM) and Cryo-Electron Microscopy (Cryo-EM)
2.6. In Vitro Tests
2.6.1. Cell Viability Assay
2.6.2. Analysis of Colony Formation (Clonogenic Assay)
2.7. In Vivo Tests
2.7.1. Anesthetic Efficacy: Tail-Flick Test
2.7.2. Anticancer Tests in Melanoma Mice
2.7.3. Tumor Regression Analysis by In Vivo Imaging (Micro-PET/CT)
2.7.4. Serum Biochemical Analytes Measurement
2.7.5. Histological Analysis
3. Results and Discussion
3.1. NLCDTX Preparation and Characterization
3.2. Hybrid Hydrogel Development and Characterization
3.3. In Vitro Tests
3.3.1. Cell Viability Assay
3.3.2. Evaluation of Cell Reproductive Viability (Clonogenic Assay)
3.4. In Vivo Assays
3.4.1. Anesthetic Efficacy Determined by the Tail-Flick Test
3.4.2. In Vivo Antitumor Efficacy in a Melanoma Model
3.4.3. Tumor Regression Analysis by In Vivo Imaging
3.5. Screening of Treatments’ Adverse Effects
3.5.1. Biochemical Analyses
3.5.2. Macroscopic Parameters
3.6. Histopathology
- Tumor
- Organs (spleen, liver, lung and kidneys)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, A.M.; Waldstein, C.; Shivalingam, B.; Carlino, M.S.; Atkinson, V.; Kefford, R.F.; McArthur, G.A.; Menzies, A.M.; Thompson, J.F.; Long, G.V. Management of melanoma brain metastases: Evidence-based clinical practice guidelines by Cancer Council Australia. Eur. J. Cancer 2021, 142, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Urban, K.; Mehrmal, S.; Uppal, P.; Giesey, R.L.; Delost, G.R. The global burden of skin cancer: A longitudinal analysis from the Global Burden of Disease Study, 1990–2017. JAAD Int. 2021, 2, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Mishra, H.; Mishra, P.K.; Ekielski, A.; Jaggi, M.; Iqbal, Z.; Talegaonkar, S. Melanoma treatment: From conventional to nanotechnology. J. Cancer Res. Clin. Oncol. 2018, 144, 2283–2302. [Google Scholar] [CrossRef]
- Rebecca, V.W.; Somasundaram, R.; Herlyn, M. Pre-clinical modeling of cutaneous melanoma. Nat. Commun. 2020, 11, 2858. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Parchur, A.K.; Jagtap, J.M.; Hansen, C.P.; Joshi, A. Hybrid Nanostructures in Targeted Drug Delivery. In Hybrid Nanostructures for Cancer Theranostics; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 139–158. [Google Scholar]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, 131–155. [Google Scholar] [CrossRef]
- Kovačević, A.B.; Müller, R.H.; Keck, C.M. Formulation development of lipid nanoparticles: Improved lipid screening and development of tacrolimus loaded nanostructured lipid carriers (NLC). Int. J. Pharm. 2020, 576, 118918. [Google Scholar] [CrossRef] [PubMed]
- Garcês, A.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef]
- Souto, E.B.; Wissing, S.; Barbosa, C.; Müller, R.H. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int. J. Pharm. 2004, 278, 71–77. [Google Scholar] [CrossRef]
- Kharkar, P.B.; Talkar, S.S.; Patravale, V.B. An industrially viable technique for fabrication of docetaxel NLCs for oncotherapy. Int. J. Pharm. 2020, 577, 119082. [Google Scholar] [CrossRef]
- Da Rocha, M.C.O.; Da Silva, P.B.; Radicchi, M.A.; Andrade, B.Y.G.; De Oliveira, J.V.; Venus, T.; Merker, C.; Estrela-Lopis, I.; Longo, J.P.F.; Báo, S.N. Docetaxel-loaded solid lipid nanoparticles prevent tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells. J. Nanobiotechnol. 2020, 18, 43. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Barani, L.; Kasaeian, A. Biomimetics in drug delivery systems: A critical review. J. Control. Release 2017, 253, 97–109. [Google Scholar] [CrossRef]
