Dermatillomania: Strategies for Developing Protective Biomaterials/Cloth
Abstract
:1. Introduction
2. Methods
3. Results
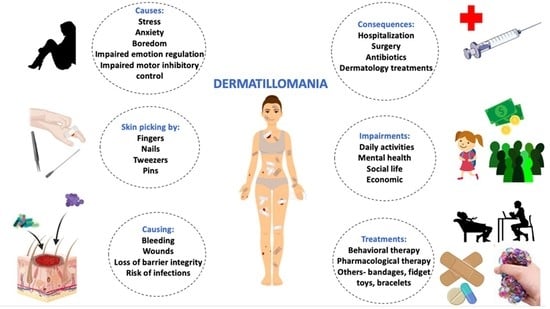
3.1. Dermatillomania as a Psychodermatologic Disorder
3.2. Prevalence
3.3. Etiology
3.4. Consequences
3.5. Diagnostic Criteria
3.6. Treatment Strategies
3.6.1. Behavioral Strategies
3.6.2. Pharmacological Strategies
3.6.3. Alternative Pharmacological Strategies
3.6.4. Other Strategies
3.7. Presenting the Need for an Alternative Treatment Strategy
3.8. Proposing Biomaterial-Based Physical Treatment Strategies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ko, S.M. Under-diagnosed psychiatric syndrome. II: Pathologic skin picking. Ann. Acad. Med. Singap. 1999, 28, 557–559. [Google Scholar]
- Weintraub, E.; Robinson, C.; Newmeyer, M. Catastrophic medical complication in psychogenic excoriation. South. Med. J. 2000, 93, 1099–1101. [Google Scholar] [CrossRef] [PubMed]
- Keuthen, N.J.; Deckersbach, T.; Wilhelm, S.; Hale, E.; Fraim, C.; Baer, L.; O’Sullivan, R.L.; Jenike, M.A. Repetitive skin-picking in a student population and comparison with a sample of self-injurious skin-pickers. Psychosomatics 2000, 41, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Odlaug, B.L.; Kim, S.W.; Grant, J.E. Quality of life and clinical severity in pathological skin picking and trichotillomania. J. Anxiety Disord. 2010, 24, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Snorrason, Í.; Smári, J.; Ólafsson, R.P. Motor inhibition, reflection impulsivity, and trait impulsivity in pathological skin picking. Behav Ther. 2011, 42, 521–532. [Google Scholar] [CrossRef]
- Ricketts, E.J.; Snorrason, Í.; Kircanski, K.; Alexander, J.R.; Thamrin, H.; Flessner, C.A.; Franklin, M.E.; Piacentini, J.; Woods, D.W. A latent profile analysis of age of onset in pathological skin picking. Compr. Psychiatry 2018, 87, 46–52. [Google Scholar] [CrossRef]
- Neziroglu, F.; Rabinowitz, D.; Breytman, A.; Jacofsky, M. Skin picking phenomenology and severity comparison, Prim Care Companion. J. Clin. Psychiatry 2008, 10, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Odlaug, B.L.; Chamberlain, S.R.; Grant, J.E. Motor inhibition and cognitive flexibility in pathologic skin picking. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.A.; Dufresne, R.G. Body dysmorphic disorder. A guide for dermatologists and cosmetic surgeons. Am. J. Clin. Dermatol. 2000, 1, 235–243. [Google Scholar] [CrossRef]
- Tucker, B.T.P.; Woods, D.W.; Flessner, C.A.; Franklin, S.A.; Franklin, M.E. The Skin Picking Impact Project: Phenomenology, interference, and treatment utilization of pathological skin picking in a population-based sample. J. Anxiety Disord. 2011, 25, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.L.; Storch, E.A.; Berlanga, L. Skin picking behaviors: An examination of the prevalence and severity in a community sample. J Anxiety Disord. 2009, 23, 314–319. [Google Scholar] [CrossRef] [Green Version]
- Campeotto, F.; Naudin, C.; Viot, G.; Dupont, C. Rectal self-mutilation, rectal bleeding and Prader-Willi syndrome. Arch. Pediatr. 2001, 8, 1075–1077. [Google Scholar] [CrossRef]
- Pozza, A.; Giaquinta, N.; Dèttore, D. Borderline, avoidant, sadistic personality traits and emotion dysregulation predict different pathological skin picking subtypes in a community sample. Neuropsychiatr. Dis. Treat. 2016, 12, 1861–1867. [Google Scholar] [CrossRef] [Green Version]
- Grant, J.E.; Christenson, G.A. Examination of gender in pathologic grooming behaviors. Psychiatr. Q. 2007, 78, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Turner, G.A.; Sutton, S.; Sharma, A. Augmentation of Venlafaxine with Aripiprazole in a Case of Treatment-resistant Excoriation Disorder. Innov. Clin. Neurosci. 2014, 11, 29–31. [Google Scholar] [PubMed]
- Calikuşu, C.; Yücel, B.; Polat, A.; Baykal, C. The relation of psychogenic excoriation with psychiatric disorders: A comparative study. Compr. Psychiatry 2003, 44, 256–261. [Google Scholar] [CrossRef]
- Yalçin, M.; Tellioğlu, E.; Yildirim, D.U.; Savrun, B.M.; Özmen, M.; Aydemir, E.H. Psychiatric Features in Neurotic Excoriation Patients: The Role of Childhood Trauma. Noro Psikiyatr. Ars. 2015, 52, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lochner, C.; Simeon, D.; Niehaus, D.J.; Stein, D.J. Trichotillomania and skin-picking: A phenomenological comparison. Depress. Anxiety 2002, 15, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, M.F.; Iwata, B.A.; Reid, D.H.; Davis, P.A. Protective equipment: Continuous and contingent application in the treatment of self-injurious behavior. J. Appl. Behav. Anal. 1982, 15, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Antoniadis, D.; Floros, G.D.; Nikolaidis, N.; Garyfallos, G. Response to agomelatine: Treatment of an obsessive skin picking episode. Ann. Clin. Psychiatry 2013, 25, 228–229. [Google Scholar] [PubMed]
- Dykens, E.; Shah, B. Psychiatric disorders in Prader-Willi syndrome: Epidemiology and management. CNS Drugs 2003, 17, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Muscatelli, F.; Abrous, D.N.; Massacrier, A.; Boccaccio, I.; Le Moal, M.; Cau, P.; Cremer, H. Disruption of the mouse Necdin gene results in hypothalamic and behavioral alterations reminiscent of the human Prader-Willi syndrome. Hum. Mol. Genet. 2000, 9, 3101–3110. [Google Scholar] [CrossRef] [Green Version]
