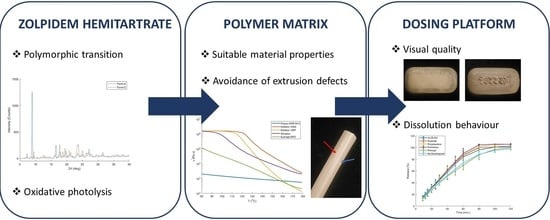
Development of a 3D-Printed Dosing Platform to Aid in Zolpidem Withdrawal Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Zolpidem Hemitartrate: Physico-Chemical Characterization
2.2.1. Thermogravimetric Analysis
2.2.2. Differential Scanning Calorimetry
2.2.3. Hot Stage Microscopy
2.2.4. Raman Spectroscopy
2.2.5. X-ray Diffraction
2.3. Zolpidem Hemitartrate: Oxidative Potential
2.4. Filament Preparation: Hot Melt Extrusion
2.4.1. Placebo Filaments
2.4.2. Drug-Loaded Filaments
2.5. Filament Characterization
2.5.1. Raman Microscopy
2.5.2. Mechanical Properties
2.5.3. Rheological Analysis
2.5.4. Karl Fischer Titration
2.5.5. Content Uniformity
2.6. Caplet 3D Printing
2.7. Caplet Characterization
2.7.1. Mass, Dimension and Hardness Analysis
2.7.2. Helium Pycnometry
2.7.3. In-Vitro Dissolution
3. Results and Discussion
3.1. Zolpidem Hemitartrate
3.1.1. Physico-Chemical Characterization
3.1.2. Photolytic Degradation
3.2. Screening and Characterization of Placebo Filaments
3.2.1. Extrusion Defects
3.2.2. Mechanical Properties
3.2.3. Moisture Absorption Analysis
3.3. Characterization and Printability of Drug-Loaded Filaments
3.3.1. Mechanical Properties and Content Uniformity
3.3.2. Rheological Properties
3.4. Caplet Printing and Characterization
3.4.1. Influence of Nozzle Size and Overlap
3.4.2. Influence of Drug Load and Caplet Size
3.5. Enhancing the Dissolution Rate
3.5.1. Caplet Modification
3.5.2. Formulation Modification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourgeois, J.; Elseviers, M.M.; Stichele, R.R.V. Benzodiazepine use in Belgian nursing homes: A closer look into indications and dosages. Pharmacoepidemiol. Prescr. 2012, 68, 833–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evrard, P.; Spinewine, A. Benzodiazepine Use and Deprescribing in Belgian Nursing Homes: Results from the COME-ON Study. Am. Geriatr. Soc. 2020, 68, 2768–2777. [Google Scholar] [CrossRef] [PubMed]
- Azermai, M.; Elseviers, M.; Petrovic, M.; Van Bortel, L.; Vander Stichele, R. Geriatric drug utilisation of psychotropics in Belgian nursing homes. J. Clin. Psychiatry 2011, 26, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Soyka, M. Treatment of Benzodiazepine Dependence. N. Engl. J. Med. 2017, 376, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, A.M.; Crowther, R.; Lotter, A.; Cheng, C.; King, D. In the treatment of insomnia. CMAJ 2000, 162, 10–15. [Google Scholar]
- Pottie, K.; Thompson, W.; Davies, S.; Grenier, J.; Sadowski, C.A.; Welch, V.; Holbrook, A.; Boyd, C.; Sweson, R.; Ma, A.; et al. Deprescribing benzodiazepine receptor agonists: Evidence-based clinical practice guideline. Can. Fam. Phys. 2018, 64, 339–351. [Google Scholar]
