Nanotechnology for Age-Related Macular Degeneration
Abstract
:1. Introduction
2. The Major Disease Mechanisms of AMD (Age-Related Macular Degeneration)
2.1. Dysregulation of Angiogenesis
2.1.1. Vascular Endothelial Growth Factor
2.1.2. Glutamine Synthetase
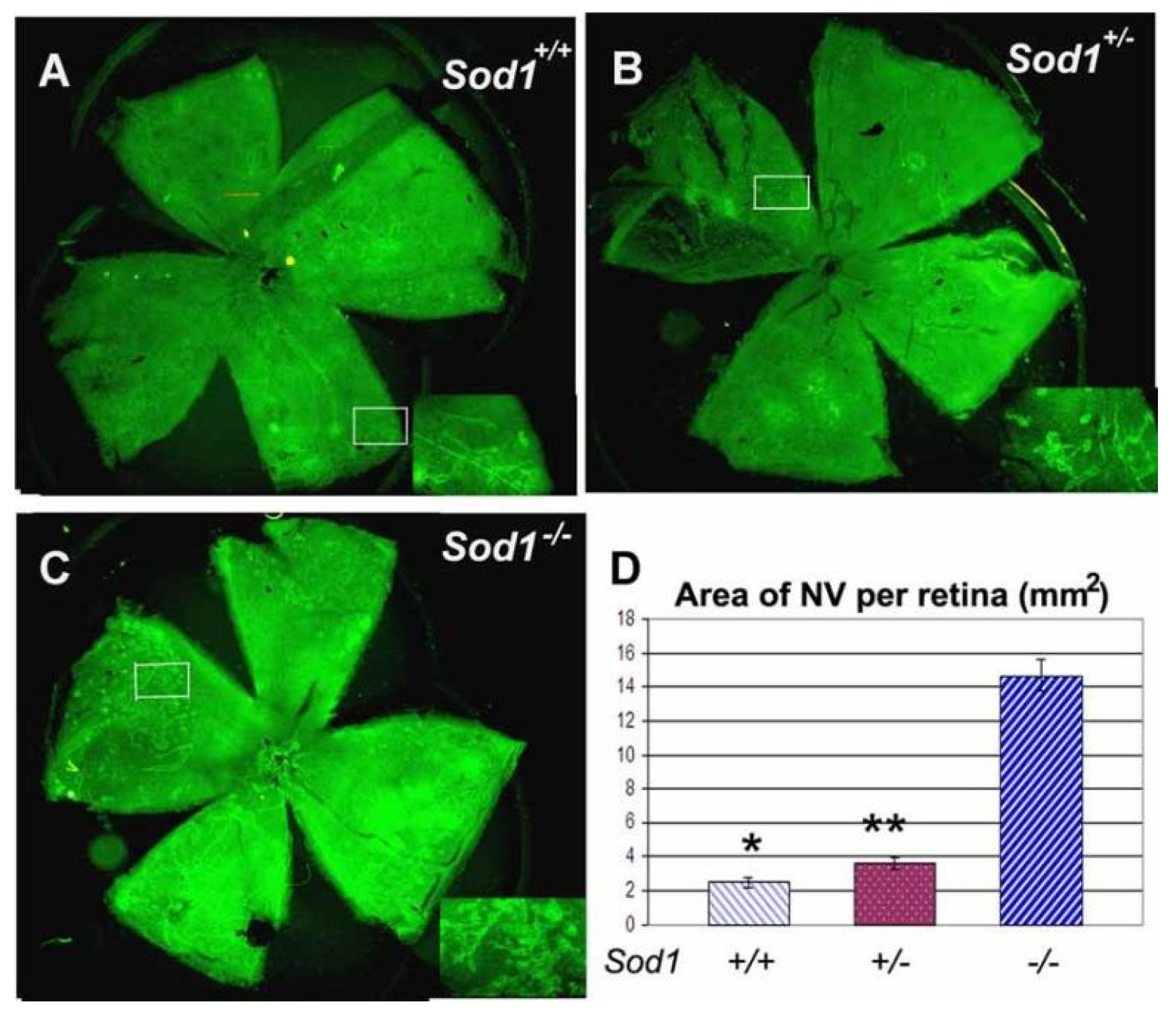
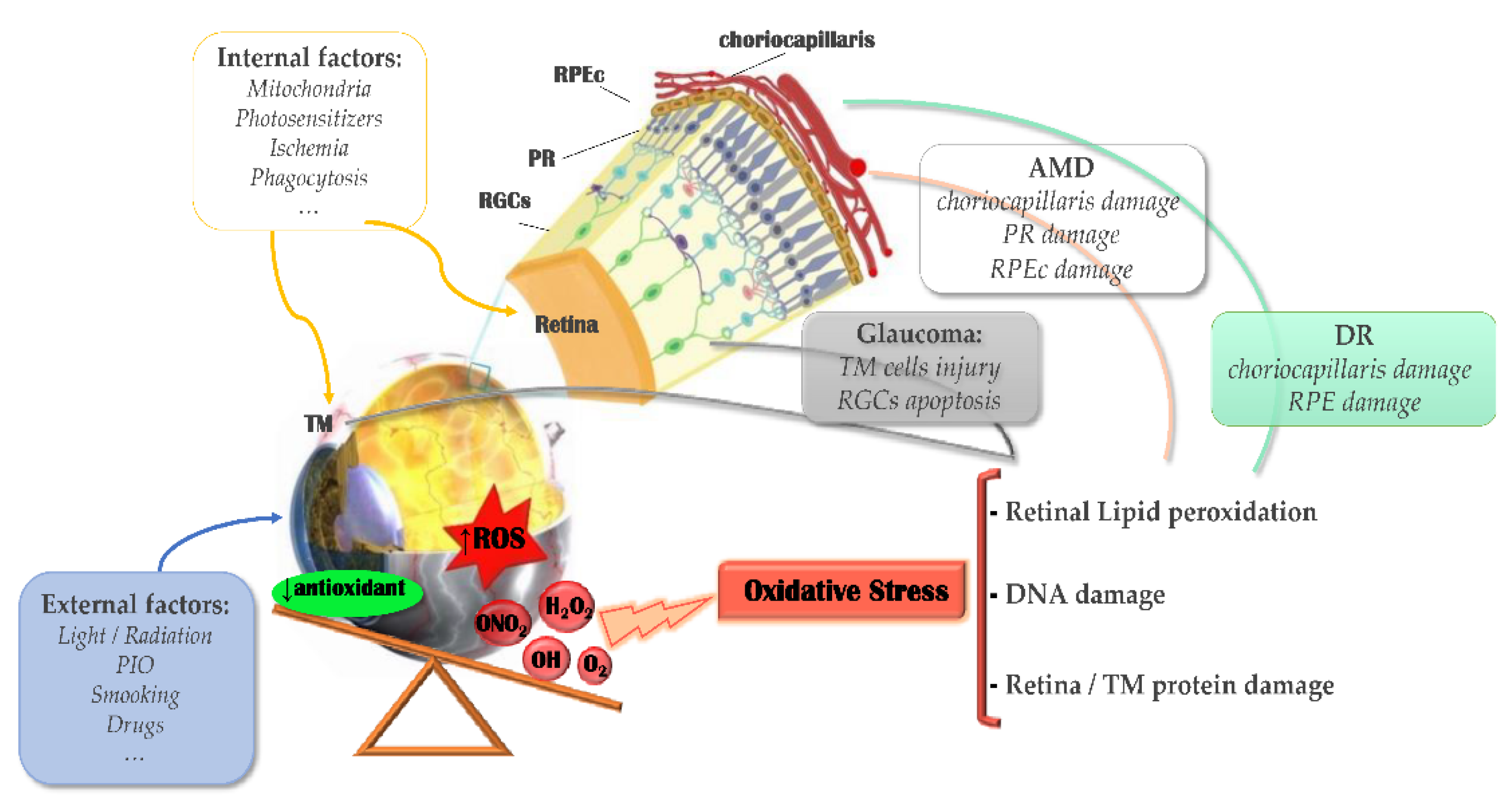
2.2. Light Damage and Oxidative Stress
2.3. Inflammation and Immune Dysfunction
3. Drug Delivery Systems for AMD
3.1. Liposomes
3.2. Nanomicelles
3.3. Nanoemulsions
3.4. Nanoparticles
3.5. Cyclodextrin
3.6. Dendrimers
3.7. Composite Drug Delivery System
4. Novel Treatment for AMD
5. Drug Delivery Systems for Gene Therapy
5.1. Adeno-Associated Virus (AAV )
5.2. Exosomes
5.3. Lipid Nanoparticles
6. Novel Targeting Strategies
6.1. Aptamer
6.2. RGD Peptide
6.3. Hyaluronic Acid
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steinmetz, J.D.; Bourne, R.R.A.; Briant, P.S.; Flaxman, S.R.; Taylor, H.R.B.; Jonas, J.B.; Abdoli, A.A.; Abrha, W.A.; Abualhasan, A.; Abu-Gharbieh, E.G.; et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Bourne, R.R.A.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.; Limburg, H.; et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e888–e897. [Google Scholar] [CrossRef] [Green Version]
- Lains, I.; Duarte, D.; Barros, A.S.; Martins, A.S.; Carneiro, T.J.; Gil, J.Q.; Miller, J.B.; Marques, M.; Mesquita, T.S.; Barreto, P.; et al. Urine Nuclear Magnetic Resonance (NMR) Metabolomics in Age-Related Macular Degeneration. J. Proteome Res. 2019, 18, 1278–1288. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Shetty, K.H.; Desai, D.T.; Shah, D.O.; Willcox, M.D.P. Recent advances in ophthalmic preparations: Ocular barriers, dosage forms and routes of administration. Int. J. Pharm. 2021, 608, 12. [Google Scholar] [CrossRef]
- Varela-Fernandez, R.; Diaz-Tome, V.; Luaces-Rodriguez, A.; Conde-Penedo, A.; Garcia-Otero, X.; Luzardo-Alvarez, A.; Fernandez-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, H.; Yu, A.Y.; Della, N.; Ozaki, K.; Luna, J.D.; Yamada, H.; Hackett, S.F.; Okamoto, N.; Zack, D.J.; Semenza, G.L.; et al. Hypoxia inducible factor-1 alpha is increased in ischemic retina: Temporal and spatial correlation with VEGF expression. Investig. Ophthalmol. Vis. Sci. 1999, 40, 182–189. [Google Scholar]
- Almuhtaseb, H.; Kanavati, S.; Rufai, S.R.; Lotery, A.J. One-year real-world outcomes in patients receiving fixed-dosing aflibercept for neovascular age related macular degeneration. Eye 2017, 31, 878–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsaid, N.; Jackson, T.L.; Elsaid, Z.; Alqathania, A.; Somavarapu, S. PLGA Microparticles Entrapping Chitosan-Based Nanoparticles for the Ocular Delivery of Ranibizumab. Mol. Pharm. 2016, 13, 2923–2940. [Google Scholar] [CrossRef] [PubMed]
- Ayata, N.; Sezer, A.D.; Bucak, S.; Turanli, E.T. Preparation and in vitro characterization of monoclonal antibody ranibizumab conjugated magnetic nanoparticles for ocular drug delivery. Braz. J. Pharm. Sci. 2020, 56, 15. [Google Scholar] [CrossRef]
- Hoshikawa, A.; Tagami, T.; Morimura, C.; Fukushige, K.; Ozeki, T. Ranibizumab biosimilar/polyethyleneglycol-conjugated gold nanoparticles as a novel drug delivery platform for age-related macular degeneration. J. Drug Deliv. Sci. Technol. 2017, 38, 45–50. [Google Scholar] [CrossRef]
- Yan, J.; Peng, X.F.; Cai, Y.L.; Cong, W.D. Development of facile drug delivery platform of ranibizumab fabricated PLGA-PEGylated magnetic nanoparticles for age-related macular degeneration therapy. J. Photochem. Photobiol. B-Biol. 2018, 183, 133–136. [Google Scholar] [CrossRef]
- Draoui, N.; de Zeeuw, P.; Carmeliet, P. Angiogenesis revisited from a metabolic perspective: Role and therapeutic implications of endothelial cell metabolism. Open Biol. 2017, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, T.; Weihrauch, D.W.; Moniz, M.C.; Tessmer, J.; Warltier, D.C.; Chilian, W.M. Angiostatin inhibits coronary angiogenesis during impaired production of nitric oxide. Circulation 2002, 105, 2185–2191. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Ren, L.; Hamblin, M.H.; Fan, Y.B. Endothelial Cell Glucose Metabolism and Angiogenesis. Biomedicines 2021, 9, 147. [Google Scholar] [CrossRef]