- Martínez-Ruvalcaba, A.; Chornet, E.; Rodrigue, D. Viscoelastic properties of dispersed chitosan/xanthan hydrogels. Carbohydr. Polym. 2007, 67, 586–595. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Matarazzo, A.P.; Elisei, L.M.S.; Carvalho, F.C.; Bonfílio, R.; Ruela, A.L.M.; Galdino, G.; Pereira, G.R. Mucoadhesive nanostructured lipid carriers as a cannabidiol nasal delivery system for the treatment of neuropathic pain. Eur. J. Pharm. Sci. 2021, 159, 105698. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.N.M.; Franz-Montan, M.; Breitkreitz, M.C.; Rodrigues da Silva, G.H.; Castro, S.R.; Guilherme, V.A.; de Araújo, D.R.; de Paula, E. Nanohybrid hydrogels designed for transbuccal anesthesia. Int. J. Nanomed. 2018, 13, 6453–6463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajinikanth, P.S.; Chellian, J. Development and evaluation of nanostructured lipid carrier-based hydrogel for topical delivery of 5-fluorouracil. Int. J. Nanomed. 2016, 11, 5067–5077. [Google Scholar] [CrossRef] [Green Version]
- Muniz, B.V.; Baratelli, D.; Di Carla, S.; Serpe, L.; da Silva, C.B.; Guilherme, V.A.; Ribeiro, L.N.M.; Cereda, C.M.S.; de Paula, E.; Volpato, M.C.; et al. Hybrid Hydrogel Composed of Polymeric Nanocapsules Co-Loading Lidocaine and Prilocaine for Topical Intraoral Anesthesia. Sci. Rep. 2018, 8, 17972. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Zhao, L.; Wang, H. Cytotoxic effects of local anesthesia through lidocaine/ropivacaine on human melanoma cell lines. Braz. J. Anesthesiol. 2016, 66, 594–602. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhao, L.; Li, M.; Yan, L.; Zhang, S.; Mi, Z.; Ren, L.; Xu, J. Lidocaine enhances the effects of chemotherapeutic drugs against bladder cancer. Sci. Rep. 2018, 8, 598. [Google Scholar] [CrossRef] [Green Version]
- Geronimo, G.; Rodrigues da Silva, G.H.; Moura, L.D.; Ribeiro, L.N.M.; Guilherme, V.A.; Mendonça, T.C.; Castro, S.R.; Breitkreitz, M.C.; de Paula, E. Development of S 75: R 25 bupivacaine-loaded lipid nanoparticles functionalized with essential oils for treating melanoma. J. Chem. Technol. Biotechnol. 2021, 96, 2197–2207. [Google Scholar] [CrossRef]
- Frames, W.L.; Zuckerman, L.M.; Mirshahidi, H.R.; Williams, N.L.; Shields, T.G.; Otoukesh, S.; Mirshahidi, S. Antiproliferative effect of bupivacaine on patient-derived sarcoma cells. Mol. Clin. Oncol. 2020, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.H.; Liu, C.C.; Xie, J.L.; Ma, B.; Cui, S.M.; Yang, G.Z.; He, S.C. The Local Anesthetic Bupivacaine Inhibits the Progression of Non-Small Cell Lung Cancer by Inducing Autophagy Through Akt/mTOR Signaling. Front. Oncol. 2021, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Zhao, H.; Hankin, J.; Chen, L.; Yao, S.; Ma, D. Local anesthetic bupivacaine induced ovarian and prostate cancer apoptotic cell death and underlying mechanisms in vitro. Sci. Rep. 2016, 6, 26277. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Li, T. Ropivacaine inhibits the proliferation and migration of colorectal cancer cells through ITGB1. Bioengineered 2021, 12, 44–53. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, L.; Cui, Q.; Iftikhar, R.; Xia, Y.; Xu, P. Repositioning Lidocaine as an Anticancer Drug: The Role Beyond Anesthesia. Front. Cell Dev. Biol. 2020, 8, 565. [Google Scholar] [CrossRef]