- Vinkers, C.H.; Olivier, B. Mechanisms underlying tolerance after long-term benzodiazepine use: A future for subtype-selective GABAA receptor modulators? Adv. Pharmacol. Sci. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Pétein, C.; Spinewine, A.; Henrard, S. Trends in benzodiazepine receptor agonists use and associated factors in the Belgian general older population: Analysis of the Belgian health interview survey data. Ther. Adv. Psychopharmacol. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Reeve, E.; To, J.; Hendrix, I.; Wiese, M.D. Patient Barriers to and Enablers of Deprescribing: A Systematic Review. Drugs Aging 2013, 30, 793–807. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Madla, C.M.; Basit, A.W.; Gaisford, S. The shape of things to come: Emerging applications of 3D printing in healthcare. AAPS Adv. Pharm. Sci. Ser. 2018, 31, 1–19. [Google Scholar] [CrossRef]
- Henry, S.; Samaro, A.; Marchesini, F.H.; Shaqour, B.; Macedo, J.; Vanhoorne, V.; Vervaet, C. Extrusion-based 3D printing of oral solid dosage forms: Material requirements and equipment dependencies. Int. J. Pharm. 2021, 598, 120361. [Google Scholar] [CrossRef]
- Korte, C.; Quodbach, J.; Korte, C.; Quodbach, J. Formulation development and process analysis of drug-loaded filaments manufactured via hot-melt extrusion for 3D-printing of medicines manufactured via hot-melt extrusion for 3D-printing of medicines. Pharm. Dev. Technol. 2018, 23, 1117–1127. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharm. 2017, 519, 186–197. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Xu, X.; Yang, G. Strategies and mechanisms to improve the printability of pharmaceutical polymers Eudragit® EPO and Soluplus®. Int. J. Pharm. 2021, 599, 120410. [Google Scholar] [CrossRef]
- Gottschalk, N.; Bogdahn, M.; Harms, M.; Quodbach, J. Brittle polymers in Fused Deposition Modeling: An improved feeding approach to enable the printing of highly drug loaded filament. Int. J. Pharm. 2021, 597. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Battu, S.K.; McGinity, J.W.; Martin, C. Pharmaceutical applications of hot-melt extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.N.; Strong, R.; Gold, S.A. A review of melt extrusion additive manufacturing processes: I. Process design and modeling. Rapid Prototyp. J. 2014, 20, 192–204. [Google Scholar] [CrossRef]
- Shaqour, B.; Samaro, A.; Verleije, B.; Beyers, K.; Vervaet, C. Production of Drug Delivery Systems Using Fused Filament Fabrication: A Systematic Review. Pharmaceutics 2020, 12, 517. [Google Scholar] [CrossRef]
- Sadia, M.; Sośnicka, A.; Arafat, B.; Isreb, A.; Ahmed, W.; Kelarakis, A.; Alhnan, M.A. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int. J. Pharm. 2016, 513, 659–668. [Google Scholar] [CrossRef]
- Pietrzak, K.; Isreb, A.; Alhnan, M.A. A flexible-dose dispenser for immediate and extended release 3D printed tablets. Eur. J. Pharm. Biopharm. 2015, 96, 380–387. [Google Scholar] [CrossRef]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Development and Optimisation of Novel Polymeric Compositions for Sustained Release Theophylline. Polymers 2020, 12, 27. [Google Scholar] [CrossRef] [Green Version]