- Symons, F.J.; Butler, M.G.; Sanders, M.D.; Feurer, I.D.; Thompson, T. Self-injurious behavior and Prader-Willi syndrome: Behavioral forms and body locations. Am. J. Ment. Retard. 1999, 104, 260–269. [Google Scholar] [CrossRef]
- Morgan, J.R.; Storch, E.A.; Woods, D.W.; Bodzin, D.; Lewin, A.B.; Murphy, T.K. A preliminary analysis of the phenomenology of skin-picking in Prader-Willi syndrome. Child Psychiatry Hum. Dev. 2010, 41, 448–463. [Google Scholar] [CrossRef] [PubMed]
- Osaba, O.; Mahr, G. Psychogenic excoriation and cancer. Psychosomatics 2002, 43, 251–252. [Google Scholar] [CrossRef]
- Wilhelm, S.; Keuthen, N.J.; Deckersbach, T.; Engelhard, I.M.; Forker, A.E.; Baer, L.; O’Sullivan, R.L.; Jenike, M.A. Self-injurious skin picking: Clinical characteristics and comorbidity. J. Clin. Psychiatry 1999, 60, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Odlaug, B.L.; Lust, K.; Schreiber, L.R.N.; Christenson, G.; Derbyshire, K.; Grant, J.E. Skin picking disorder in university students: Health correlates and gender differences. Gen. Hosp. Psychiatry 2013, 35, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Grant, J.E.; Leppink, E.W.; Tsai, J.; Chamberlain, S.R.; Redden, S.A.; Curley, E.E.; Odlaug, B.L.; Keuthen, N.J. Does comorbidity matter in body-focused repetitive behavior disorders. Ann. Clin. Psychiatry 2016, 28, 175–181. [Google Scholar]
- Mutasim, D.F.; Adams, B.B. The psychiatric profile of patients with psychogenic excoriation. J. Am. Acad. Dermatol. 2009, 61, 611–613. [Google Scholar] [CrossRef]
- Yadav, S.; Narang, T.; Kumaran, M.S. Psychodermatology: A comprehensive review. Indian J. Dermatol. Venereol. Leprol. 2013, 79, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Keuthen, N.J.; Koran, L.M.; Aboujaoude, E.; Large, M.D.; Serpe, R.T. The prevalence of pathologic skin picking in US adults. Compr. Psychiatry 2010, 51, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Deckersbach, T.; Wilhelm, S.; Keuthen, N.J.; Baer, L.; Jenike, M.A. Cognitive-behavior therapy for self-injurious skin picking. A case series. Behav. Modif. 2002, 26, 361–377. [Google Scholar] [CrossRef]
- Yeh, A.H.; Taylor, S.; Thordarson, D.S.; Corcoran, K.M. Efficacy of telephone-administered cognitive behaviour therapy for obsessive-compulsive spectrum disorders: Case studies. Cogn. Behav. Ther. 2003, 32, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.C.; Sharma, N.L. Effectiveness of fluoxetine in the treatment of skin-picking. Indian J. Psychiatry 2005, 47, 241–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiegel, D.R.; Finklea, L. The recognition and treatment of pathological skin picking: A potential neurobiological underpinning of the efficacy of pharmacotherapy in impulse control disorders. Psychiatry (Edgmont) 2009, 6, 38–42. [Google Scholar]
- Warnock, J.K.; Kestenbaum, T. Pharmacologic treatment of severe skin-picking behaviors in Prader-Willi syndrome. Two case reports. Arch. Dermatol. 1992, 128, 1623–1625. [Google Scholar] [CrossRef]
- Prochwicz, K.; Kałużna-Wielobób, A.; Kłosowska, J. Skin picking in a non-clinical sample of young Polish adults. Prevalence and characteristics. Compr. Psychiatry 2016, 71, 77–85. [Google Scholar] [CrossRef]
- Gupta, M.A.; Gupta, A.K.; Haberman, H.F. Neurotic excoriations: A review and some new perspectives. Compr. Psychiatry 1986, 27, 381–386. [Google Scholar] [CrossRef] [Green Version]
- Ehsani, A.H.; Toosi, S.; Shahshahani, M.M.; Arbabi, M.; Noormohammadpour, P. Psycho-cutaneous disorders: An epidemiologic study. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.O.; Köhler, C.A.; Stubbs, B.; Nunes-Neto, P.R.; Koyanagi, A.; Quevedo, J.; Soares, J.C.; Hyphantis, T.N.; Marazziti, D.; Maes, M.; et al. Skin picking disorder: Prevalence, correlates, and associations with quality of life in a large sample. CNS Spectr. 2018, 23, 311–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibovici, V.; Koran, L.M.; Murad, S.; Siam, I.; Odlaug, B.L.; Mandelkorn, U.; Feldman-Weisz, V.; Keuthen, N.J. Excoriation (skin-picking) disorder in adults: A cross-cultural survey of Israeli Jewish and Arab samples. Compr. Psychiatry 2015, 58, 102–107. [Google Scholar] [CrossRef]
- Solley, K.; Turner, C. Prevalence and correlates of clinically significant body-focused repetitive behaviors in a non-clinical sample. Compr. Psychiatry 2018, 86, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calikusu, C.; Kucukgoncu, S.; Tecer, Ö.; Bestepe, E. Skin picking in Turkish students: Prevalence, characteristics, and gender differences. Behav. Modif. 2012, 36, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Bohne, A.; Wilhelm, S.; Keuthen, N.J.; Baer, L.; Jenike, M.A. Skin picking in German students. Prevalence, phenomenology, and associated characteristics. Behav. Modif. 2002, 26, 320–339. [Google Scholar] [CrossRef]
- Yeo, S.K.; Lee, W.K. The relationship between adolescents’ academic stress, impulsivity, anxiety, and skin picking behavior. Asian J. Psychiatr. 2017, 28, 111–114. [Google Scholar] [CrossRef]
