pH-Sensitive Alginate/Carboxymethyl Chitosan/Aminated Chitosan Microcapsules for Efficient Encapsulation and Delivery of Diclofenac Sodium
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of Alg-CMCs Single PEC Microcapsules
2.3. Preparation of Alg-CMCs@AmCs Dual PECs Microcapsules
2.4. DS-Drug Loading Process
2.5. Microcapsules Characterization
2.6. Swelling Studies
2.7. DS-Drug Release Studies
2.8. In Vitro Biodegradation Study
2.9. Statistical Analysis
3. Results and Discussion
3.1. FT-IR Analysis
3.2. TGA Analysis
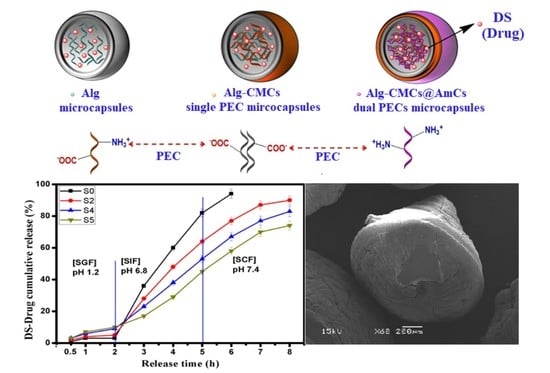
3.3. Morphological Analysis
3.4. In Vitro Swelling and pH Sensitivity
3.4.1. Swelling at pH 1.2
3.4.2. Swelling at pH 7.4
3.5. Evaluation of DS-Loading
3.6. In Vitro DS-Drug Release
3.7. Cumulative DS-Drug Release in Simulated GI-Tract
3.8. Biodegradability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bae, Y.H.; Park, K. Advanced Drug Delivery 2020 and Beyond: Perspectives on the Future. Adv. Drug Deliv. Rev. 2020, 158, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; González-Ortiz, A.; Lopez Romo, I.; V Barrera, E. Development of Functionalized Carbon Nano-onions Reinforced Zein Protein Hydrogel Interfaces for Controlled Drug Release. Pharmaceutics 2019, 11, 621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikmen, G.; Genç, L.; Güney, G. Advantage and Disadvantage in Drug Delivery Systems. J. Mater. Sci. Eng. 2011, 5, 468. [Google Scholar]
- Sung, Y.K.; Kim, S.W. Recent Advances in Polymeric Drug Delivery Systems. Biomater. Res. 2020, 24, 1–12. [Google Scholar] [CrossRef]
- Sun, X.; Wang, N.; Yang, L.-Y.; Ouyang, X.-K.; Huang, F. Folic Acid and PEI Modified Mesoporous Silica for Targeted Delivery of Curcumin. Pharmaceutics 2019, 11, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef] [Green Version]
- Schmaljohann, D. Thermo-and pH-responsive Polymers in Drug Delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Polymeric Hydrogels as Technology Platform for Drug Delivery Applications. Gels 2017, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306. [Google Scholar] [CrossRef]
- d’Ayala, G.G.; Malinconico, M.; Laurienzo, P. Marine Derived Polysaccharides for Biomedical Applications: Chemical Modification Approaches. Molecules 2008, 13, 2069–2106. [Google Scholar] [CrossRef] [Green Version]
- Pawar, S.N.; Edgar, K.J. Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Xiao, Y.; Liu, E.; Su, Z.; Meng, X.; Liu, B. Preparation of Ca-alginate-whey Protein Isolate Microcapsules for Protection and Delivery of L. bulgaricus and L. paracasei. Int. J. Biol. Macromol. 2020, 163, 1361–1368. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based Hydrogels as Drug Delivery Vehicles in Cancer Treatment and Their Applications in Wound Dressing and 3D Bioprinting. J. Biol. Eng. 2020, 14, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I.; Taylor, C. Chemical, Physical and Biological Properties of Alginates and Their Biomedical Implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Sun, X.; Liu, C.; Omer, A.M.; Yang, L.-Y.; Ouyang, X.-K. Dual-layered pH-sensitive Alginate/Chitosan/Kappa-carrageenan Microbeads for Colon-targeted Release of 5-fluorouracil. Int. J. Biol. Macromol. 2019, 132, 487–494. [Google Scholar] [CrossRef]