- Mirshahidi, S.; Shields, T.; de Necochea-Campion, R.; Yuan, X.; Janjua, A.; Williams, N.; Mirshahidi, H.; Reeves, M.; Duerksen-Hughes, P.; Zuckerman, L.S. Bupivacaine and Lidocaine Induce Apoptosis in Osteosarcoma Tumor Cells. Clin. Orthop. Relat. Res. 2021, 479, 180–194. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, Y.; Chen, Y.J.; Liu, Q. Anti-tumor effects of lidocaine on human gastric cancer cells in vitro. Bratisl. Med. J. 2019, 120, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Viderman, D.; Nurpeissov, A.; Bilotta, F. Intravenous lidocaine in the management of severe brain tumor-associated headache. J. Clin. Anesth. 2019, 55, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.N.M.; Franz-Montan, M.; Breitkreitz, M.C.; Alcântara, A.C.S.; Castro, S.R.; Guilherme, V.A.; Barbosa, R.M.; de Paula, E. Nanostructured lipid carriers as robust systems for topical lidocaine-prilocaine release in dentistry. Eur. J. Pharm. Sci. 2016, 93, 192–202. [Google Scholar] [CrossRef]
- Rodrigues da Silva, G.H.; Geronimo, G.; Ribeiro, L.N.M.; Guilherme, V.A.; de Moura, L.D.; Bombeiro, A.L.; Oliveira, J.D.; Breitkreitz, M.C.; de Paula, E. Injectable in situ forming nanogel: A hybrid Alginate-NLC formulation extends bupivacaine anesthetic effect. Mater. Sci. Eng. C 2020, 109, 110608. [Google Scholar] [CrossRef]
- Nahak, P.; Karmakar, G.; Roy, B.; Guha, P.; Sapkota, M.; Koirala, S.; Chang, C.-H.; Panda, A.K. Physicochemical studies on local anaesthetic loaded second generation nanolipid carriers. RSC Adv. 2015, 5, 26061–26070. [Google Scholar] [CrossRef]
- Riss, T.; Niles, A.; Moravec, R.; Karassina, N.; Vidugiriene, J. Cytotoxicity Assays: In Vitro Methods to Measure Dead Cells; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Shin, S.; Cho, C.; Yang, H. Development of lidocaine gels for enhanced local anesthetic action. Int. J. Pharm. 2004, 287, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yoshikawa, N.; Yamaguchi, Y.; Kagota, S.; Shinozuka, K.; Kunitomo, M. Characterization of mouse melanoma cell lines by their mortal malignancy using an experimental metastatic model. Life Sci. 2002, 70, 791–798. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Liu, D.; Liu, C.; Juan, Z.; Zhang, N. Docetaxel-loaded-lipid-based-nanosuspensions (DTX-LNS): Preparation, pharmacokinetics, tissue distribution and antitumor activity. Int. J. Pharm. 2011, 413, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.R.; Piroyan, A.; Ganta, S.; Morse, A.B.; Candiloro, K.M.; Solon, A.L.; Nack, A.H.; Galati, C.A.; Bora, C.; Maglaty, M.A.; et al. In Vitro and In Vivo evaluation of a novel folate-targeted theranostic nanoemulsion of docetaxel for imaging and improved anticancer activity against ovarian cancers. Cancer Biol. Ther. 2018, 19, 554–564. [Google Scholar] [CrossRef] [Green Version]
- Estebe, J.P. Intravenous lidocaine. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Dagher, F.B.; Yared, G.M.; Machtou, P. An evaluation of 2% lidocaine with different concentrations of epinephrine for inferior alveolar nerve block. J. Endod. 1997, 23, 178–180. [Google Scholar] [CrossRef]
- Santos, M.S.C.; Gouvêa, A.L.; de Moura, L.D.; Paterno, L.G.; de Souza, P.E.N.; Bastos, A.P.; Damasceno, E.A.M.; Veiga-Souza, F.H.; de Azevedo, R.B.; Báo, S.N. Nanographene oxide-methylene blue as phototherapies platform for breast tumor ablation and metastasis prevention in a syngeneic orthotopic murine model. J. Nanobiotechnol. 2018, 16, 9. [Google Scholar] [CrossRef] [Green Version]