- Rohlenova, K.; Goveia, J.; Garcia-Caballero, M.; Subramanian, A.; Kalucka, J.; Treps, L.; Falkenberg, K.D.; de Rooij, L.; Zheng, Y.F.; Lin, L.; et al. Single-Cell RNA Sequencing Maps Endothelial Metabolic Plasticity in Pathological Angiogenesis. Cell Metab. 2020, 31, 862–877.e14. [Google Scholar] [CrossRef]
- De Bock, K.; Georgiadou, M.; Carmeliet, P. Role of Endothelial Cell Metabolism in Vessel Sprouting. Cell Metab. 2013, 18, 634–647. [Google Scholar] [CrossRef] [Green Version]
- Cohen, E.B.; Geck, R.C.; Toker, A. Metabolic pathway alterations in microvascular endothelial cells in response to hypoxia. PLoS ONE 2020, 15, e0232072. [Google Scholar] [CrossRef]
- Joyal, J.S.; Sun, Y.; Gantner, M.L.; Shao, Z.; Evans, L.P.; Saba, N.; Fredrick, T.; Burnim, S.; Kim, J.S.; Patel, G.; et al. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat. Med. 2016, 22, 439–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruning, U.; Morales-Rodriguez, F.; Kalucka, J.; Goveia, J.; Taverna, F.; Queiroz, K.C.S.; Dubois, C.; Cantelmo, A.R.; Chen, R.Y.; Loroch, S.; et al. Impairment of Angiogenesis by Fatty Acid Synthase Inhibition Involves mTOR Malonylation. Cell Metab. 2018, 28, 866–880. [Google Scholar] [CrossRef] [Green Version]
- Schoors, S.; Bruning, U.; Missiaen, R.; Queiroz, K.C.S.; Borgers, G.; Elia, I.; Zecchin, A.; Cantelmo, A.R.; Christen, S.; Goveia, J.; et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature 2015, 520, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Vandekeere, S.; Kalucka, J.; Bierhansl, L.; Zecchin, A.; Bruning, U.; Visnagri, A.; Yuldasheva, N.; Goveia, J.; Cruys, B.; et al. Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J. 2017, 36, 2334–2352. [Google Scholar] [CrossRef]
- Dong, A.; Xie, B.; Shen, J.; Yoshida, T.; Yokoi, K.; Hackett, S.F.; Campochiaro, P.A. Oxidative Stress Promotes Ocular Neovascularization of the article. J. Cell. Physiol. 2009, 219, 544–552. [Google Scholar] [CrossRef] [Green Version]
- de Jong, P. Mechanisms of disease: Age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1474–1485. [Google Scholar] [CrossRef]
- Dammak, A.; Huete-Toral, F.; Carpena-Torres, C.; Martin-Gil, A.; Pastrana, C.; Carracedo, G. From Oxidative Stress to Inflammation in the Posterior Ocular Diseases: Diagnosis and Treatment. Pharmaceutics 2021, 13, 1376. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, S.J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 2001, 20, 175–208. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Asp. Med. 2012, 33, 399–417. [Google Scholar] [CrossRef] [Green Version]
- Nowak, J.Z. Age-related macular degeneration (AMD): Pathogenesis and therapy. Pharmacol. Rep. 2006, 58, 353–363. [Google Scholar]
- Kaarniranta, K.; Salminen, A.; Eskelinen, E.L.; Kopitz, J. Heat shock proteins as gatekeepers of proteolytic pathways-Implications for age-related macular degeneration (AMD). Ageing Res. Rev. 2009, 8, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hackett, S.F.; Mincey, A.; Lai, H.; Campochiaro, P.A. Effects of different types of oxidative stress in RPE cells. J. Cell. Physiol. 2006, 206, 119–125. [Google Scholar] [CrossRef]
- Tsutsui, H. Mitochondrial oxidative stress and heart failure. Intern. Med. 2006, 45, 809–813. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef] [Green Version]
- Meeus, M.; Nijs, J.; Hermans, L.; Goubert, D.; Calders, P. The role of mitochondrial dysfunctions due to oxidative and nitrosative stress in the chronic pain or chronic fatigue syndromes and fibromyalgia patients: Peripheral and central mechanisms as therapeutic targets? Expert Opin. Ther. Targets 2013, 17, 1081–1089. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A.; Ascaso, F.J.; Huerva, V. Age-Related Macular Degeneration in the Aspect of Chronic Low-Grade Inflammation (Pathophysiological ParaInflammation). Mediat. Inflamm. 2014, 2014, 930671. [Google Scholar] [CrossRef]