- Polk, A.; Amsden, B.; De Yao, K.; Peng, T.; Goosen, M. Controlled Release of Albumin from Chitosan-alginate Microcapsules. J. Pharm. Sci. 1994, 83, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Rafi, A.A.; Mahkam, M. Preparation of Magnetic pH-sensitive Microcapsules with an Alginate Base as Colon Specific Drug Delivery Systems Through an Entirely Green Route. RSC Adv. 2015, 5, 4628–4638. [Google Scholar] [CrossRef]
- Omer, A.M.; Tamer, T.; Hassan, M.; Rychter, P.; Eldin, M.M.; Koseva, N. Development of Amphoteric Alginate/Aminated Chitosan Coated Microbeads for Oral Protein Delivery. Int. J. Biol. Macromol. 2016, 92, 362–370. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, W.; Ke, Z.; Li, Y.; Zhou, Z. In Vitro Release and Antioxidant Activity of Satsuma Mandarin (Citrus Reticulata Blanco cv. Unshiu) Peel Flavonoids Encapsulated by Pectin Nanoparticles. Int. J. Food Sci. Technol. 2017, 52, 2362–2373. [Google Scholar]
- Mohy Eldin, M.S.; Omer, A.M.; Wassel, M.; Tamer, T.; Abd-Elmonem, M.; Ibrahim, S. Novel Smart pH Sensitive Chitosan Grafted Alginate Hydrogel Microcapsules for Oral Protein Delivery: II. Evaluation of the Swelling Behavior. Int. J. Pharm. Pharm. Sci. 2015, 7, 331–337. [Google Scholar]
- George, M.; Abraham, T.E. Polyionic Hydrocolloids for the Intestinal Delivery of Protein Drugs: Alginate and Chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef]
- Abreu, F.R.D.; Campana-Filho, S.P. Preparation and Characterization of Carboxymethylchitosan. Polímeros 2005, 15, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Siahaan, P.; Mentari, N.C.; Wiedyanto, U.O.; Hudiyanti, D.; Hildayani, S.Z.; Laksitorini, M.D. The Optimum Conditions of Carboxymethyl Chitosan Synthesis on Drug Delivery Application and Its Release of Kinetics Study. Indones. J. Chem. 2017, 17, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Omer, A.; Hu, Z.; Yang, L.-Y.; Ji, C.; Ouyang, X.-K. Fabrication of Magnetic Bentonite/Carboxymethyl Chitosan/Sodium Alginate Hydrogel Beads for Cu (II) Adsorption. Int. J. Biol. Macromol. 2019, 135, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl Cellulose-based Materials for Infection Control and Wound Healing: A Review. Int. J. Biol. Macromol. 2020, 146, 963–975. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Zhang, Z. Calcium-carboxymethyl Chitosan Hydrogel Beads for Protein Drug Delivery System. J. Appl. Polym. Sci. 2007, 103, 3164–3168. [Google Scholar] [CrossRef]
- Wang, L.C.; Chen, X.G.; Yu, L.J.; Li, P.W. Controlled Drug Release Through Carboxymethyl-chitosan/Poly (Vinyl Alcohol) Blend Films. Polym. Eng. Sci. 2007, 47, 1373–1379. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Liang, H.-F.; Chung, C.-K.; Chen, M.-C.; Sung, H.-W. Physically Crosslinked Alginate/N, O-carboxymethyl Chitosan Hydrogels with Calcium for Oral Delivery of Protein Drugs. Biomaterials 2005, 26, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.X.; Omer, A.; Hu, Z.-H.; Wang, Y.G.; Yu, D.; Ouyang, X.-K. Efficient Adsorption of Diclofenac Sodium from Aqueous Solutions Using Magnetic Amine-functionalized Chitosan. Chemosphere 2019, 217, 270–278. [Google Scholar] [CrossRef]
- Tamer, T.M.; Hassan, M.A.; Omer, A.M.; Valachová, K.; Eldin, M.S.M.; Collins, M.N.; Šoltés, L. Antibacterial and Antioxidative Activity of O-amine Functionalized Chitosan. Carbohydr. Polym. 2017, 169, 441–450. [Google Scholar] [CrossRef]
- Tamer, T.M.; Valachová, K.; Mohyeldin, M.S.; Soltes, L. Free Radical Scavenger Activity of Chitosan and Its Aminated Derivative. J. Appl. Pharm. Sci. 2016, 6, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Kenawy, E.; Abdel-Hay, F.; Tamer, T.; Ibrahim, E.A.-E.; Eldin, M.M. Antimicrobial Activity of Novel Modified Aminated Chitosan with Aromatic Esters. Polym. Bull. 2020, 77, 1631–1647. [Google Scholar] [CrossRef]