- Bland, J.M.; Altman, D.G. Survival probabilities (the Kaplan-Meier method). BMJ 1998, 317, 1572. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Zhang, L.-F.; Zhang, H.-W.; Hu, S.; Lu, M.-H.; Liang, S.; Li, B.; Li, Y.; Li, D.; Wang, E.-D.; et al. A novel miR-155/miR-143 cascade controls glycolysis by regulating hexokinase 2 in breast cancer cells. EMBO J. 2012, 31, 1985–1998. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.E.S.; Mosci, C.; Souza, E.M.; Brunetto, S.Q.; Etchebehere, E.; Santos, A.O.; Camacho, M.R.; Miranda, E.; Lima, M.C.L.; Amorim, B.J.; et al. Proposal for a Quantitative 18F-FDG PET/CT Metabolic Parameter to Assess the Intensity of Bone Involvement in Multiple Myeloma. Sci. Rep. 2019, 9, 16429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albano, J.M.R.; de Morais Ribeiro, L.N.; Couto, V.M.; Messias, M.B.; Rodrigues da Silva, G.H.; Breitkreitz, M.C.; de Paula, E.; Pickholz, M. Rational design of polymer-lipid nanoparticles for docetaxel delivery. Colloids Surf. B Biointerfaces 2019, 175, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, V.A.; Ribeiro, L.N.M.; Alcântara, A.C.S.; Castro, S.R.; Rodrigues da Silva, G.H.; da Silva, C.G.; Breitkreitz, M.C.; Clemente-Napimoga, J.; Macedo, C.G.; Abdalla, H.B.; et al. Improved efficacy of naproxen-loaded NLC for temporomandibular joint administration. Sci. Rep. 2019, 9, 11160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues da Silva, G.H.; Geronimo, G.; García-López, J.P.; Ribeiro, L.N.M.; de Moura, L.D.; Breitkreitz, M.C.; Feijóo, C.G.; de Paula, E. Articaine in functional NLC show improved anesthesia and anti-inflammatory activity in zebrafish. Sci. Rep. 2020, 10, 19733. [Google Scholar] [CrossRef]
- Pleguezuelos-Villa, M.; Nácher, A.; Hernández, M.J.; Busó, M.A.O.V.; Barrachina, M.; Peñalver, N.; Díez-Sales, O. A novel lidocaine hydrochloride mucoadhesive films for periodontal diseases. J. Mater. Sci. Mater. Med. 2019, 30, 14. [Google Scholar] [CrossRef]
- Yaşayan, G.; Karaca, G.; Akgüner, Z.P.; Bal Öztürk, A. Chitosan/collagen composite films as wound dressings encapsulating allantoin and lidocaine hydrochloride. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 623–635. [Google Scholar] [CrossRef]
- Vigato, A.A.; Querobino, S.M.; de Faria, N.C.; Candido, A.C.B.B.; Magalhães, L.G.; Cereda, C.M.S.; Tófoli, G.R.; Campos, E.V.R.; Machado, I.P.; Fraceto, L.F.; et al. Physico-Chemical Characterization and Biopharmaceutical Evaluation of Lipid-Poloxamer-Based Organogels for Curcumin Skin Delivery. Front. Pharmacol. 2019, 10, 1006. [Google Scholar] [CrossRef] [PubMed]
- Vigato, A.A.; Querobino, S.M.; de Faria, N.C.; de Freitas, A.C.P.; Leonardi, G.R.; de Paula, E.; Cereda, C.M.S.; Tófoli, G.R.; de Araujo, D.R. Synthesis and characterization of nanostructured lipid-poloxamer organogels for enhanced skin local anesthesia. Eur. J. Pharm. Sci. 2019, 128, 270–278. [Google Scholar] [CrossRef]
- Ćirić, A.; Medarević, Đ.; Čalija, B.; Dobričić, V.; Mitrić, M.; Djekic, L. Study of chitosan/xanthan gum polyelectrolyte complexes formation, solid state and influence on ibuprofen release kinetics. Int. J. Biol. Macromol. 2020, 148, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Muri, H.; Hoang, L.; Hjelme, D. Mapping Nanoparticles in Hydrogels: A Comparison of Preparation Methods for Electron Microscopy. Appl. Sci. 2018, 8, 2446. [Google Scholar] [CrossRef] [Green Version]
- Jores, K.; Mehnert, W.; Drechsler, M.; Bunjes, H.; Johann, C.; Mäder, K. Investigations on the structure of solid lipid nanoparticles (SLN) and oil-loaded solid lipid nanoparticles by photon correlation spectroscopy, field-flow fractionation and transmission electron microscopy. J. Control. Release 2004, 95, 217–227. [Google Scholar] [CrossRef]