- Isreb, A.; Baj, K.; Wojsz, M.; Isreb, M.; Peak, M.; Alhnan, M.A. 3D printed oral theophylline doses with innovative ‘radiator-like’ design: Impact of polyethylene oxide (PEO) molecular weight. Int. J. Pharm. 2019, 564, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Gültekin, H.E.; Tort, S.; Acartürk, F. An Effective Technology for the Development of Immediate Release Solid Dosage Forms Containing Low-Dose Drug: Fused Deposition Modeling 3D Printing. Pharm. Res. 2019, 36, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gioumouxouzis, C.I.; Tzimtzimis, E.; Katsamenis, O.L.; Dourou, A.; Markopoulou, C.; Bouropoulos, N.; Tzetzis, D.; Fatouros, D.G. Fabrication of an osmotic 3D printed solid dosage form for controlled release of active pharmaceutical ingredients. Eur. J. Pharm. Sci. 2020, 143, 105176. [Google Scholar] [CrossRef]
- Dumpa, N.R.; Bandari, S.; Repka, M.A. Novel gastroretentive floating pulsatile drug delivery system produced via hot-melt extrusion and fused deposition modeling 3D printing. Pharmaceutics 2020, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Murthy, M.V.; Krishnaiah, C.; Kumar, R.; Mukkanti, K. Development of stability-indicating UPLC method for determining zolpidem tartrate and its product related variants in drug substance and drug products. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 836–851. [Google Scholar] [CrossRef]
- Malesevic, M.M.; Ivanovic, L.Z.; Rotic, A.P.; Adisic, M.R.; Ausevic, M.L. Stress Degradation Studies on Zolpidem Tartrate Using LC—DAD and LC—MS Methods. Acta Chromatogr. 2014, 26, 81–96. [Google Scholar] [CrossRef] [Green Version]
- Mahapatra, A.K.; Sameeraja, N.H.; Murthy, P.N. Development of Modified-Release Tablets of Zolpidem Tartrate by Biphasic Quick/Slow Delivery System. AAPS PharmSciTech 2015, 16, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Seshadri, V.C.; Manohari, P.J. Formulation and evaluation of pulsatile delivery system of zolpidem tartrate. World J. Pharm. Pharm. Sci. 2017, 6, 1200–1213. [Google Scholar] [CrossRef]
- Andreas, C.J.; Pepin, X.; Markopoulos, C.; Vertzoni, M.; Reppas, C.; Dressman, J.B. Mechanistic investigation of the negative food effect of modified release zolpidem. Eur. J. Pharm. Sci. 2017, 102, 284–298. [Google Scholar] [CrossRef]
- Malesevic, M.; Zivanovic, L. Multiobjective Optimization Approach in Evaluation of Chromatographic Behaviour of Zolpidem Tartrate and Its Degradation Products. Chromatographia 2011, 74, 197–208. [Google Scholar] [CrossRef]
- Verhoeven, E.; Beer, T.R.M.D.; Mooter, G.V.D.; Remon, J.P.; Vervaet, C. Influence of formulation and process parameters on the release characteristics of ethylcellulose sustained-release mini-matrices produced by hot-melt extrusion. Eur. J. Pharm. Biopharm. 2008, 69, 312–319. [Google Scholar] [CrossRef]
- Gupta, S.S.; Parikh, T.; Meena, A.K.; Mahajan, N.; Vitez, I.; Serajuddin, A.T. Effect of carbamazepine on viscoelastic properties and hot melt extrudability of Soluplus®. Int. J. Pharm. 2015, 478, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Samaro, A.; Janssens, P.; Vanhoorne, V.; Van Renterghem, J.; Eeckhout, M.; Cardon, L.; De Beer, T.; Vervaet, C. Screening of pharmaceutical polymers for extrusion-Based Additive Manufacturing of patient-tailored tablets. Int. J. Pharm. 2020, 586. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.R.; Taylor, P.A.; MacGregor, J.F. Missing data methods in PCA and PLS: Score calculations with incomplete observations. Chemom. Intell. Lab. Syst. 1996, 35, 45–65. [Google Scholar] [CrossRef]