- Sun, X.; Yu, D.; Ying, Z.; Pan, C.; Wang, N.; Huang, F.; Ling, J.; Ouyang, X.-K. Fabrication of Ion-Crosslinking Aminochitosan Nanoparticles for Encapsulation and Slow Release of Curcumin. Pharmaceutics 2019, 11, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuvaraja, G.; Chen, D.-Y.; Pathak, J.L.; Long, J.; Subbaiah, M.V.; Wen, J.-C.; Pan, C.-L. Preparation of Novel Aminated Chitosan Schiff’s Base Derivative for the Removal of Methyl Orange Dye from Aqueous Environment and Its Biological Applications. Int. J. Biol. Macromol. 2020, 146, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, G.; Bouropoulos, N. Swelling Studies and In Vitro Release of Verapamil from Calcium Alginate and Calcium Alginate–chitosan Beads. Int. J. Pharm. 2006, 323, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Anitha, A.; Maya, S.; Deepa, N.; Chennazhi, K.; Nair, S.; Jayakumar, R. Curcumin-loaded N, O-carboxymethyl Chitosan Nanoparticles for Cancer Drug Delivery. J. Biomater. Sci. Polym. Ed. 2012, 23, 1381–1400. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.M.; Ziora, Z.M.; Tamer, T.M.; Khalifa, R.E.; Hassan, M.A.; Mohy-Eldin, M.S.; Blaskovich, M.A. Formulation of Quaternized Aminated Chitosan Nanoparticles for Efficient Encapsulation and Slow Release of Curcumin. Molecules 2021, 26, 449. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Wang, J.; Zhu, S.; Liu, S.; Yu, X.; Li, S. Synthesis and Characterization of Novel Carboxymethyl Chitosan Grafted Polylactide Hydrogels for Controlled Drug delivery. Polym. Adv. Technol. 2015, 26, 924–931. [Google Scholar] [CrossRef]
- Muzzarelli, C.; Tosi, G.; Francescangeli, O.; Muzzarelli, R.A. Alkaline Chitosan Solutions. Carbohydr. Res. 2003, 338, 2247–2255. [Google Scholar] [CrossRef]
- Abruzzo, A.; Bigucci, F.; Cerchiara, T.; Saladini, B.; Gallucci, M.; Cruciani, F.; Vitali, B.; Luppi, B. Chitosan/Alginate Complexes for Vaginal Delivery of Chlorhexidine Digluconate. Carbohydr. Polym. 2013, 91, 651–658. [Google Scholar] [CrossRef]
- Tamer, T.M.; Omer, A.M.; Hassan, M.A.; Hassan, M.E.; Sabet, M.M.; Eldin, M.M. Development of Thermo-sensitive Poly N-isopropyl Acrylamide Grafted Chitosan Derivatives. J. Appl. Pharm. Sci. 2015, 5, 1–6. [Google Scholar]
- Patel, N.; Lalwani, D.; Gollmer, S.; Injeti, E.; Sari, Y.; Nesamony, J. Development and Evaluation of a Calcium Alginate Based Oral Ceftriaxone Sodium Formulation. Prog. Biomater. 2016, 5, 117–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohy Eldin, M.S.; Omer, A.M.; Soliman, E.A.; Hassan, E.A. Superabsorbent Polyacrylamide Grafted Carboxymethyl Cellulose pH Sensitive Hydrogel: I. Preparation and Characterization. Desalination Water Treat. 2013, 51, 3196–3206. [Google Scholar] [CrossRef]
- Mohy Eldin, M.; Soliman, E.; Hashem, A.; Tamer, T. Antimicrobial Activity of Novel Aminated Chitosan Derivatives for Biomedical Applications. Adv. Polym. Technol. 2012, 31, 414–428. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Cornejo, D.R.; Petri, D.F.S. Alginate/Magnetite Hybrid Beads for Magnetically Stimulated Release of Dopamine. Colloids Surf. B Biointerfaces 2016, 138, 94–101. [Google Scholar] [CrossRef]
- Smrdel, P.; Bogataj, M.; Mrhar, A. The Influence of Selected Parameters on the Size and Shape of Alginate Beads Prepared by Ionotropic Gelation. Sci. Pharm. 2008, 76, 77–90. [Google Scholar] [CrossRef]