- Anderson, D.H.; Radeke, M.J.; Gallo, N.B.; Chapin, E.A.; Johnson, P.T.; Curletti, C.R.; Hancox, L.S.; Hu, J.; Ebright, J.N.; Malek, G.; et al. The pivotal role of the complement system in aging and age-related macular degeneration: Hypothesis re-visited. Prog. Retin. Eye Res. 2010, 29, 95–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armento, A.; Ueffing, M.; Clark, S.J. The complement system in age-related macular degeneration. Cell. Mol. Life Sci. 2021, 78, 4487–4505. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Connor, K.M.; Lambris, J.D. The Challenges and Promise of Complement Therapeutics for Ocular Diseases. Front. Immunol. 2019, 10, 1007. [Google Scholar] [CrossRef]
- Rodrigues, E.B. Inflammation in dry age-related macular degeneration. Ophthalmologica 2007, 221, 143–152. [Google Scholar] [CrossRef]
- Anderson, D.H.; Mullins, R.F.; Hageman, G.S.; Johnson, L.V. Perspective—A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 2002, 134, 411–431. [Google Scholar] [CrossRef]
- Elsaid, N.; Somavarapu, S.; Jackson, T.L. Cholesterol-poly(ethylene) glycol nanocarriers for the transscleral delivery of sirolimus. Exp. Eye Res. 2014, 121, 121–129. [Google Scholar] [CrossRef]
- Behroozi, F.; Abdkhodaie, M.J.; Abandansari, H.S.; Satarian, L.; Ashtiani, M.K.; Jaafari, M.R.; Baharvand, H. Smart liposomal drug delivery for treatment of oxidative stress model in human embryonic stem cell-derived retinal pigment epithelial cells. Int. J. Pharm. 2018, 548, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.R.; Tan, D.W.N.; Ramon, M.R.M.; Natarajan, J.V.; Agrawal, R.; Wong, T.T.; Venkatraman, S.S. Characterization of liposomal carriers for the trans-scleral transport of Ranibizumab. Sci. Rep. 2017, 7, 16803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, H.J.; Wang, Y.Y.; Chu, Y.C.; Jiang, Y.; Hua, H.C.; Chu, L.X.; Wang, K.L.; Wang, A.P.; Liu, W.H.; Li, Y.X.; et al. Multivesicular liposomes for sustained release of bevacizumab in treating laser-induced choroidal neovascularization. Drug Deliv. 2018, 25, 1372–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karumanchi, D.K.; Skrypai, Y.; Thomas, A.; Gaillard, E.R. Rational design of liposomes for sustained release drug delivery of bevacizumab to treat ocular angiogenesis. J. Drug Deliv. Sci. Technol. 2018, 47, 275–282. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Fisichella, V.; Fidilio, A.; Geraci, F.; Lazzara, F.; Leggio, G.M.; Salomone, S.; Drago, F.; Pignatello, R.; Caraci, F.; et al. Topical Ocular Delivery of TGF-beta 1 to the Back of the Eye: Implications in Age-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2017, 18, 2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.W.; Yeh, M.K.; Shiau, C.Y.; Chiang, C.H.; Lu, D.W. Efficient downregulation of VEGF in retinal pigment epithelial cells by integrin ligand-labeled liposome-mediated siRNA delivery. Int. J. Nanomed. 2013, 8, 2613–2627. [Google Scholar] [CrossRef] [Green Version]
- Takashima, Y.; Tsuchiya, T.; Igarashi, Y.; Kanazawa, T.; Okada, H.; Urtti, A. Non-Invasive Ophthalmic Liposomes for Nucleic Acid Delivery to Posterior Segment of Eye. Yakugaku Zasshi-J. Pharm. Soc. Jpn. 2012, 132, 1365–1370. [Google Scholar] [CrossRef] [Green Version]
- Chaw, S.Y.; Novera, W.; Chacko, A.M.; Wong, T.T.L.; Venkatraman, S. In vivo fate of liposomes after subconjunctival ocular delivery. J. Control. Release 2021, 329, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Balazs, D.A.; Godbey, W. Liposomes for use in gene delivery. J. Drug Deliv. 2011, 2011, 326497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordling-David, M.M.; Golomb, G. Gene Delivery by Liposomes. Isr. J. Chem. 2013, 53, 737–747. [Google Scholar] [CrossRef]
- Vaishya, R.D.; Gokulgandhi, M.; Patel, S.; Minocha, M.; Mitra, A.K. Novel Dexamethasone-Loaded Nanomicelles for the Intermediate and Posterior Segment Uveitis. AAPS Pharmscitech 2014, 15, 1238–1251. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.Y.; Nan, K.H.; Lee, S.; Beadle, J.R.; Hou, H.Y.; Freeman, W.R.; Hostetler, K.Y.; Cheng, L.Y. Micelle formulation of hexadecyloxypropyl-cidofovir (HDP-CDV) as an intravitreal long-lasting delivery system. Eur. J. Pharm. Biopharm. 2015, 89, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Alshamrani, M.; Sikder, S.; Coulibaly, F.; Mandal, A.; Pal, D.; Mitra, A.K. Self-Assembling Topical Nanomicellar Formulation to Improve Curcumin Absorption Across Ocular Tissues. AAPS Pharmscitech 2019, 20, 254. [Google Scholar] [CrossRef]