- ISO. Plastics—Determination of tensile properties—Part 1: General principles. ISO 2008, 527-1, 13. [Google Scholar]
- Mezger, T.G. The Rheology Handbook; Vincentz Network GmBH: Hannover, Germany, 2009; Volume 38, pp. 790–797. [Google Scholar] [CrossRef]
- Coogan, T.J.; Kazmer, D.O. In-line rheological monitoring of fused deposition modeling. J. Rheol. 2018, 63, 141–155. [Google Scholar] [CrossRef]
- Aronhime, J.; Leonov, D.; Meszaros-SOS, E. Zolpidem Hemitartrate. Patent EP1292304B1, 11 February 2005. [Google Scholar]
- Khankari, R.K.; Law, D.; Grant, D.J. Determination of water content in pharmaceutical hydrates by differential scanning calorimetry. Int. J. Pharm. 1992, 82, 117–127. [Google Scholar] [CrossRef]
- Li, Y.; Pang, H.; Guo, Z.; Lin, L.; Dong, Y.; Li, G.; Lu, M.; Wu, C. Interactions between drugs and polymers influencing hot melt extrusion. J. Pharm. Pharmacol. 2014, 66, 148–166. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies. Tables and Charts; John Wiley & Sons, Ltd.: West-Sussex, UK, 2001; p. 347. [Google Scholar]
- Zeany, B.A.E.; Moustafa, A.A.; Farid, N.F. Determination of zolpidem hemitartrate by quantitative HPTLC and LC. J. Pharm. Biomed. Anal. 2003, 33, 393–401. [Google Scholar] [CrossRef]
- Jørgensen, A.; Rantanen, J.; Karjalainen, M.; Khriachtchev, L.; Räsänen, E.; Yliruusi, J. Hydrate Formation during Wet Granulation Studied by Spectroscopic Methods and Multivariate Analysis. Pharm. Res. 2002, 19, 1285–1291. [Google Scholar] [CrossRef]
- Waterman, K.C.; Adami, R.C.; Alsante, K.M.; Hong, J.; Landis, M.S.; Lombardo, F.; Roberts, C.J. Stabilization of Pharmaceuticals to Oxidative Degradation. Pharm. Dev. Technol. 2002, 7, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Ahlneck, C.; Zografi, G. The molecular basis of moisture effects on the physical and chemical stability of drugs in the solid state. Int. J. Pharm. 1990, 62, 87–95. [Google Scholar] [CrossRef]
- Safari, G.H.; Hoseini, M.; Seyedsalehi, M.; Kamani, H.; Jaafari, J.; Mahvi, A.H. Photocatalytic degradation of tetracycline using nanosized titanium dioxide in aqueous solution. Int. J. Environ. Sci. Technol. 2015, 12, 603–616. [Google Scholar] [CrossRef] [Green Version]
- Seburg, R.A.; Ballard, J.M.; Hwang, T.l.; Sullivan, C.M. Photosensitized degradation of losartan potassium in an extemporaneous suspension formulation. J. Pharm. Biomed. Anal. 2006, 42, 411–422. [Google Scholar] [CrossRef]
- Hovorka, S.W.; Schöneich, C. Oxidative degradation of pharmaceuticals: Theory, mechanisms and inhibition. J. Pharm. Sci. 2001, 90, 253–269. [Google Scholar] [CrossRef]
- Sadeghian, Z.; Lotfi, B.; Enayati, M.H.; Beiss, P. Microstructural and mechanical evaluation of Al—TiB 2 nanostructured composite fabricated by mechanical alloying. J. Alloys Compd. 2011, 509, 7758–7763. [Google Scholar] [CrossRef]