- Rodrigues da Silva, G.H.; Lemes, J.B.P.; Geronimo, G.; Lima, F.F.; Moura, L.D.; Santos, A.C.; Carvalho, N.S.; Malange, K.F.; Breitkreitz, M.C.; Parada, C.A.; et al. Lipid nanoparticles loaded with butamben and designed to improve anesthesia at inflamed tissues. Biomater. Sci. 2021, 9, 3378–3389. [Google Scholar] [CrossRef]
- Che, C.L.; Zhang, Y.M.; Zhang, H.H.; Sang, Y.L.; Lu, B.; Dong, F.-S.; Zhang, L.-J.; Lv, F.-Z. DNA microarray reveals different pathways responding to paclitaxel and docetaxel in non-small cell lung cancer cell line. Int. J. Clin. Exp. Pathol. 2013, 6, 1538. [Google Scholar]
- Attia, R.; Tolba, M.; Trivedi, R.; Tadros, M.; Arafa, H.; Abdel-Naim, A. The chemomodulatory effects of glufosfamide on docetaxel cytotoxicity in prostate cancer cells. PeerJ 2016, 4, e2168. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Z.; Wang, L.; Zhang, C.; Zhang, N. Nanostructured lipid carriers as novel carrier for parenteral delivery of docetaxel. Colloids Surf. B Biointerfaces 2011, 85, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Vohra, T.; Kaur, I.; Heer, H.; Rayasa, R.M. Nanolipid carrier-based thermoreversible gel for localized delivery of docetaxel to breast cancer. Cancer Nanotechnol. 2013, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karniel, M.; Beitner, R. Local Anesthetics Induce a Decrease in the Levels of Glucose 1,6-Bisphosphate, Fructose 1,6-Bisphosphate, and ATP, and in the Viability of Melanoma Cells. Mol. Genet. Metab. 2000, 69, 40–45. [Google Scholar] [CrossRef]
- Desai, S.P.; Kojima, K.; Vacanti, C.A.; Kodama, S. Lidocaine inhibits NIH-3T3 cell multiplication by increasing the expression of cyclin-dependent kinase inhibitor 1A (p21). Anesth. Analg. 2008, 107, 1592–1597. [Google Scholar] [CrossRef]
- Rafehi, H.; Orlowski, C.; Georgiadis, G.T.; Ververis, K.; El-Osta, A.; Karagiannis, T.C. Clonogenic assay: Adherent cells. J. Vis. Exp. 2011, 49, e2573. [Google Scholar] [CrossRef]
- Choi, Y.J.; Park, J.H.; Han, J.W.; Kim, E.; Jae-Wook, O.; Lee, S.Y.; Kim, J.H.; Gurunathan, S. Differential cytotoxic potential of silver nanoparticles in human ovarian cancer cells and ovarian cancer stem cells. Int. J. Mol. Sci. 2016, 17, 2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peira, E.; Chirio, D.; Battaglia, L.; Barge, A.; Chegaev, K.; Gigliotti, C.L.; Ferrara, B.; Dianzani, C.; Gallarate, M.; Luca Gigliotti, C. Micro and Nano Carriers Solid lipid nanoparticles carrying lipophilic derivatives of doxorubicin: Preparation, characterization, and in vitro cytotoxicity studies Solid lipid nanoparticles carrying lipophilic derivatives of doxorubicin: Preparation, characterization, and in vitro cytotoxicity studies. J. Microencapsul. 2016, 33, 381–390. [Google Scholar] [CrossRef]
- Bannon, A.W.; Malmberg, A.B. Models of Nociception: Hot-Plate, Tail-Flick, and Formalin Tests in Rodents. Curr. Protoc. Neurosci. 2007. [Google Scholar] [CrossRef] [PubMed]
- Atabaki, R.; Hassanpour-Ezatti, M. Improvement of Lidocaine Local Anesthetic Action Using Lallemantia royleana Seed Mucilage as an Excipient. Iran. J. Pharm. Res. 2014, 13, 1431–1436. [Google Scholar] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Chellat, F.; Tabrizian, M.; Dumitriu, S.; Chornet, E.; Magny, P.; Rivard, C.-H.; Yahia, H. In vitro and in vivo biocompatibility of chitosan-xanthan polyionic complex. J. Biomed. Mater. Res. 2000, 51, 107–116. [Google Scholar] [CrossRef]
- Chamaraux-Tran, T.N.; Mathelin, C.; Aprahamian, M.; Joshi, G.P.; Tomasetto, C.; Diemunsch, P.; Akladios, C. Antitumor effects of lidocaine on human breast cancer cells: An in vitro and in vivo experimental trial. Anticancer Res. 2018, 38, 95–105. [Google Scholar] [CrossRef]