- Gote, V.; Mandal, A.; Alshamrani, M.; Pal, D. Self-Assembling Tacrolimus Nanomicelles for Retinal Drug Delivery. Pharmaceutics 2020, 12, 1072. [Google Scholar] [CrossRef]
- Vaishya, R.D.; Khurana, V.; Patel, S.; Mitra, A.K. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2014, 6, 422–437. [Google Scholar] [CrossRef] [Green Version]
- Hagigit, T.; Abdulrazik, M.; Valamanesh, F.; Behar-Cohen, F.; Benita, S. Ocular antisense oligonucleotide delivery by cationic nanoemulsion for improved treatment of ocular neovascularization: An in-vivo study in rats and mice. J. Control. Release 2012, 160, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Nakrani, H.; Raval, M.; Sheth, N. Development of loteprednol etabonate-loaded cationic nanoemulsified in-situ ophthalmic gel for sustained delivery and enhanced ocular bioavailability. Drug Deliv. 2016, 23, 3712–3723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Y.; Zhang, A.; Sun, R.; Xu, J.W.; Yin, T.; He, H.B.; Gou, J.X.; Kong, J.; Zhang, Y.; Tang, X. Penetratin-modified lutein nanoemulsion in-situ gel for the treatment of age-related macular degeneration. Expert Opin. Drug Deliv. 2020, 17, 603–619. [Google Scholar] [CrossRef]
- Lim, C.; Kim, D.-W.; Sim, T.; Hoang, N.H.; Lee, J.W.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Preparation and characterization of a lutein loading nanoemulsion system for ophthalmic eye drops. J. Drug Deliv. Sci. Technol. 2016, 36, 168–174. [Google Scholar] [CrossRef]
- Laradji, A.M.; Kolesnikov, A.V.; Karakocak, B.B.; Kefalov, V.J.; Ravi, N. Redox-Responsive Hyaluronic Acid-Based Nanogels for the Topical Delivery of the Visual Chromophore to Retinal Photoreceptors. ACS Omega 2021, 6, 6172–6184. [Google Scholar] [CrossRef]
- Du, M.B.; Shen, S.; Liang, L.N.; Xu, K.; He, A.P.; Yao, Y.; Liu, S.Z. Evaluations of the Chuanqi Ophthalmic Microemulsion In Situ Gel on Dry Age-Related Macular Degeneration Treatment. Evid.-Based Complement. Altern. Med. 2020, 2020, 3805967. [Google Scholar] [CrossRef]
- Al-Kassas, R.S.; El-Khatib, M.M. Ophthalmic controlled release in situ gelling systems for ciprofloxacin based on polymeric carriers. Drug Deliv. 2009, 16, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Bharadwaj, S.; Lee, K.E.; Kang, S.G. Therapeutic nanoemulsions in ophthalmic drug administration: Concept in formulations and characterization techniques for ocular drug delivery. J. Control. Release 2020, 328, 895–916. [Google Scholar] [CrossRef] [PubMed]
- Mundada, A.S.; Avari, J.G. In Situ Gelling Polymers in Ocular Drug Delivery Systems: A Review. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 85–118. [Google Scholar] [CrossRef] [PubMed]
- Bolla, P.K.; Gote, V.; Singh, M.; Patel, M.; Clark, B.A.; Renukuntla, J. Lutein-Loaded, Biotin-Decorated Polymeric Nanoparticles Enhance Lutein Uptake in Retinal Cells. Pharmaceutics 2020, 12, 798. [Google Scholar] [CrossRef] [PubMed]
- Narvekar, P.; Bhatt, P.; Fnu, G.; Sutariya, V. Axitinib-Loaded Poly(Lactic-Co-Glycolic Acid) Nanoparticles for Age-Related Macular Degeneration: Formulation Development and In Vitro Characterization. Assay Drug Dev. Technol. 2019, 17, 167–177. [Google Scholar] [CrossRef]
- Liu, J.X.; Zhang, X.Y.; Li, G.; Xu, F.; Li, S.; Teng, L.S.; Li, Y.X.; Sun, F.Y. Anti-Angiogenic Activity Of Bevacizumab-Bearing Dexamethasone-Loaded PLGA Nanoparticles For Potential Intravitreal Applications. Int. J. Nanomed. 2019, 14, 8819–8834. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Hurley, B.; Liu, Y.; Leonard, B.; Griffith, M. Controlled release of bevacizumab through nanospheres for extended treatment of age-related macular degeneration. Open Ophthalmol. J. 2012, 6, 54–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.L.; Si, T.; Fischer, A.J.; Letson, A.; Yuan, S.; Roberts, C.J.; Xu, R.X. Coaxial Electrospray of Ranibizumab-Loaded Microparticles for Sustained Release of Anti-VEGF Therapies. PLoS ONE 2015, 10, e0135608. [Google Scholar] [CrossRef] [Green Version]