- Siddiqui, E.U.; Naeem, S.S.; Naqvi, H.; Ahmed, B. Prevalence of body-focused repetitive behaviors in three large medical colleges of Karachi: A cross-sectional study. BMC Res. Notes 2012, 5, 614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, C.; Lipner, S.R. Insights into recurrent body-focused repetitive behaviors: Evidenced by New York Times commenters. Arch. Dermatol. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Anzengruber, F.; Ruhwinkel, K.; Ghosh, A.; Klaghofer, R.; Lang, U.E.; Navarini, A.A. Wide range of age of onset and low referral rates to psychiatry in a large cohort of acne excoriée at a Swiss tertiary hospital. J. Dermatolog. Treat. 2018, 29, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.Y.; Smith, L.L. Obsessive-compulsive disorders in the pediatric dermatology practice. Pediatr. Dermatol. 1991, 8, 107–113. [Google Scholar] [CrossRef]
- Roberts, S.; O’Connor, K.; Aardema, F.; Bélanger, C. The impact of emotions on body-Focused repetitive behaviors: Evidence from a non-treatment-seeking sample. J. Behav. Ther. Exp. Psychiatry 2015, 46, 189–197. [Google Scholar] [CrossRef]
- Snorrason, I.; Ricketts, E.J.; Flessner, C.A.; Franklin, M.E.; Stein, D.J.; Woods, D.W. Skin picking disorder is associated with other body-focused repetitive behaviors: Findings from an internet study. Ann. Clin. Psychiatry 2012, 24, 292–299. [Google Scholar]
- Odlaug, B.L.; Grant, J.E. Childhood-onset pathologic skin picking: Clinical characteristics and psychiatric comorbidity. Compr. Psychiatry 2007, 48, 388–393. [Google Scholar] [CrossRef]
- Bienvenu, O.J.; Wang, Y.; Shugart, Y.Y.; Welch, J.M.; Grados, M.A.; Fyer, A.J.; Rauch, S.L.; McCracken, J.T.; Rasmussen, S.A.; Murphy, D.L.; et al. Sapap3 and pathological grooming in humans: Results from the OCD collaborative genetics study. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009, 150, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Monzani, B.; Rijsdijk, F.; Cherkas, L.; Harris, J.; Keuthen, N.; Mataix-Cols, D. Prevalence and heritability of skin picking in an adult community sample: A twin study. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012, 159, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.; Buot-Smith, T. Naltrexone and fluoxetine in Prader-Willi syndrome. J. Am. Acad. Child Adolesc. Psychiatry 1993, 32, 870–873. [Google Scholar] [CrossRef]
- Blanch, J.; Grimalt, F.; Massana, G.; Navarro, V. Efficacy of olanzapine in the treatment of psychogenic excoriation. Br. J. Dermatol. 2004, 151, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.E.; Odlaug, B.L.; Hampshire, A.; Schreiber, L.R.N.; Chamberlain, S.R. White matter abnormalities in skin picking disorder: A diffusion tensor imaging study. Neuropsychopharmacology 2013, 38, 763–769. [Google Scholar] [CrossRef] [Green Version]
- Roos, A.; Grant, J.E.; Fouche, J.-P.; Stein, D.J.; Lochner, C. A comparison of brain volume and cortical thickness in excoriation (skin picking) disorder and trichotillomania (hair pulling disorder) in women. Behav. Brain Res. 2015, 279, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Schienle, A.; Potthoff, J.; Wabnegger, A. Voxel-based morphometry analysis of structural brain scans in skin-picking disorder. Compr. Psychiatry 2018, 84, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Harries, M.D.; Chamberlain, S.R.; Redden, S.A.; Odlaug, B.L.; Blum, A.W.; Grant, J.E. A structural MRI study of excoriation (skin-picking) disorder and its relationship to clinical severity. Psychiatry Res. Neuroimaging 2017, 269, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Wabnegger, A.; Schienle, A. The Role of the Cerebellum in Skin-Picking Disorder. Cerebellum 2019, 18, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Sneddon, J.; Sneddon, I. Acne excoriée: A protective device. Clin. Exp. Dermatol. 1983, 8, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Kent, A.; Drummond, L.M. Acne excoriée—A case report of treatment using habit reversal. Clin. Exp. Dermatol. 1989, 14, 163–164. [Google Scholar] [CrossRef]
- Schepis, B.; Failla, P.; Siragusa, M.; Romano, C. Skin-picking: The best cutaneous feature in the recognization of Prader-Willi syndrome. Int. J. Dermatol. 1994, 33, 866–867. [Google Scholar] [CrossRef]
- Phillips, K.A.; Taub, S.L. Skin picking as a symptom of body dysmorphic disorder. Psychopharmacol. Bull. 1995, 31, 279–288. [Google Scholar] [PubMed]
- O’Sullivan, R.L.; Phillips, K.A.; Keuthen, N.J.; Wilhelm, S. Near-fatal skin picking from delusional body dysmorphic disorder responsive to fluvoxamine. Psychosomatics 1999, 40, 79–81. [Google Scholar] [CrossRef]
- Banga, A.; Connor, D.F. Effectiveness of naltrexone for treating pathologic skin picking behavior in an adolescent with Prader-Willi syndrome. J. Child Adolesc. Psychopharmacol. 2012, 22, 396–398. [Google Scholar] [CrossRef]
- Bain, M.A.; Vincent, J. Management of a Complex Excoriation Disorder-induced Wound with a Viable Cryopreserved Placental Membrane. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1132. [Google Scholar] [CrossRef]