- Rauwendaal, C.; Gramann, P.J.; Davis, B.A.; Osswald, T.A. Polymer Extrusion, 5th ed.; Hanser: Auburn, AL, USA, 2014; Volume 65, pp. 1–943. [Google Scholar]
- Ilyés, K.; Kovács, N.K.; Balogh, A.; Borbás, E.; Farkas, B.; Casian, T.; Marosi, G.; Tomuță, I.; Nagy, Z.K. The applicability of pharmaceutical polymeric blends for the fused deposition modelling (FDM) 3D technique: Material considerations–printability–process modulation, with consecutive effects on in vitro release, stability and degradation. Eur. J. Pharm. Sci. 2019, 129, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Kolter, K.; Karl, M.; Gryczke, A. Hot-Melt Extrusion with BASF Pharma Polymers—Extrusion Compendium; BASF SF: Ludwigshafen, Germany, 2012; pp. 1–201. [Google Scholar]
- El Kissi, N.; Piau, J.M.; Toussaint, F. Sharkskin and cracking of polymer melt extrudates. J. Non-Newton. Fluid Mech. 1997, 68, 271–290. [Google Scholar] [CrossRef]
- Sharma, J.; Clarke, N. Miscibility Determination of a Lower Critical Solution Temperature Polymer Blend by Rheology. J. Phys. Chem. B 2004, 108, 13220–13230. [Google Scholar] [CrossRef]
- Macedo, J.; Samaro, A.; Vanhoorne, V.; Vervaet, C.; Pinto, J. Processability of poly (vinyl alcohol) Based Filaments With Paracetamol Prepared by Hot-Melt Extrusion for Additive Manufacturing. J. Pharm. Sci. 2020, 109, 3636–3644. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused- filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, G.; Samaro, A.; Grymonpre, W.; Vanhoorne, V.; Van Snick, B.; Boone, M.N.; Hellemans, T.; Van Hoorebeke, L.; Remon, J.P.; Vervaet, C. 3D printing of high drug loaded dosage forms using thermoplastic polyurethanes. Int. J. Pharm. 2018, 536, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.Q.; Zhang, J.; Nyavanandi, D.; Bandari, S.; Repka, M.A. Hot melt extrusion paired fused deposition modeling 3D printing to develop hydroxypropyl cellulose based floating tablets of cinnarizine. Carbohydr. Polym. 2020, 246, 116519. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.R.; Song, E.S.; Kwon, J.; Lee, J.H.; Park, J.B. Fabrication of Intragastric Floating, Controlled Release 3D Printed Theophylline Tablets Using Hot-Melt Extrusion and Fused Deposition Modeling. Pharmaceutics 2020, 12, 77. [Google Scholar] [CrossRef] [Green Version]
- Bawuah, P.; Markl, D.; Turner, A.; Evans, M.; Portieri, A.; Farrell, D.; Lucas, R.; Anderson, A.; Goodwin, D.J.; Zeitler, J.A. A Fast and Non-destructive Terahertz Dissolution Assay for Immediate Release Tablets. J. Pharm. Sci. 2021, 110, 2083–2092. [Google Scholar] [CrossRef]
- Sadia, M.; Arafat, B.; Ahmed, W.; Forbes, R.T.; Alhnan, M.A. Channelled tablets: An innovative approach to accelerating drug release from 3D printed tablets. J. Control. Release 2018, 269, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Kempin, W.; Domsta, V.; Grathoff, G.; Brecht, I.; Semmling, B.; Tillmann, S.; Weitschies, W.; Seidlitz, A. Immediate Release 3D-Printed Tablets Produced Via Fused Deposition Modeling of a Thermo-Sensitive Drug. Pharm. Res. 2018, 35, 1–12. [Google Scholar] [CrossRef]