- Sousa, F.; Cruz, A.; Fonte, P.; Pinto, I.M.; Neves-Petersen, M.T.; Sarmento, B. A new paradigm for antiangiogenic therapy through controlled release of bevacizumab from PLGA nanoparticles. Sci. Rep. 2017, 7, 3736. [Google Scholar] [CrossRef] [PubMed]
- Varshochian, R.; Riazi-Esfahani, M.; Jeddi-Tehrani, M.; Mahmoudi, A.R.; Aghazadeh, S.; Mahbod, M.; Movassat, M.; Atyabi, F.; Sabzevari, A.; Dinarvand, R. Albuminated PLGA nanoparticles containing bevacizumab intended for ocular neovascularization treatment. J. Biomed. Mater. Res. Part A 2015, 103, 3148–3156. [Google Scholar] [CrossRef]
- Bourges, J.L.; Gautier, S.E.; Delie, F.; Bejjani, R.A.; Jeanny, J.C.; Gurny, R.; BenEzra, D.; Behar-Cohen, F.F. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3562–3569. [Google Scholar] [CrossRef]
- Kim, H.; Csaky, K.G. Nanoparticle-integrin antagonist C16Y peptide treatment of choroidal neovascularization in rats. J. Control. Release 2010, 142, 286–293. [Google Scholar] [CrossRef]
- Wang, Y.F.; Liu, C.H.; Ji, T.J.; Mehta, M.; Wang, W.P.; Marino, E.; Chen, J.; Kohane, D.S. Intravenous treatment of choroidal neovascularization by photo-targeted nanoparticles. Nat. Commun. 2019, 10, 804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyfoddin, A.; Shaw, J.; Al-Kassas, R. Solid lipid nanoparticles for ocular drug delivery. Drug Deliv. 2010, 17, 467–489. [Google Scholar] [CrossRef]
- Singh, K.H.; Shinde, U.A. Development and Evaluation of Novel Polymeric Nanoparticles of Brimonidine Tartrate. Curr. Drug Deliv. 2010, 7, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kam, J.H.; Lynch, A.; Begum, R.; Cunea, A.; Jeffery, G. Topical cyclodextrin reduces amyloid beta and inflammation improving retinal function in ageing mice. Exp. Eye Res. 2015, 135, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Darzi, N.; Mast, N.; Petrov, A.M.; Pikuleva, I.A. 2-Hydroxypropyl-beta-cyclodextrin reduces retinal cholesterol in wild-type and Cyp27a1(−/−)Cyp46a1(−/−) mice with deficiency in the oxysterol production. Br. J. Pharmacol. 2021, 178, 3220–3234. [Google Scholar] [CrossRef]
- Kaur, I.P.; Chhabra, S.; Aggarwal, D. Role of cyclodextrins in ophthalmics. Curr. Drug Deliv. 2004, 1, 351–360. [Google Scholar] [CrossRef]
- Marano, R.J.; Toth, I.; Wimmer, N.; Brankov, M.; Rakoczy, P.E. Dendrimer delivery of an anti-VEGF oligonucleotide into the eye: A long-term study into inhibition of laser-induced CNV, distribution, uptake and toxicity. Gene Ther. 2005, 12, 1544–1550. [Google Scholar] [CrossRef]
- Yavuz, B.; Pehlivan, S.B.; Bolu, B.S.; Sanyal, R.N.; Vural, I.; Unlu, N. Dexamethasone—PAMAM dendrimer conjugates for retinal delivery: Preparation, characterization and in vivo evaluation. J. Pharm. Pharmacol. 2016, 68, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.S.; Wei, Y.Y.; Wu, Q.W.; Zhou, K.; Liu, T.; Zhang, Y.F.; Jiang, N.; Xiao, W.; Chen, J.J.; Liu, Q.H.; et al. Liposomes for effective drug delivery to the ocular posterior chamber. J. Nanobiotechnol. 2019, 17, 64. [Google Scholar] [CrossRef] [Green Version]
- Yao, W.J.; Sun, K.X.; Liu, Y.; Liang, N.; Mu, H.J.; Yao, C.; Liang, R.C.; Wang, A.P. Effect of Poly(amidoamine) Dendrimers on Corneal Penetration of Puerarin. Biol. Pharm. Bull. 2010, 33, 1371–1377. [Google Scholar] [CrossRef] [Green Version]
- Xin, G.; Zhang, M.; Zhong, Z.H.; Tang, L.; Feng, Y.L.; Wei, Z.L.; Li, S.Y.; Li, Y.P.; Zhang, J.H.; Zhang, B.L.; et al. Ophthalmic Drops with Nanoparticles Derived from a Natural Product for Treating Age-Related Macular Degeneration. ACS Appl. Mater. Interfaces 2020, 12, 57710–57720. [Google Scholar] [CrossRef]