- Hawatmeh, A.; Al-Khateeb, A. An unusual complication of dermatillomania. Quant. Imaging Med. Surg. 2017, 7, 166–168. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.I.; Garrison, R.C.; Thompson, G. A near fatal case of pathological skin picking. Am. J. Case Rep. 2013, 14, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Galdyn, I.A.; Chidester, J.; Martin, M.C. The reconstructive challenges and approach to patients with excoriation disorder. J. Craniofac. Surg. 2015, 26, 824–825. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.A.; Putnam, P.E.; Kocoshis, S.A.; Rowe, M.; Hanchett, J.M. Rectal bleeding in Prader-Willi syndrome. Pediatrics 1996, 97, 265–267. [Google Scholar] [PubMed]
- Alexandrov, P.; Tan, W.P.; Elterman, L. Genital Dermatillomania. Curr. Urol. 2017, 11, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Al Assad, W.; Marinos, A. An unusual aetiology of back pain. BMJ Case Rep. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Schlessinger, D.I.; Gray, J.; Speiser, J.; Lake, E. Ulcerated forehead nodule in an intravenous heroin user. JAAD Case Rep. 2019, 5, 63–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culver, A.L.; Hernandez, A.; Paek, S.Y. Neurosis and true dermatosis: A case of ossified pilomatricoma developing within a self-inflicted ulcer. Dermatol. Online J. 2019, 25, 11. [Google Scholar]
- Keuthen, N.J.; Deckersbach, T.; Wilhelm, S.; Engelhard, I.; Forker, A.; O’Sullivan, R.L.; Jenike, M.A.; Baer, L. The Skin Picking Impact Scale (SPIS): Scale development and psychometric analyses. Psychosomatics 2001, 42, 397–403. [Google Scholar] [CrossRef]
- Grant, J.E.; Redden, S.A.; Leppink, E.W.; Odlaug, B.L.; Chamberlain, S.R. Psychosocial dysfunction associated with skin picking disorder and trichotillomania. Psychiatry Res. 2016, 239, 68–71. [Google Scholar] [CrossRef] [Green Version]
- Flessner, C.A.; Woods, D.W. Phenomenological characteristics, social problems, and the economic impact associated with chronic skin picking. Behav. Modif. 2006, 30, 944–963. [Google Scholar] [CrossRef]
- Toro-Martínez, E. DSM-5: OCD and related disorders. Vertex 2014, 25, 63–67. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Twohig, M.P.; Woods, D.W. Habit reversal as a treatment for chronic skin picking in typically developing adult male siblings. J. Appl. Behav. Anal. 2001, 34, 217–220. [Google Scholar] [CrossRef] [Green Version]
- Nirmal, C.; Shenoi, S.D.; Rai, S.; Sreejayan, K.; Savitha, S. “Look beyond skin”: Psychogenic excoriation—A series of five cases. Indian J Dermatol. 2013, 58, 246. [Google Scholar] [CrossRef]
- Percinel, I.; Yazici, K.U. Glutamatergic dysfunction in skin-picking disorder: Treatment of a pediatric patient with N-acetylcysteine. J. Clin. Psychopharmacol. 2014, 34, 772–774. [Google Scholar] [CrossRef]
- Shenefelt, P.D. Biofeedback, cognitive-behavioral methods, and hypnosis in dermatology: Is it all in your mind? Dermatol. Ther. 2003, 16, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Schuck, K.; Keijsers, G.P.J.; Rinck, M. The effects of brief cognitive-behaviour therapy for pathological skin picking: A randomized comparison to wait-list control. Behav. Res. Ther. 2011, 49, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Jafferany, M.; Shireen, F.; Ibrahim, A. An open-label trial of topiramate in the treatment of skin picking in pervasive developmental disorder not otherwise specified. Prim. Care Companion J. Clin. Psychiatry 2010, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twohig, M.P.; Hayes, S.C.; Masuda, A. A preliminary investigation of acceptance and commitment therapy as a treatment for chronic skin picking. Behav. Res. Ther. 2006, 44, 1513–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruensgaard, K. Psychotherapeutic strategy and neurotic excoriations. Int. J. Dermatol. 1991, 30, 198–203. [Google Scholar] [CrossRef]
- Nakell, S. A healing herd: Benefits of a psychodynamic group approach in treating body-focused repetitive behaviors. Int. J. Group Psychother. 2015, 65, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Shenefelt, P.D. Using hypnosis to facilitate resolution of psychogenic excoriations in acne excoriée. Am. J. Clin. Hypn. 2004, 46, 239–245. [Google Scholar] [CrossRef]
- O’Connor, K.; Lavoie, M.; Desaulniers, B.; Audet, J.-S. Cognitive psychophysiological treatment of bodily-focused repetitive behaviors in adults: An open trial. J. Clin. Psychol. 2018, 74, 273–285. [Google Scholar] [CrossRef]
- Clay, C.J.; Clohisy, A.M.; Ball, A.M.; Haider, A.F.; Schmitz, B.A.; Kahng, S. Further Evaluation of Presentation Format of Competing Stimuli for Treatment of Automatically Maintained Challenging Behavior. Behav. Modif. 2018, 42, 382–397. [Google Scholar] [CrossRef] [PubMed]