- Čalija, B.; Cekić, N.; Savić, S.; Krajišnik, D.; Daniels, R.; Milić, J. An Investigation of Formulation Factors Affecting Feasibility of Alginate-chitosan Microparticles for Oral delivery of Naproxen. Arch. Pharmacal. Res. 2011, 34, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Maity, S.; Chakraborty, S.; Rudra, R.; Ghodadara, H.; Solanki, M.; Chakraborti, A.S.; Prajapati, A.; Kundu, P. Oral Delivery of Quercetin to Diabetic Animals Using Novel pH Responsive Carboxypropionylated Chitosan/Alginate microparticles. RSC Adv. 2016, 6, 73210–73221. [Google Scholar] [CrossRef]
- Ganji, F.; Vasheghani, F.S.; Vasheghani, F.E. Theoretical Description of Hydrogel Swelling: A Review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Mohy Eldin, M.S.; Omer, A.M.; Wassel, M.; Tamer, T.; Abd-Elmonem, M.; Ibrahim, S. Novel Smart pH Sensitive Chitosan Grafted Alginate Hydrogel Microcapsules for Oral Protein Delivery: I. Preparation and Characterization. Int. J. Pharm. Pharm. Sci. 2015, 7, 320–326. [Google Scholar]
- Szabó, L.; Gerber-Lemaire, S.; Wandrey, C. Strategies to Functionalize the Anionic Biopolymer Na-Alginate without Restricting Its Polyelectrolyte Properties. Polymers 2020, 12, 919. [Google Scholar] [CrossRef] [Green Version]
- Amin, L.; Jesmeen, T.; Bishwajit Sutradhar, K.; Mannan, A. Development and In Vitro Evaluation of Diclofenac Sodium Loaded Mucoadhesive Microsphere with Natural Gum for Sustained Delivery. Curr. Drug Deliv. 2013, 10, 765–770. [Google Scholar] [CrossRef]
- Manjunatha, K.; Ramana, M.; Satyanarayana, D. Design and Evaluation of Diclofenac Sodium Controlled Drug Delivery Systems. Indian J. Pharm. Sci. 2007, 69, 384. [Google Scholar] [CrossRef] [Green Version]
- Trevisol, T.C.; Scartazzini, L.; Valerio, A.; Guelli Ulson de Souza, S.M.A.; Bierhalz, A.C.K.; Valle, J.A.B. Diclofenac Release from Alginate/Carboxymethyl Cellulose Mono and Bilayer Films for Wound Dressing Applications. Cellulose 2020, 27, 6629–6642. [Google Scholar] [CrossRef]
- Rasel, M.A.T.; Hasan, M. Formulation and Evaluation of Floating Alginate Beads of Diclofenac Sodium. Dhaka Univ. J. Pharm. Sci. 2012, 11, 29–35. [Google Scholar] [CrossRef]
- Manjanna, K.; Shivakumar, B.; Pramod Kumar, T. Diclofenac Sodium Microbeads for Oral Sustained Drug Delivery. Int. J. Pharmtech Res. 2009, 1, 317–327. [Google Scholar]
- Taher, M.; Omer, A.M.M.; Hamed, A.; Ali, A.; Tamer, T.; Mohy Eldin, M.S. Development of Smart Alginate/Chitosan Grafted Microcapsules for Colon Site-specific Drug Delivery. Egypt. J. Chem. 2019, 62, 1037–1045. [Google Scholar]
- Sinha, P.; Ubaidulla, U.; Hasnain, M.S.; Nayak, A.K.; Rama, B. Alginate-okra Gum Blend Beads of Diclofenac Sodium from Aqueous Template Using ZnSO4 as a Cross-linker. Int. J. Biol. Macromol. 2015, 79, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.S.; Ahmad, M.; Minhas, M.U.; Tulain, R.; Barkat, K.; Khalid, I.; Khalid, Q. Chitosan/Xanthan Gum Based Hydrogels as Potential Carrier for an Antiviral Drug: Fabrication, Characterization, and Safety Evaluation. Front. Chem. 2020, 8, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.-S.; Liu, S.-Q.; Ng, S.Y.; Froix, M.; Ohno, T.; Heller, J. Controlled Release of Interleukin-2 for Tumour Immunotherapy Using Alginate/Chitosan Porous Microspheres. J. Control. Release 1997, 43, 65–74. [Google Scholar] [CrossRef]
- Bajpai, S.; Tankhiwale, R. Investigation of Water Uptake Behavior and Stability of Calcium Alginate/chitosan Bi-polymeric Beads: Part-1. React. Funct. Polym. 2006, 66, 645–658. [Google Scholar] [CrossRef]