- Hirani, A.; Grover, A.; Lee, Y.W.; Pathak, Y.; Sutariya, V. Triamcinolone acetonide nanoparticles incorporated in thermoreversible gels for age-related macular degeneration. Pharm. Dev. Technol. 2016, 21, 61–67. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, M.; Lester, K.L.; Han, Z.C. Light-induced Nrf2(−/−) mice as atrophic age-related macular degeneration model and treatment with nanoceria laden injectable hydrogel. Sci. Rep. 2019, 9, 14573. [Google Scholar] [CrossRef] [PubMed]
- Osswald, C.R.; Guthrie, M.J.; Avila, A.; Valio, J.A.; Mieler, W.F.; Kang-Mieler, J.J. In Vivo Efficacy of an Injectable Microsphere-Hydrogel Ocular Drug Delivery System. Curr. Eye Res. 2017, 42, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Osswald, C.R.; Kang-Mieler, J.J. Controlled and Extended In Vitro Release of Bioactive Anti-Vascular Endothelial Growth Factors from a Microsphere-Hydrogel Drug Delivery System. Curr. Eye Res. 2016, 41, 1216–1222. [Google Scholar] [CrossRef]
- Osswald, C.R.; Kang-Mieler, J.J. Controlled and Extended Release of a Model Protein from a Microsphere-Hydrogel Drug Delivery System. Ann. Biomed. Eng. 2015, 43, 2609–2617. [Google Scholar] [CrossRef] [PubMed]
- Gebler, C.; Lohoff, T.; Paszkowski-Rogacz, M.; Mircetic, J.; Chakraborty, D.; Camgoz, A.; Hamann, M.V.; Theis, M.; Thiede, C.; Buchholz, F. Inactivation of Cancer Mutations Utilizing CRISPR/Cas9. JNCI-J. Natl. Cancer Inst. 2017, 109, djw183. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.L.; Tu, Z.C.; Sun, Q.; Li, X.J. CRISPR/Cas9: Implications for Modeling and Therapy of Neurodegenerative Diseases. Front. Mol. Neurosci. 2016, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- McAndrews, K.M.; Xiao, F.; Chronopoulos, A.; LeBleu, V.S.; Kugeratski, F.G.; Kalluri, R. Exosome-mediated delivery of CRISPR/Cas9 for targeting of oncogenic KrasG12D in pancreatic cancer. Life Sci. Alliance 2021, 4. [Google Scholar] [CrossRef]
- Sabit, H.; Abdel-Ghany, S.; Tombuloglu, H.; Cevik, E.; Alqosaibi, A.; Almulhim, F.; Al-Muhanaa, A. New insights on CRISPR/Cas9-based therapy for breast Cancer. Genes Environ. 2021, 43, 13. [Google Scholar] [CrossRef] [PubMed]
- Men, K.; Duan, X.M.; He, Z.Y.; Yang, Y.; Yao, S.H.; Wei, Y.Q. CRISPR/Cas9-mediated correction of human genetic disease. Sci. China-Life Sci. 2017, 60, 447–457. [Google Scholar] [CrossRef]
- Gupta, A.; Kafetzis, K.N.; Tagalakis, A.D.; Yu-Wai-Man, C. RNA therapeutics in ophthalmology-translation to clinical trials. Exp. Eye Res. 2021, 205, 108482. [Google Scholar] [CrossRef] [PubMed]
- Yiu, G.; Tieu, E.; Nguyen, A.T.; Wong, B.; Smit-McBride, Z. Genomic Disruption of VEGF-A Expression in Human Retinal Pigment Epithelial Cells Using CRISPR-Cas9 Endonuclease. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5490–5497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Park, S.W.; Kim, J.H.; Lee, S.H.; Kim, D.; Koo, T.; Kim, K.E.; Kim, J.H.; Kim, J.S. Genome surgery using Cas9 ribonucleoproteins for the treatment of age-related macular degeneration. Genome Res. 2017, 27, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.G.; Zhou, G.H.; Wu, W.Y.; Duan, Y.J.; Ma, G.E.; Song, J.Y.; Xiao, R.; Vandenberghe, L.; Zhang, F.; D’Amore, P.A.; et al. Genome editing abrogates angiogenesis in vivo. Nat. Commun. 2017, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Y.; Duan, Y.J.; Ma, G.E.; Zhou, G.H.; Park-Windhol, C.; D’Amore, P.A.; Lei, H.T. AAV-CRISPR/Cas9-Mediated Depletion of VEGFR2 Blocks Angiogenesis In Vitro. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6082–6090. [Google Scholar] [CrossRef] [Green Version]
- Benati, D.; Patrizi, C.; Recchia, A. Gene editing prospects for treating inherited retinal diseases. J. Med. Genet. 2020, 57, 437–444. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Lin, C.Y.; Gootenberg, J.S.; Konermann, S.; Trevino, A.E.; Scott, D.A.; Inoue, A.; Matoba, S.; Zhang, Y.; et al. Double Nicking by RNA-Guided CRISPR Cas9 for Enhanced Genome Editing Specificity. Cell 2013, 154, 1380–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vakulskas, C.A.; Dever, D.P.; Rettig, G.R.; Turk, R.; Jacobi, A.M.; Collingwood, M.A.; Bode, N.M.; McNeill, M.S.; Yan, S.Q.; Camarena, J.; et al. A high-fidelity Cas9 mutant delivered as a ribonucleoprotein complex enables efficient gene editing in human hematopoietic stem and progenitor cells. Nat. Med. 2018, 24, 1216–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merienne, N.; Vachey, G.; de Longprez, L.; Meunier, C.; Zimmer, V.; Perriard, G.; Canales, M.; Mathias, A.; Herrgott, L.; Beltraminelli, T.; et al. The Self-Inactivating KamiCas9 System for the Editing of CNS Disease Genes. Cell Rep. 2017, 20, 2980–2991. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhang, L.; Liu, H.; Cheng, K. Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic applications. J. Control. Release 2017, 266, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Dreismann, A.K.; McClements, M.E.; Barnard, A.R.; Orhan, E.; Hughes, J.P.; Lachmann, P.J.; MacLaren, R.E. Functional expression of complement factor I following AAV-mediated gene delivery in the retina of mice and human cells. Gene Ther. 2021, 28, 265–276. [Google Scholar] [CrossRef]
- Ling, S.; Yang, S.; Hu, X.; Yin, D.; Dai, Y.; Qian, X.; Wang, D.; Pan, X.; Hong, J.; Sun, X.; et al. Lentiviral delivery of co-packaged Cas9 mRNA and a Vegfa-targeting guide RNA prevents wet age-related macular degeneration in mice. Nat. Biomed. Eng. 2021, 5, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Mollhoff, I.N.; Nguyen, U.; Nguyen, A.; Stucka, N.; Tieu, E.; Manna, S.; Meleppat, R.K.; Zhang, P.F.; Nguyen, E.L.; et al. Factors Impacting Efficacy of AAV-Mediated CRISPR-Based Genome Editing for Treatment of Choroidal Neovascularization. Mol. Ther. Methods Clin. Dev. 2020, 17, 409–417. [Google Scholar] [CrossRef]
- Fuentes, C.M.; Schaffer, D.V. Adeno-associated virus-mediated delivery of CRISPR-Cas9 for genome editing in the central nervous system. Curr. Opin. Biomed. Eng. 2018, 7, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.Y.; Bang, J.Y.; Choi, A.J.; Yoon, J.; Lee, W.C.; Choi, S.; Yoon, S.; Kim, H.C.; Baek, J.H.; Park, H.S.; et al. Exosomal Proteins in the Aqueous Humor as Novel Biomarkers in Patients with Neovascular Age-related Macular Degeneration. J. Proteome Res. 2014, 13, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Chiechi, A.; Couch, R.; Liotta, L.A.; Espina, V. Retinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stress. Exp. Cell Res. 2013, 319, 2113–2123. [Google Scholar] [CrossRef] [Green Version]
- Hajrasouliha, A.R.; Jiang, G.M.; Lu, Q.X.; Lu, H.Y.; Kaplan, H.J.; Zhang, H.G.; Shao, H. Exosomes from Retinal Astrocytes Contain Antiangiogenic Components That Inhibit Laser-induced Choroidal Neovascularization. J. Biol. Chem. 2013, 288, 28058–28067. [Google Scholar] [CrossRef] [Green Version]
- Duan, L.; Ouyang, K.; Wang, J.H.; Xu, L.M.; Xu, X.; Wen, C.I.; Xie, Y.X.; Liang, Y.J.; Xia, J. Exosomes as Targeted Delivery Platform of CRISPR/Cas9 for Therapeutic Genome Editing. ChemBioChem 2021. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Raiker, R.S.; Jay, S.M. Exogenous DNA Loading into Extracellular Vesicles via Electroporation is Size-Dependent and Enables Limited Gene Delivery. Mol. Pharm. 2015, 12, 3650–3657. [Google Scholar] [CrossRef] [Green Version]
- Finn, J.D.; Smith, A.R.; Patel, M.C.; Shaw, L.; Youniss, M.R.; van Heteren, J.; Dirstine, T.; Ciullo, C.; Lescarbeau, R.; Seitzer, J.; et al. A Single Administration of CRISPR/Cas9 Lipid Nanoparticles Achieves Robust and Persistent In Vivo Genome Editing. Cell Rep. 2018, 22, 2227–2235. [Google Scholar] [CrossRef] [Green Version]
- Sung, D.; Sun, Z.H.; Jiang, H.F.; Vaidya, A.M.; Xin, R.; Ayat, N.R.; Schilb, A.L.; Qiao, P.L.; Han, Z.; Naderi, A.; et al. Synthesis and Evaluation of pH-Sensitive Multifunctional Lipids for Efficient Delivery of CRISPR/Cas9 in Gene Editing. Bioconjug. Chem. 2019, 30, 667–678. [Google Scholar] [CrossRef] [Green Version]
- del Pozo-Rodriguez, A.; Delgado, D.; Gascon, A.R.; Solinis, M.A. Lipid Nanoparticles as Drug/Gene Delivery Systems to the Retina. J. Ocul. Pharmacol. Ther. 2013, 29, 173–188. [Google Scholar] [CrossRef]