- Prochwicz, K.; Kłosowska, J.; Kałużna-Wielobób, A. The relationship between emotion regulation strategies, personality traits and skin picking behaviours in a non-clinical sample of Polish adults. Psychiatry Res. 2018, 264, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Shenefelt, P.D. Mindfulness-Based Cognitive Hypnotherapy and Skin Disorders. Am. J. Clin. Hypn. 2018, 61, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Keuthen, N.; Greenberg, E. Assessment and treatment of trichotillomania (hair pulling disorder) and excoriation (skin picking) disorder. Clin. Dermatol. 2018, 36, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Tiger, J.H.; Fisher, W.W.; Bouxsein, K.J. Therapist- and self-monitored DRO contingencies as a treatment for the self-injurious skin picking of a young man with Asperger syndrome. J. Appl. Behav. Anal. 2009, 42, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Tomas-Aragones, L.; Consoli, S.M.; Consoli, S.G.; Poot, F.; Taube, K.-M.; Linder, M.D.; Jemec, G.B.E.; Szepietowski, J.C.; de Korte, J.; Lvov, A.N.; et al. Self-Inflicted Lesions in Dermatology: A Management and Therapeutic Approach—A Position Paper from the European Society for Derma-tology and Psychiatry. Acta Derm. Venereol. 2017, 97, 159–172. [Google Scholar] [CrossRef] [Green Version]
- Arnold, L.M. Phenomenology and therapeutic options for dermatotillomania. Expert Rev. Neurother. 2002, 2, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Sasso, D.A.; Kalanithi, P.S.A.; Trueblood, K.V.; Pittenger, C.; Kelmendi, B.; Wayslink, S.; Malison, R.T.; Krystal, J.H.; Coric, V. Beneficial effects of the glutamate-modulating agent riluzole on disordered eating and pathological skin-picking behaviors. J. Clin. Psychopharmacol. 2006, 26, 685–687. [Google Scholar] [CrossRef]
- Odlaug, B.L.; Grant, J.E. N-acetyl cysteine in the treatment of grooming disorders. J. Clin. Psychopharmacol. 2007, 27, 227–229. [Google Scholar] [CrossRef]
- Gupta, M.A. Emotional regulation, dissociation, and the self-induced dermatoses: Clinical features and implications for treatment with mood stabilizers. Clin. Dermatol. 2013, 31, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Angulo, M. An open-label pilot study of N-acetylcysteine for skin-picking in Prader-Willi syndrome. Am. J. Med. Genet. A 2014, 164, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.E.; Chamberlain, S.R.; Redden, S.A.; Leppink, E.W.; Odlaug, B.L.; Kim, S.W. N-Acetylcysteine in the Treatment of Excoriation Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2016, 73, 490–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, J.E.; Odlaug, B.L.; Chamberlain, S.R.; Keuthen, N.J.; Lochner, C.; Stein, D.J. Skin picking disorder. Am. J. Psychiatry 2012, 169, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Silva-Netto, R.; Jesus, G.; Nogueira, M.; Tavares, H. N-acetylcysteine in the treatment of skin-picking disorder. Braz. J. Psychiatry 2014, 36, 101. [Google Scholar] [CrossRef] [Green Version]
- Kiliç, F.; Keleş, S. Repetitive Behaviors Treated with N-Acetylcysteine: Case Series. Clin. Neuropharmacol. 2019, 42, 139–141. [Google Scholar] [CrossRef] [PubMed]
- George, N.M.; Whitaker, J.; Vieira, G.; Geronimo, J.T.; Bellinger, D.A.; Fletcher, C.A.; Garner, J.P. Antioxidant Therapies for Ulcerative Dermatitis: A Potential Model for Skin Picking Disorder. PLoS ONE 2015, 10, e0132092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallinat, D.; Moessner, M.; Haenssle, H.A.; Winkler, J.K.; Backenstrass, M.; Bauer, S. SaveMySkin: An Internet-based self-help intervention for skin picking. Study protocol for a randomized pilot study. Contemp. Clin. Trials Commun. 2019, 13, 100315. [Google Scholar] [CrossRef]
- Gallinat, C.; Moessner, M.; Haenssle, H.A.; Winkler, J.K.; Backenstrass, M.; Bauer, S. An Internet-Based Self-Help Intervention for Skin Picking (SaveMySkin): Pilot Randomized Controlled Trial. J. Med. Internet Res. 2019, 21, e15011. [Google Scholar] [CrossRef] [Green Version]
- Flessner, C.A.; Mouton-Odum, S.; Stocker, A.J.; Keuthen, N.J. StopPicking.com: Internet-based treatment for self-injurious skin picking. Dermatol. Online J. 2007, 13, 3. [Google Scholar]
- Habitaware. Available online: https://habitaware.com/ (accessed on 15 June 2019).
- Child Mind Institute. Available online: https://matter.childmind.org/tingle.html (accessed on 15 June 2019).
- Luiselli, J.K. Contingent glove wearing for the treatment of self-excoriating behavior in a sensory-impaired adolescent. Behav. Modif. 1989, 13, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, A.; Fantuzzo, J.W. Contingent application of a protective device to treat the severe self-biting behavior of a disturbed autistic child. J. Behav. Ther. Exp. Psychiatry 1984, 15, 79–83. [Google Scholar] [CrossRef]
- Selles, R.R.; McGuire, J.F.; Small, B.J.; Storch, E.A. A systematic review and meta-analysis of psychiatric treatments for excoriation (skin-picking) disorder. Gen. Hosp. Psychiatry 2016, 41, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Enta, T. Dermacase. Neurotic excoriation. Can. Fam. Physician 1996, 42, 1682–1688. [Google Scholar]
- Gupta, M.A.; Gupta, A.K. Olanzapine may be an effective adjunctive therapy in the management of acne excoriée: A case report. J. Cutan. Med. Surg. 2001, 5, 25–27. [Google Scholar] [CrossRef]
- Yousef, H.; Alhajj, M.; Sharma, S. Anatomy, Skin (Integument), Epidermis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020; Bookshelf ID: NBK279255. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK470464/ (accessed on 15 June 2019).