- Raff, A.B.; Thomas, C.N.; Chuang, G.S.; Avram, M.M.; Le, M.H.; Anderson, R.R.; Purschke, M. Lidocaine-induced potentiation of thermal damage in skin and carcinoma cells. Lasers Surg. Med. 2019, 51, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Long, J.; Etxeberria, A.E.; Nand, A.V.; Bunt, C.R.; Ray, S.; Seyfoddin, A. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater. Sci. Eng. C. 2019, 104, 109873. [Google Scholar] [CrossRef]
- Ten Tije, A.J.; Verweij, J.; Loos, W.J.; Sparreboom, A. Pharmacological effects of formulation vehicles: Implications for cancer chemotherapy. Clin. Pharmacokinet. 2003, 42, 665–685. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.Y.; Hung, C.F.; Hwang, T.L.; Huang, Y.L. Physicochemical characteristics and in vivo deposition of liposome-encapsulated tea catechins by topical and intratumor administrations. J. Drug Target. 2005, 13, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.X.; Haebe, S.; Lee, A.S.; Benedikt Westphalen, C.; Norton, J.A.; Jiang, W.; Levy, R. Intratumoral immunotherapy for early-stage solid tumors. Clin. Cancer Res. 2020, 26, 3091–3099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.R.; Shin, J.H.; Park, J.H.; Song, S.U.; Choi, G.S. Combined treatment with intratumoral injection of dendritic cells and topical application of imiquimod for murine melanoma. Clin. Exp. Dermatol. 2007, 32, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yang, Y.; Yang, K.; Wu, Y.; Boone, J.M.; Cherry, S.R. A microPET/CT system for in vivo small animal imaging. Phys. Med. Biol. 2007, 52, 3881. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.; Erdi, Y.; Gonen, M.; Riedel, E.; Yeung, H.; Macapinlac, H.; Chisin, R.; Larson, S. FDG-PET standardized uptake values in normal anatomical structures using iterative reconstruction segmented attenuation correction and filtered back-projection. Eur. J. Nucl. Med. 2001, 28, 155–164. [Google Scholar] [CrossRef]
- Li, Y.; Zschaeck, S.; Lin, Q.; Chen, S.; Chen, L.; Wu, H. Metabolic parameters of sequential 18F-FDG PET/CT predict overall survival of esophageal cancer patients treated with (chemo-) radiation. Radiat. Oncol. 2019, 14, 35. [Google Scholar] [CrossRef]
- Tseng, J.R.; Kang, K.W.; Dandekar, M.; Yaghoubi, S.; Lee, J.H.; Christensen, J.G.; Muir, S.; Vincent, P.W.; Michaud, N.R.; Gambhir, S.S. Preclinical Efficacy of the c-Met Inhibitor CE-355621 in a U87 MG Mouse Xenograft Model Evaluated by 18F-FDG Small-Animal PET. J. Nucl. Med. 2008, 49, 129–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhakuni, G.S.; Bedi, O.; Bariwal, J.; Deshmukh, R.; Kumar, P. Animal models of hepatotoxicity. Inflamm. Res. 2015, 65, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.S.; Ferguson, M.A.; Bonventre, J.V. Biomarkers of Acute Kidney Injury. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.-J. Acute kidney injury. Nat. Rev. Dis. Prim. 2021, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hansson, G.K. Effect of Sex and Age on Serum Biochemical Reference Ranges in C57BL/6J Mice. Comp. Med. 2004, 54, 176–178. [Google Scholar] [PubMed]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Saleh, J. Murine models of melanoma. Pathol. -Res. Pract. 2018, 214, 1235–1238. [Google Scholar] [CrossRef]
- Definition of Clark Level V Skin Cancer-NCI. Dictionary of Cancer Terms-National Cancer Institute. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/clark-level-v-skin-cancer (accessed on 30 April 2021).