- Wang, Y.H.; Rajala, A.; Rajala, R.V.S. Lipid Nanoparticles for Ocular Gene Delivery. J. Funct. Biomater. 2015, 6, 379–394. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.H.; Liu, A.Q.; Zhang, H.; Wang, M.; Tang, Q.; Huang, Y.F.; Wang, L.Q. Inhibition of retinal neovascularization by VEGF siRNA delivered via bioreducible lipid-like nanoparticles. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2407–2418. [Google Scholar] [CrossRef]
- Lino, C.A.; Harper, J.C.; Carney, J.P.; Timlin, J.A. Delivering CRISPR: A review of the challenges and approaches. Drug Deliv. 2018, 25, 1234–1257. [Google Scholar] [CrossRef] [Green Version]
- Leaderer, D.; Cashman, S.M.; Kumar-Singh, R. Topical application of a G-Quartet aptamer targeting nucleolin attenuates choroidal neovascularization in a model of age-related macular degeneration. Exp. Eye Res. 2015, 140, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Vivanco-Rojas, O.; Garcia-Bermudez, M.Y.; Iturriaga-Goyon, E.; Rebollo, W.; Buentello-Volante, B.; Magana-Guerrero, F.S.; Bates, P.; Perez-Torres, A.; Garfias, Y. Corneal neovascularization is inhibited with nucleolin-binding aptamer, AS1411. Exp. Eye Res. 2020, 193, 107977. [Google Scholar] [CrossRef]
- Evaluation of intracellular uptake of cyclic RGD peptides in integrin αvβ3-expressing tumor cells. J. Radiopharm. Mol. Probes 2020, 6, 34–43. Available online: https://kmbase.medric.or.kr/Main.aspx?d=KMBASE&i=1145520200060020034&m=VIEW (accessed on 22 November 2021).
- Singh, S.R.; Grossniklaus, H.E.; Kang, S.J.; Edelhauser, H.F.; Ambati, B.K.; Kompella, U.B. Intravenous transferrin, RGD peptide and dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009, 16, 645–659. [Google Scholar] [CrossRef] [Green Version]
- Sim, C.; Park, J.Y.; Park, J.H.; Hong, H.; Ahn, S.J.; Woo, S.J.; Park, K.H.; Kim, H. Development and Evaluation of a polysiRNA delivery system to the retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4952. [Google Scholar]


| Drug | Product | Time and Indication | Target | Company |
|---|---|---|---|---|
| Brolucizumab | Beovu® | 7 October 2019 America, wAMD 17 February 2020 EU, wAMD | VEGFA | Alcon Laboratories; Novartis |
| Bevacizumab | Avastin® | 26 February 2004 America, wAMD 26 February 2010 China, wAMD | VEGFA | Genentech; Chugai Pharmaceutical |
| Ranibizumab | Lucentis® | 30 June 2006 America-Genentech, wAMD, RVO, DME, mCNV 22 January 2007 EU-Novartis, wAMD, DME 21 January 2009 Japan-Novartis, AMD, DME, CNV, RVO 31 December 2011 China-Novartis, wAMD, RVO, CNV | VEGFA | Genentech; Novartis; Roche |
| Aflibercept | Eylea® | 18 November 2011 America-Regeneron Pharmaceuticals, wAMD 28 September 2012 Japan-Bayer, AMD, macular edema 21 November 2012 EU-Bayer, wAMD, DME, macular edema 2 February 2018, 30 November 2018 China-Bayer, DME, wAMD | VEGFA/B, PGF | Regeneron Pharmaceuticals; Bayer |
| Conbercept | Langmu® | 27 November 2013 China wAMD 24 May 2017 China pmCNV 17 May 2019 China DME | VEGFA/B, PGF | KangHong Pharmaceutical Group; RemeGen |
| Pegaptanib Sodium | Macugen®/PrMacugen® | 17 September 2004 America, wAMD 31 January 2006 EU, wAMD | VEGF | Valeant |
| Anecortave Acetate | Retaane® | 16 December 2005 Australia, auxiliary ranibizumab treatment wAMD | VEGF | Alcon Laboratories |
| Verteporfin | Visudyne® | 16 December 1999 Switzerland-Novartis 12 April 2000 America-Valeant 27 July 2000 EU-Novartis 16 October 2003 Japan-Novartis 15 May 2015 China-Novartis, PM, AMD | Photosensitizers | Novartis, Novelion, Therapeutics, Valeant |
| VistaMR | Vistaplus® | 2014, AMD | MR | Eye Co |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Li, G.; Liu, J.; Li, X.; Zhang, S.; Sun, F.; Liu, W. Nanotechnology for Age-Related Macular Degeneration. Pharmaceutics 2021, 13, 2035. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13122035
Yang B, Li G, Liu J, Li X, Zhang S, Sun F, Liu W. Nanotechnology for Age-Related Macular Degeneration. Pharmaceutics. 2021; 13(12):2035. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13122035
Chicago/Turabian StyleYang, Bo, Ge Li, Jiaxin Liu, Xiangyu Li, Shixin Zhang, Fengying Sun, and Wenhua Liu. 2021. "Nanotechnology for Age-Related Macular Degeneration" Pharmaceutics 13, no. 12: 2035. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13122035