- Boer, M.; Duchnik, E.; Maleszka, R.; Marchlewicz, M. Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Postepy Dermatol. Alergol. 2016, 33, 1–5. [Google Scholar] [CrossRef]
- Annaidh, A.N.; Bruyère, K.; Destrade, M.; Gilchrist, M.D.; Otténio, M. Characterization of the anisotropic mechanical properties of excised human skin. J. Mech. Behav. Biomed. Mater. 2012, 5, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, Z.W.K.; Li, Z.; Owh, C.; Chee, P.L.; Ye, E.; Kai, D.; Yang, D.-P.; Loh, X.J. Using Artificial Skin Devices as Skin Replacements: Insights into Superficial Treatment. Small 2019, 15, e1805453. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, A.K.; Rotaru, G.-M.; Derler, S.; Spano, F.; Camenzind, M.; Annaheim, S.; Stämpfli, R.; Schmid, M.; Rossi, R.M. Materials used to simulate physical properties of human skin. Skin Res. Technol. 2016, 22, 3–14. [Google Scholar] [CrossRef]
- Figueira, D.R.; Miguel, S.P.; de Sá, K.D.; Correia, I.J. Production and characterization of polycaprolactone- hyaluronic acid/chitosan- zein electrospun bilayer nanofibrous membrane for tissue regeneration. Int. J. Biol. Macromol. 2016, 93, 1100–1110. [Google Scholar] [CrossRef]
- Yu, B.; Kang, S.-Y.; Akthakul, A.; Ramadurai, N.; Pilkenton, M.; Patel, A.; Nashat, A.; Anderson, D.G.; Sakamoto, F.H.; Gilchrest, B.A.; et al. An elastic second skin. Nat. Mater. 2016, 15, 911–918. [Google Scholar] [CrossRef] [Green Version]
- Pawlaczyk, M.; Lelonkiewicz, M.; Wieczorowski, M. Age-dependent biomechanical properties of the skin. Postepy Dermatol. Alergol. 2013, 30, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, A.; Zhou, S. One-component waterborne in vivo cross-linkable polysiloxane coatings for artificial skin. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1725–1737. [Google Scholar] [CrossRef]
- Antosik, A.K.; Piątek, A.; Wilpiszewska, K. Carboxymethylated starch and cellulose derivatives-based film as human skin equivalent for adhesive properties testing. Carbohydr. Polym. 2019, 222, 115014. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Jemec, G.B.E. Mechanical properties and barrier function of healthy human skin. Acta Derm. Venereol. 2006, 86, 308–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queen, E.; Gaylor, J.D.; Evans, J.H.; Courtney, J.M.; Reid, W.H. The preclinical evaluation of the water vapour transmission rate through burn wound dressings. Biomaterials 1987, 8, 367–371. [Google Scholar] [CrossRef]
- Xia, D.-L.; Chen, Y.-P.; Wang, Y.-F.; Li, X.-D.; Bao, N.; He, H.; Gu, H.-Y. Fabrication of Waterproof, Breathable Composite Liquid Dressing and Its Application in Diabetic Skin Ulcer Repair. Adv. Skin. Wound Care 2016, 29, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Bouthillette, M.; Beccati, D.; Akthakul, A.; Ramadurai, N.; Nashat, A.; Langer, R.; Anderson, R.R.; Sakamoto, F.H. A crosslinked polymer skin barrier film for moderate to severe atopic dermatitis: A pilot study in adults. J. Am. Acad. Dermatol. 2020, 82, 895–901. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.; Ahumada, M.; Franco, W.; Mah, T.-F.; Seymour, R.; Suuronen, E.J.; Alarcon, E.I. Sprayable peptide-modified silver nanoparticles as a barrier against bacterial colonization. Nanoscale 2016, 8, 19200–19203. [Google Scholar] [CrossRef]
- Kang, J.; Dietz, M.J.; Li, B. Antimicrobial peptide LL-37 is bactericidal against Staphylococcus aureus biofilms. PLoS ONE 2019, 14, e0216676. [Google Scholar] [CrossRef] [Green Version]
- Lei, Z.; Wu, P. A supramolecular biomimetic skin combining a wide spectrum of mechanical properties and multiple sensory capabilities. Nat. Commun. 2018, 9, 1134. [Google Scholar] [CrossRef]
- Wang, R.; Li, Q.; Chi, B.; Wang, X.; Xu, Z.; Xu, Z.; Chen, S.; Xu, H. Enzyme-induced dual-network ε-poly-l-lysine-based hydrogels with robust self-healing and antibacterial performance. Chem. Commun. (Camb.) 2017, 53, 4803–4806. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Liu, L.; Yu, J.; Ding, B. Human Skin-Like, Robust Waterproof, and Highly Breathable Fibrous Membranes with Short Perfluorobutyl Chains for Eco-Friendly Protective Textiles. ACS Appl. Mater. Interfaces 2018, 10, 30887–30894. [Google Scholar] [CrossRef]
- Muwaffak, Z.; Goyanes, A.; Clark, V.; Basit, A.W.; Hilton, S.T.; Gaisford, S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int. J. Pharm. 2017, 527, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Feula, A.; Tang, X.; Giannakopoulos, I.; Chippindale, A.M.; Hamley, I.W.; Greco, F.; Buckley, C.P.; Siviour, C.R.; Hayes, W. An adhesive elastomeric supramolecular polyurethane healable at body temperature. Chem. Sci. 2016, 7, 4291–4300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholas, M.N.; Jeschke, M.G.; Amini-Nik, S. Methodologies in creating skin substitutes. Cell. Mol. Life Sci. 2016, 73, 3453–3472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, S.P.; Zhang, Y.Z.; Lim, C.T. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Aston Chemical. Available online: http://www.aston-chemicals.com/single-product?id=387 (accessed on 15 June 2019).
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. Engl. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, E.I.; Vulesevic, B.; Argawal, A.; Ross, A.; Bejjani, P.; Podrebarac, J.; Ravichandran, R.; Phopase, J.; Suuronen, E.J.; Griffith, M. Coloured cornea replacements with anti-infective properties: Expanding the safe use of silver nanoparticles in regenerative medicine. Nanoscale 2016, 8, 6484–6489. [Google Scholar] [CrossRef]
- Ahumada, M.; Bohne, C.; Oake, J.; Alarcon, E.I. Protein capped nanosilver free radical oxidation: Role of biomolecule capping on nanoparticle colloidal stability and protein oxidation. Chem. Commun. (Camb.) 2018, 54, 4724–4727. [Google Scholar] [CrossRef] [PubMed]
- Houston-Hicks, M.; Lura, D.J.; Highsmith, M.J. Play Hands Protective Gloves: Technical Note on Design and Concept. Technol. Innov. 2016, 18, 207–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Dermatillomania | Biomaterial Based Therapies | ||
|---|---|---|---|
| Database | PubMed | PubMed | Google Scholar |
| Search date | April 2020 | May 2020 | May 2020 |
| Keywords | Dermatillomania, excoriation disorder, skin picking disorder, neurotic excoriation, psychogenic excoriation, acne excoriee | Second skin, extra skin, artificial skin, synthetic skin, skin substitute, breathable, polymers, cloth, textile, antibacterial. | Polymer, antimicrobial, biomaterial, on-skin, wearable, aesthetic, water resistant, water proof |