- Verma, N.; Cowperthwaite, M.C.; Burnett, M.G.; Markey, M.K. Differentiating tumor recurrence from treatment necrosis: A review of neuro-oncologic imaging strategies. Neuro-Oncology 2013, 15, 515–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsson, G.; Gullberg, B.; Hafström, L. Estimation of liver tumor volume using different formulas-An experimental study in rats. J. Cancer Res. Clin. Oncol. 1983, 105, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Ladstein, R.G.; Bachmann, I.M.; Straume, O.; Akslen, L.A. Tumor Necrosis Is a Prognostic Factor in Thick Cutaneous Melanoma. Am. J. Surg. Pathol. 2012, 36, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, D.; Bogenrieder, T.; Elder, D.; Herlyn, M. Melanoma-stroma interactions: Structural and functional aspects. Lancet Oncol. 2002, 3, 35–43. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ju, M.K.; Jeon, H.M.; Jeong, E.K.; Lee, Y.J.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Regulation of Tumor Progression by Programmed Necrosis. Oxid. Med. Cell. Longev. 2018, 28. [Google Scholar] [CrossRef] [Green Version]
- Vakkila, J.; Lotze, M.T. Inflammation and necrosis promote tumour growth. Nat. Rev. Immunol. 2004, 4, 641–648. [Google Scholar] [CrossRef]
- Winkelmann, C.T.; Daibes Figueroa, S.; Rold, T.L.; Volkert, W.A.; Hoffman, T.J. Microimaging Characterization of a B16-F10 Melanoma Metastasis Mouse Model. Mol. Imaging 2006, 5, 105–114. [Google Scholar] [CrossRef]







| Formulation | Size (nm) | PDI | ZP (mV) | %EE | Drug Loading (%) |
|---|---|---|---|---|---|
| NLCCTRL | 222.6 ± 8.5 | 0.15 ± 0.04 | −25.9 ± 0.2 | - | - |
| NLCDTX | 214.0 ± 10.9 | 0.09 ± 0.01 | −24.2 ± 0.3 | 97.3 ± 2.6 | 7.47 ± 0.26 |
| Non-Cancerous Cell Lines | Cancer Cell Lines | |||||||
|---|---|---|---|---|---|---|---|---|
| NIH-3T3 (Murine) | HaCaT (Human) | B16-F10 (Murine) | SK-MEL-103 (Human) | |||||
| Formulations | IC50 of DTX (µmol L−1) | IC50 of LDC (mmol L−1) | IC50 of DTX (µmol L−1) | IC50 of LDC (mmol L−1) | IC50 of DTX (µmol L−1) | IC50 of LDC (mmol L−1) | IC50 of DTX (µmol L−1) | IC50 of LDC (mmol L−1) |
| DTXT-HYD | 0.32 ± 0.53 | - | 0.03 ± 0.03 | - | 0.40 ± 0.48 | - | 0.33 ± 0.45 | - |
| LDC | - | 4.95 ± 5.60 | - | 2.67 ± 2.92 | - | 6.13 ± 5.44 | - | 3.26 ± 1.84 |
| NLCDTX | 2.71 ± 4.53 | - | 2.29 ± 2.84 | - | 3.53 ± 4.27 | - | 5.27 ± 3.47 | - |
| NLCDTX + LDC | 2.63 ± 2.95 | 3.29 ± 3.69 | 1.83 ± 3.92 | 2.83 ± 3.53 | 2.08 ± 1.76 | 1.30 ± 2.21 | 1.32 ± 2.25 | 2.18 ± 3.21 |
| HGel-NLCDTX | 1.24 ± 2.70 | - | 3.72 ± 3.09 | - | 2.15 ± 1.54 | - | 1.55 ± 1.00 | - |
| HGel-NLCDTX-LDC | 0.71 ± 1.43 | 0.86 ± 1.73 | 0.29 ± 0.53 | 0.34 ± 0.68 | 0.81 ± 1.34 | 0.97 ± 1.65 | 0.28 ± 0.23 | 0.32 ± 0.10 |
| Formulation | MPE ± SD (%) | AUC ± SD | Time of Anesthesia (min) |