- Sankalia, M.G.; Mashru, R.C.; Sankalia, J.M.; Sutariya, V.B. Reversed Chitosan–alginate Polyelectrolyte Complex for Stability Improvement of Alpha-amylase: Optimization and Physicochemical Characterization. Eur. J. Pharm. Biopharm. 2007, 65, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Lončarević, A.; Ivanković, M.; Rogina, A. Lysozyme-induced Degradation of Chitosan: The Characterisation of Degraded Chitosan Scaffolds. J. Tissue Repair Regen. 2017, 1, 12. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.M.; Song, D.K.; Oh, S.H.; Lee-Yoon, D.S.; Bae, E.H.; Lee, J.H. In Vitro and In Vivo Degradation Behavior of Acetylated Chitosan Porous Beads. J. Biomater. Sci. Polym. Ed. 2008, 19, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.; Omer, A.M.; Tamer, T.; Elmeligy, M.; Mohy Eldin, M.S. Fabrication of Biodegradable Gelatin/Chitosan/Cinnamaldehyde Crosslinked Membranes for Antibacterial Wound Dressing Applications. Int. J. Biol. Macromol. 2019, 139, 440–448. [Google Scholar] [CrossRef] [PubMed]







| Samples | Compositions | Diameter (mm) | |
|---|---|---|---|
| Wet Microcapsules | Dried Microcapsules | ||
| S0 | Alg-CMCs (2:0) | 3891 ± 0.005 a | 0.562 ± 0.090 |
| S1 | Alg-CMCs (1.5:0.5) | 3821 ± 0.012 | 0.522 ± 0.041 |
| S2 | Alg-CMCs (1:1) | 3684 ± 0.020 | 0.5421 ± 0.082 |
| S3 | Alg-CMCs (0.5:1.5) | 3735 ± 0.070 | 0.515 ± 0.120 |
| S4 | Alg-CMCs (1:1)@ 0.25% AmCs | 3133 ± 0.081 | 0.498 ± 0.061 |
| S5 | Alg-CMCs (1:1)@ 0.5% AmCs | 2915 ± 0.050 | 0.491 ± 0.020 |
| S6 | Alg-CMCs (1:1)@ 1%AmCs | 2711 ± 0.010 | 0.484 ± 0.071 |
| Sample | Weight Loss (%) | T50%°C | |
|---|---|---|---|
| 25–120 °C | 220–350 °C | ||
| CMCs | 9.51 | 29.11 | 335.61 |
| AmCs | 11.12 | 22.27 | 346.69 |
| Alg (S0) | 11.7 | 24.222 | 365.39 |
| S2 | 12.38 | 26.240 | 377.90 |
| S4 | 13.67 | 27.576 | 384.55 |
| S5 | 13.87 | 27.509 | 383.09 |
| Drug Delivery System | DS-Loading Efficiency (%) | Ref. |
|---|---|---|
| Alginate microspheres | 49.97 | [54] |
| Sodium alginate beads | 59.88–74.44 | [55] |
| Alginate/carboxymethyl cellulose mono and bilayer films | 57.5–77.3 | [56] |
| Floating Alginate Beads | 75–88.9 | [57] |
| Sodium alginate-Pectin microbeads | 70.40–88.20 | [58] |
| Alginate grafted chitosan microcapsules | 89.0 | [59] |
| Alginate-okra gum blend beads | 89.27 | [60] |
| Alg-CMCs@AmCs microcapsules | 95.45 | this study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omer, A.M.; Ahmed, M.S.; El-Subruiti, G.M.; Khalifa, R.E.; Eltaweil, A.S. pH-Sensitive Alginate/Carboxymethyl Chitosan/Aminated Chitosan Microcapsules for Efficient Encapsulation and Delivery of Diclofenac Sodium. Pharmaceutics 2021, 13, 338. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13030338
Omer AM, Ahmed MS, El-Subruiti GM, Khalifa RE, Eltaweil AS. pH-Sensitive Alginate/Carboxymethyl Chitosan/Aminated Chitosan Microcapsules for Efficient Encapsulation and Delivery of Diclofenac Sodium. Pharmaceutics. 2021; 13(3):338. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13030338
Chicago/Turabian StyleOmer, Ahmed M., Maha S. Ahmed, Gehan M. El-Subruiti, Randa E. Khalifa, and Abdelazeem S. Eltaweil. 2021. "pH-Sensitive Alginate/Carboxymethyl Chitosan/Aminated Chitosan Microcapsules for Efficient Encapsulation and Delivery of Diclofenac Sodium" Pharmaceutics 13, no. 3: 338. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13030338