| Search method | dermatillomania[All Fields] OR “excoriation disorder”[All Fields] OR skin-picking[All Fields] OR “neurotic excoriation”[All Fields] OR “psychogenic excoriation”[All Fields] OR “acne excoriee”[All Fields] | 1. “second skin”[All Fields] OR “extra skin”[All Fields] OR “artificial skin”[All Fields] OR “Synthetic skin”[All Fields] OR “skin substitute”[all fields] AND (“20 May 2015”[PDAT]: “17 May 2015”[PDAT]) AND (“20 May 2015”[PDat]: “17 May 2015”[PDat]) 2. breathable[All Fields] AND (“polymers”[MeSH Terms] OR “polymers”[All Fields]) AND (“skin”[MeSH Terms] OR “skin”[All Fields]) 3. (((((((“anti bacterial agents”[Pharmacological Action] OR “anti-bacterial agents”[MeSH Terms]) OR (“anti bacterial”[All Fields] AND “agents”[All Fields])) OR “anti bacterial agents”[All Fields]) OR “antibacterial”[All Fields]) OR “antibacterials”[All Fields]) OR “antibacterially”[All Fields]) AND ((((((((“clothing”[MeSH Terms] OR “clothing”[All Fields]) OR “clothes”[All Fields]) OR “clothings”[All Fields]) OR “textiles”[MeSH Terms]) OR “textiles”[All Fields]) OR “cloth”[All Fields]) OR “clothed”[All Fields]) OR “cloths”[All Fields])) AND (“skin”[MeSH Terms] OR “skin”[All Fields]). | Find articles with all of the words polymer antimicrobial biomaterial on-skin wearable aesthetic and with at least one of the words water-resistant waterproof appearing anywhere in the articles |
| Results | 439 | 963 | - |
| Screening strategy | All types of articles including case studies, research papers and review articles were referred to. | Title/abstract was read and suitable research articles with products having properties similar to the listed ideal properties were chosen accordingly. | Title/abstract was read and suitable research articles with products having properties similar to the listed ideal properties were chosen accordingly |
| Ideal Physical Barrier Product | |
|---|---|
| Properties | Functions |
| Biocompatible | Non-toxic and non-allergenic on topical application to skin. |
| Wearable, waterproof, detergent resistant and easily removable | To prevent skin picking, consciously or subconsciously, at all times and allow already damaged skin to heal. To allow normal functioning of body without interfering with daily life activities. |
| Skin camouflaging or aesthetically appealing | To prevent attention seeking and improve psychological and social quality of life. |
| Mimicking mechanical properties of skin | Mechanical strength to resist tearing (product acting as a substitute to skin) caused by skin picking behavior. |
| Breathable | To allow optimal transepithelial water loss or MVTR, to promote wound healing and prevent skin maceration (causing uneven and easily peelable skin) which can be a trigger for skin picking. |
| Anti-microbial | To prevent microbial infections in the already damaged skin. |
| Self-healing material | To allow reuse of product if mechanically damaged by skin picking behavior. |
| Biodegradable | To prevent environmental pollution. |
| Cost-effective | To allow affordable, regular usage due to the chronic and recurrent nature of skin picking. |
| Properties of Skin | References | |
|---|---|---|
| Skin structure and thickness | ||
| Stratum corneum | 14 μm | [123] |
| Epidermis | 20–150 μm | [123] |
| Dermis | 1–4 mm | [123] |
| Hypodermis | >1 mm | [123] |
| Mechanical properties of human skin | ||
| Tensile strength | 5–30 MPa | [124] |
| Elastic modulus | 0.42 to 0.85 MPa from torsion tests 4.6 to 20 MPa from mechanical equipment 0.05 to 0.15 MPa from suction tests | [125] [124] [126] |
| Fracture strain | 140–180% | [125] |
| Tear energy (fracture toughness values) by scissors | 1700–2600 J/m2 | [122] |
| Other properties | ||
| Moisture vapor transmission rate (MVTR) | 10–75 g/m2/h | [127] |
| Skin roughness | Rz (Average roughness of skin surface) = 84.3 ± 12.3 μm Ra (difference between the tallest “peak” and the deepest “valley” in the surface) = 6.7 ± 0.6 μm | [128] |
| Product and Production Technique | Components | Properties | Intended Use | Suggested Future Studies or Improvements for Use in SPD | Upscale | Ref. |
|---|---|---|---|---|---|---|
| Emulsion: Two step emulsification forming an in situ crosslinked polymer layer. Addition of aqueous phase to silicone phase in a mixer and homogenization. Emulsion 1 applied on skin first and then emulsion 2. Platinum catalyzes crosslinking polymer layer by hydrosilylation | Emulsion 1: Water-in-silicone emulsion with polysiloxane reactive polymer blend (vinyl dimethicone and hydrogen dimethicone) containing 27% (w/w) fumed silica in continuous phase Emulsion 2: water-in-silicone emulsion with platinum catalyst (200 ppm w/w) and nylon 10–12 in continuous phase | * In situ polymer crosslinked in 2 minutes * Mechanical properties: elastic modulus = 0.48 MPa, fracture strain = 826%, adhesive strength = 78 N/mm, elastic recoil with minimal strain-energy loss. * Thickness of film = approx. 40 μm. * Biodegradable and biocompatible * Wearable for up to 16 hours, easy removal without damaging skin and breathable. * Polymer film intact even with daily activities like swimming and running * Water-resistant and detergent resistant, rub and wash resistant. * Aesthetically appealing. Gives appearance of natural skin. | Restores compromised skin barrier function; Can be used for pharmaceutical delivery and wound dressings. Used in successfully treating AD patients as an adjuvant treatment [132] | -Incorporating nanoparticles in the film can be a means to combine other actives such as antimicrobials, vitamins, wound healing factors and nutrients. -Improving the current once daily application mode | Feasible | [125] |
| Emulsion: One step emulsion system forming in vivo crosslinkable polysiloxane coating. Preparation of catalyst capsules dispersion by solvent evaporation method. Addition of aqueous phase to silicone phase in a Mixer and homogenization to obtain V and H emulsions. K,V,H parts are blended in 0.25/9.1/0.9 ratio. These three parts are isolated from each other by a continuous water phase. | (K) Karstedt (Pt) catalyst capsules dispersion (K) (V) Vinyl dimethicone emulsion = 30% w/w (V) (H) Hydrogen dimethicone emulsion = 30% w/w (H) | * In situ cross-linked polymer formed with tack free time of 10–30 min * Tensile strength = 0.55 MPa, elongation at break = 250%, elastic modulus = 0.47MPa * Thickness of dried film = 50 μm * Biocompatible and safe * Skin adherent and wearable * Easy single step application * Comparable to WVTR of human skin * Gives appearance of natural skin. * Water proof and high adhesion strength to human skin and also can be peeled off without irritating or harming the underlying skin. | Suggested as base materials for dermatological drug carrier, wearable electronic skin and wound dressing. | Incorporation of nanoparticles in the film to introduce antimicrobial and wound healing activities. * More studies on daily wear and wear time. | Feasible | [127] |