|---|---|---|---|
| HGel-LDC | 88.48 ± 11.39 | 3545.8 ± 821.1 | 150 |
| HGel-NLCCTRL-LDC | 88.85 ± 15.84 | 3882.8 ± 805.2 | 150 |
| HGel-NLCDTX-LDC | 88.06 ± 11.65 | 4377.8 ± 950.8 | 150 |
| Micro-PET/CT | Caliper | ||||||
|---|---|---|---|---|---|---|---|
| Formulations | %ID[T/BG]Max | %ID[T/BG]Mean | %ID[T/L]Max | %ID[T/L]Mean | MTV | TLG | TV (mm³) |
| Positive Control | 9.169 | 11.336 | 2.812 | 2.570 | 0.633 | 1.558 | 3741.68 |
| DTXT-HYD IT | 2.012 | 3.354 | 1.457 | 1.795 | 0.051 | 0.105 | 41.87 |
| HGel-LDC TP | 8.350 | 8.512 | 2.498 | 2.209 | 0.199 | 0.288 | 752.33 |
| HGel-NLCDTX TP | 5.205 | 4.752 | 1.060 | 0.731 | 0.728 | 1.287 | 544.01 |
| HGel-NLCDTX-LDC TP | 3.641 | 4.129 | 0.518 | 0.390 | 0.034 | 0.095 | 155.23 |
| NLCDTX IT + HGel-LDC TP | 1.230 | 1.806 | 0.410 | 0.423 | 0.012 | 0.011 | 42.60 |
| Groups | ALT (UI/L) (Rf:44.0–87.0) | AST (UI/L) (Rf:55.0–251.0) | Creatinine (mg/dL) (Rf: 0.48–1.10) | Urea (mg/dL) (Rf: 18.0–31.0) |
|---|---|---|---|---|
| Naive | 52.0 ± 6.2 | 144.0 ± 10.5 | 0.66 ± 0.26 | 48.0 ± 18.0 |
| Positive Control | 171.0 ± 8.4 | 20.0 ± 5.7 | 0.68 ± 0.12 | 57.0 ± 14.1 |
| DTXT-HYD IT | 28.0 ± 11.0 | 144.0 ± 3.4 | 0.15 ± 0.09 | 66.0 ± 17.9 |
| HGel-LDC TP | 135.0 ± 16.0 | 36.0 ± 9.0 | 0.57 ± 0.10 | 52.0 ± 16.3 |
| HGel-NLCDTX TP | 34.0 ± 12.0 | 186.0 ± 6.2 | 0.57 ± 0.21 | 43.0 ± 15.5 |
| HGel-NLCDTX-LDC TP | 98.0 ± 14.4 | 213.0 ± 11.4 | 0.38 ± 0.14 | 52.0 ± 15.0 |
| NLCDTXIT + HGel-LDC TP | 45.0 ± 17.0 | 51.0 ± 8.4 | 0.52 ± 0.13 | 45.0 ± 19.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Moura, L.D.; Ribeiro, L.N.M.; de Carvalho, F.V.; Rodrigues da Silva, G.H.; Lima Fernandes, P.C.; Brunetto, S.Q.; Ramos, C.D.; Velloso, L.A.; de Araújo, D.R.; de Paula, E. Docetaxel and Lidocaine Co-Loaded (NLC-in-Hydrogel) Hybrid System Designed for the Treatment of Melanoma. Pharmaceutics 2021, 13, 1552. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13101552
de Moura LD, Ribeiro LNM, de Carvalho FV, Rodrigues da Silva GH, Lima Fernandes PC, Brunetto SQ, Ramos CD, Velloso LA, de Araújo DR, de Paula E. Docetaxel and Lidocaine Co-Loaded (NLC-in-Hydrogel) Hybrid System Designed for the Treatment of Melanoma. Pharmaceutics. 2021; 13(10):1552. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13101552
Chicago/Turabian Stylede Moura, Ludmilla David, Lígia N. M. Ribeiro, Fabíola V. de Carvalho, Gustavo H. Rodrigues da Silva, Priscila C. Lima Fernandes, Sérgio Q. Brunetto, Celso D. Ramos, Lício A. Velloso, Daniele R. de Araújo, and Eneida de Paula. 2021. "Docetaxel and Lidocaine Co-Loaded (NLC-in-Hydrogel) Hybrid System Designed for the Treatment of Melanoma" Pharmaceutics 13, no. 10: 1552. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13101552