- Arafat, B.; Wojsz, M.; Isreb, A.; Forbes, R.T.; Isreb, M.; Ahmed, W.; Arafat, T.; Alhnan, M.A. Tablet fragmentation without a disintegrant: A novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets. Eur. J. Pharm. Sci. 2018, 118, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.C.R.; Chaves, P.S.; Goyanes, A.; Vukosavljevic, B.; Buanz, A.; Windbergs, M.; Basit, A.W.; Gaisford, S. 3D printed tablets loaded with polymeric nanocapsules: An innovative approach to produce customized drug delivery systems. Int. J. Pharm. 2017, 528, 268–279. [Google Scholar] [CrossRef]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]













| Solution | Solid State | |||||
|---|---|---|---|---|---|---|
| Peak | Light | Dark | Light | Dark | 40% RH | Sealed |
| RRT | 7 days | 7 days | 3 days | 3 days | 7 days | 3 months |
| 0.69 | 7.2% | / | 0.1% | / | 0.6% | 0.2% |
| 0.75 | 4.0% | / | / | / | / | / |
| 0.85 | 5.5% | / | 0.1% | / | / | / |
| 1 | 78.7% | 100% | 99.7% | 100% | 99.4% | 99.8% |
| (58.1%) | (96.6%) | (94.2%) | (99.8%) | (96.0%) | (96.9%) | |
| 2.05 | 4.6% | / | 0.2% | / | / | / |
| Blend | Young’s Modulus MPa | Stress at Break MPa | Strain at Break % | Energy to Break × J/m |
|---|---|---|---|---|
| Placebo filaments | ||||
| K12PF:PEO (70:30)(120) * | 722.0 ± 63.1 | 3.5 ± 0.7 | 0.7 ± 0.2 | 2.4 ± 0.8 |
| K12PF:PEO (70:30)(160) * | 401.2 ± 26.2 | 3.8 ± 0.9 | 0.8 ± 0.2 | 2.4 ± 0.6 |
| K12PF:PEO (30:70)(140) | 507.2 ± 105.2 | 14.0 ± 1.4 | 3.3 ± 0.3 | 26.0 ± 4.8 |
| K12PF:PEO (30:70)(160) | 448.9 ± 140.7 | 14.1 ± 1.6 | 3.6 ± 0.5 | 28.0 ± 7.0 |
| KVA64:PEO (70:30)(140) * | 859.7 ± 60.4 | 9.1 ± 1.4 | 1.4± 0.2 | 7.2 ± 2.0 |
| KVA64:PEO (70:30)(180) | 714.1 ± 92.1 | 16.8 ± 2.1 | 3.9 ± 0.3 | 38.0 ± 5.7 |
| KVA64:PEO (30:70)(160) | 531.5 ± 22.1 | 17.8 ± 0.5 | 4.3 ± 0.5 | 42.0 ± 6.5 |
| SOL:PEO (70:30) | 649.0 ± 34.8 | 11.7 ± 3.3 | 2.3 ± 0.8 | 15.7 ± 5.8 |
| SOL:PEO (30:70) | 400.8 ± 58.3 | 16.5 ± 3.2 | 5.8 ± 0.6 | 57.8 ± 9.1 |
| EPO:PEO (70:30) | 400.1 ± 33.4 | 14.0 ± 0.6 | 4.3 ± 0.5 | 36.0 ± 5.3 |
| EPO:PEO (30:70) | 754.1 ± 132.3 | 20.9 ± 0.9 | 4.9 ± 0.3 | 57.0 ± 10.1 |
| Drug-loaded filaments | ||||
| EPO:PEO:ZHT (69.3:29.7:1) | 522.1 ± 120.1 | 13.0 ± 1.7 | 2.4 ± 0.5 | 13.8 ± 3.4 |
| EPO:PEO:ZHT (63:27:10) | 517.2 ± 47.0 | 9.7 ± 1.1 | 2.3 ± 0.3 | 11.9 ± 2.1 |
| Drug-loaded filaments with disintegrant | ||||
| EPO:PEO:ZHT 69.3:29.7:1 | ||||
| AcDiSol (4%) | 473.0 ± 159.6 | 11.2 ± 2.6 | 2.6 ± 0.7 | 15.8 ± 9.3 |
| AcDiSol (8%) | 744.0 ± 129.6 | 10.3 ± 2.7 | 1.6 ± 0.6 | 9.6 ± 6.3 |
| AcDiSol (12%) | 577.1 ± 151.2 | 13.2 ± 2.0 | 2.9 ± 0.8 | 21.7 ± 8.7 |
| AcDiSol (16%) | 565.4 ± 132.2 | 12.3 ± 0.7 | 13.8 ± 2.6 | 175.1 ± 32.8 |
| Explotab (8%) | 442.20 ± 125.2 | 12.1 ± 3.4 | 3.8 ± 1.6 | 28.7 ± 7.4 |
| Polyplasdone (8%) | 539.3 ± 145.4 | 10.0 ± 2.1 | 2.0 ± 0.7 | 10.9 ± 5.9 |
| Primojel (8%) | 795.3 ± 125.5 | 11.2 ± 3.2 | 1.7 ± 0.6 | 11.2 ± 7.0 |
| Primellose (8%) | 245.5 ± 57.1 | 12.7 ± 1.8 | 5.8 ± 0.4 | 43.5 ± 6.0 |