| Spray based Suspension Addition of LL37-SH to citrate@AgNPs and incubation followed by crosslinking of type1 collagen with addition of glutaraldehyde. Addition of excess glycine to quench glutaraldehyde.Final formulation has a total silver concentration of 100 um | Type 1 medical grade collagen, LL37-SH (antimicrobial peptide) Citrate capped Silver nanoparticles | * Non toxic * Antimicrobial properties (P.aeruginosa) [133] Staphylococcus aureus [134]. * Silver NP have a wide spectrum antimicrobial property. * Sprayable on wounds * Remains in place when sprayed into skin wound * Minimal organ infiltration upon spraying on wound. | Spray-on topical application for prophylactics and infection control in infected wounds | In addition, this technology maybe developed for spraying on clothes or products in contact with skin, to achieve antimicrobial properties and prevent infections. | Feasible | [133] |
| Hydrogel: Polyelectrolyte and self-healable. One-step random copolymerization of AA and DMAPS monomers | Acrylic acid (AA) and 3-dimethyl(methacryloyloxyethyl) ammonium propane sulfonate (DMAPS) | * Viscoelastic behavior with solid like elasticity and liquid-like plasticity * Imitates mechanical properties of natural skin Wide spectrum time-dependent mechanical properties with Compressive modulus of 27.6 KPa * Flexible reconfiguration ability: Can be reconfigured to fabricate a thin layer of transparent hydrogel skin. Can be adapted to irregular surfaces and was shown to be compliant with prosthetic finger locomotion. * Robust elasticity * Extremely stretchable: can be stretched more than 10000% the original length without fracture elongation of >100 without fracture * Fast autonomous self-healable within 2 hours. * Recyclable: It can recover >90% G’ in 10 dehydration-hydration cycles. | Used on prosthetic finger to sense train and temperature stimuli through capacitive and resistive sensors respectively. To be used to construct deformable sensory systems in the next generation of soft intelligent robots and smart wearable devices for IoT applications. | This technology may be improved to form self-healing patches or apparels that can be stuck at regularly skin picking areas which may be helpful. Long term wearability and biocompatibility on skin to be assessed. | Feasible | [135] |
| Hydrogel:Enzyme-induced dual-network EPL based hydrogels Self-healing (EDH). Radical polymerization of NVP and NMA under EPL to form single network EPL-G-POLY(NVP-co-NMA) hydrogels. Followed by Plasma amine oxidase (PAO) catalyzing in situ Schiff base reaction to form double network hydrogel | 1-vinyl-2-pyrrolidinone (NVP) N-methylol acrylamide (NMA) Epsilon-poly-l-lysine (EPL) | * Biocompatible * Self-healing synthetic material. High autonomous self-healing efficiency of 95% without any external stimuli * Broad spectrum antimicrobial activity against both Gram-negative and Gram-positive bacteria. * Enhances wound healing with minimal inflammatory response. Wound closure rate of 97% * Robust mechanical strength ~0.11 MPa * EPL exhibits potential adhesive property | Suggested use and great potential in myriad biomedical fields, such as wound repair, artificial skin and tissue engineering | May be developed into patches or films for application over picked skin for wound healing and protect that area from being picked by individuals consciously or unconsciously. | Feasible | [136] |
| Fibrous membrane Electrospinning (of PU/C4FPU/AgNO3 in N, N-dimethylacetamide) | Polyurethane elastomer (C4FPU) possessing double terminal short perfluoro butyl (−C4F9) chain Polyurethane (PU) Silver nitrate (AgNO3) | * Eco-friendly * Water proof (water resistant property of 102.8 kPa) * Breathable (WVTR of 12.9 kg.m−2·d−1) * High mechanical property of 9.8 MPa * Anti-bacterial activity (against S. aureus and E. coli) | Suggested for developing protective garments/textile | More studies on alteration of properties with respect to wash-reuse cycles to develop into aesthetic apparels. Incorporation of nanoparticles into nanofibers for other desired functions. | Feasible | [137] |
| 3D printed wound dressing 3D scanning of physical object or body part and 3D printing of wound dressings using prepared silver-loaded PCL filament, copper-loaded PCL filament zinc-loaded PCL filament | Polycaprolactone Silver nitrate Copper sulphate (II) pentahydrate Zinc oxide | * Biocompatible and biodegradable * Flexible due to elastomeric properties of PCL * Personalized treatment: Personalized wound dressings anatomically adaptable * Bactericidal properties of Silver loaded PCL dressing and copper loaded PCL dressing * Dressings can be tailored to shape, size and with antimicrobial agents. | Customizable wound dressing | Evaluation of safety and wearable time for this type of wound dressing. Can be used for developing patches or other apparels to promote wound healing and prevent bacterial infections. | Feasible | [138] |
| Type | Examples | Reference |
|---|---|---|
| Natural polymers | Collagen, hyaluronic acid, chitosan, gelatin, elastin, pullulan, alginate, dextran, cellulose, agar, agarose, carrageenan, pectin, keratin, fibrin, silk fibroin, egg shell membrane, Heparin | [123,140,141,142] |
| Synthetic polymers | Polyurethane, poly (l-lactic acid)(PLLA), poly(glycolide-co-l-lactide) (PLGA), poly(ethylene glycol) (PEG), polycaprolactone (PCL), poly(N,N-diethylacrylamide), poly(N-vinyl-2-pyrrolidone), polyvinyl alcohol (PVA), polyacrylic acid (PAA), silicones (polydimethylsiloxanes) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravipati, P.; Conti, B.; Chiesa, E.; Andrieux, K. Dermatillomania: Strategies for Developing Protective Biomaterials/Cloth. Pharmaceutics 2021, 13, 341. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13030341
Ravipati P, Conti B, Chiesa E, Andrieux K. Dermatillomania: Strategies for Developing Protective Biomaterials/Cloth. Pharmaceutics. 2021; 13(3):341. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13030341
Chicago/Turabian StyleRavipati, Priusha, Bice Conti, Enrica Chiesa, and Karine Andrieux. 2021. "Dermatillomania: Strategies for Developing Protective Biomaterials/Cloth" Pharmaceutics 13, no. 3: 341. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13030341