| Blend | Moisture Content | |
|---|---|---|
| After Extrusion | 7d at RH 60% | |
| % | % | |
| K12PF:PEO (70:30) | 1.94 | 14.32 |
| K12PF:PEO (30:70) | 1.20 | 6.83 |
| KVA64:PEO (70:30) | 1.54 | 8.70 |
| KVA64:PEO (30:70) | 1.20 | 4.28 |
| SOL:PEO (70:30) | 1.27 | 4.63 |
| SOL:PEO (30:70) | 1.24 | 3.08 |
| EPO:PEO (70:30) | 1.39 | 1.56 |
| EPO:PEO (30:70) | 0.89 | 1.63 |
| Blend | Cross-Model Parameters | |||
|---|---|---|---|---|
| (Pa × s) | (Pa) | n | Ea (kJ/mol) | |
| EPO:PEO (70:30) | 1417 | 3072 | 0.467 | 119.51 |
| EPO:PEO:ZHT (69.3:29.7:1) | 1432 | 5641 | 0.426 | 119.96 |
| EPO:PEO:ZHT (63:27:10) | 1010 | 6309 | 0.387 | 136.81 |
| Nozzle Size | Weight | Length | Width | Height | Hardness | Porosity |
|---|---|---|---|---|---|---|
| Ø | mg | mm | mm | mm | N | % |
| 0.25 | 113.16 ± 0.96 | 10.90 ± 0.02 | 5.39 ± 0.02 | 4.44 ± 0.03 | 17.50 ± 2.80 | 59.65 ± 1.51 |
| 0.40 | 118.34 ± 2.36 | 10.76 ± 0.06 | 5.35 ± 0.03 | 4.49 ± 0.02 | 23.10 ± 1.91 | 60.86 ± 0.68 |
| 0.60 | 124.20 ± 4.66 | 10.75 ± 0.03 | 5.42 ± 0.02 | 4.57 ± 0.09 | 29.30 ± 2.91 | 48.56 ± 2.75 |
| Size | Weight | Length | Width | Height |
|---|---|---|---|---|
| mm | mg | mm | mm | mm |
| 11.0 × 5.5 × 4.4 | 93.90 ± 2.10 | 10.87 ± 0.08 | 5.36 ± 0.01 | 4.36 ± 0.02 |
| 19.2 × 9.6 × 7.68 | 1028.10 ± 25.70 | 19.04 ± 0.06 | 9.44 ± 0.02 | 7.55 ± 0.12 |
| Blend | Weight mg | Length mm | Width mm | Height mm |
|---|---|---|---|---|
| Channeled caplets | 132.8 ± 5.0 | 10.80 ± 0.01 | 5.40 ± 0.02 | 4.24 ± 0.01 |
| AcDiSol (4%) | 103.6 ± 2.8 | 10.88 ± 0.02 | 5.39 ± 0.01 | 4.32 ± 0.02 |
| AcDiSol (8%) | 104.0 ± 3.7 | 10.89 ± 0.03 | 5.42 ± 0.02 | 4.32 ± 0.03 |
| AcDiSol (12%) | 107.2 ± 3.6 | 10.89 ± 0.02 | 5.41 ± 0.02 | 4.32 ± 0.04 |
| AcDiSol (16%) | 108.2 ± 1.8 | 10.86 ± 0.04 | 5.41 ± 0.01 | 4.33 ± 0.03 |
| Explotab (8%) | 115.0 ± 3.1 | 10.91 ± 0.04 | 5.43 ± 0.01 | 4.36 ± 0.03 |
| Polyplasdone (8%) | 99.6 ± 3.1 | 10.88 ± 0.03 | 5.42 ± 0.02 | 4.32 ± 0.03 |
| Primojel (8%) | 113.7 ± 4.7 | 10.91 ± 0.03 | 5.42 ± 0.02 | 4.36 ± 0.04 |
| Primellose (8%) | 91.0 ± 3.2 | 10.81 ± 0.03 | 5.35 ± 0.01 | 4.27 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henry, S.; De Vadder, L.; Decorte, M.; Francia, S.; Van Steenkiste, M.; Saevels, J.; Vanhoorne, V.; Vervaet, C. Development of a 3D-Printed Dosing Platform to Aid in Zolpidem Withdrawal Therapy. Pharmaceutics 2021, 13, 1684. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13101684
Henry S, De Vadder L, Decorte M, Francia S, Van Steenkiste M, Saevels J, Vanhoorne V, Vervaet C. Development of a 3D-Printed Dosing Platform to Aid in Zolpidem Withdrawal Therapy. Pharmaceutics. 2021; 13(10):1684. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13101684
Chicago/Turabian StyleHenry, Silke, Lien De Vadder, Milan Decorte, Susanna Francia, Magali Van Steenkiste, Jan Saevels, Valérie Vanhoorne, and Chris Vervaet. 2021. "Development of a 3D-Printed Dosing Platform to Aid in Zolpidem Withdrawal Therapy" Pharmaceutics 13, no. 10: 1684. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13101684