1. Introduction
To this day, cancer remains one of the most severe diseases, even though mortality has decreased overall due to steady reductions in smoking and advances in early detection and treatments for patients [
1]. However, some death rates increased from 2012–2016, such as for cancers of the liver, pancreas, uterus, brain and nervous system [
1]. Common methods in cancer therapy, such as the use of chemotherapeutic substances, are not specific to cancer cells but affect healthy cells as well and harm the patient to a large extent. A way to prevent the unnecessary harm of the human body is the specific targeting of the tumour by using, for example, fusion protein engineered antibodies [
2]. These approaches still face the problem of degradation because the endogenous defence detects the antibodies that carry the drug as an intruder. A different approach is the encapsulation of pharmaceutical ingredients in liposomes which can be delivered to the target cell without previous degradation [
3]. Liposomes consist of phospholipids that play a key role in the food and pharma industry because of their ubiquity in all organisms and their absolute safety [
4]. They are often utilized as natural emulsifiers. Using PLs as emulsifiers or building blocks, they contribute to the properties of the delivery system. The amphiphilic characteristics of the phospholipids are the constitutive base of all biological membranes and allow the carrying of an active ingredient inside, while transport through the blood circulation is possible without provoking an immediate immune response [
4].
Manufacturing methods for liposomes and other drug delivery vehicles have been the subject of intensive research for over 30 years. A conventional laboratory method for the production of liposomes is the film method, in which the phospholipids, possibly with a hydrophobic active ingredient, are dissolved in a solvent, e.g., ethanol or a mixture of methanol and chloroform. The solvent is then removed in the rotary evaporator so that the lipids form ordered bilayers at the wall of the flask. The hydrophilic active ingredient is encapsulated by adding the aqueous solvent. The lipids start swelling and form a heterogeneous suspension of multilamellar vesicles (liposomes) in which the active ingredient is encapsulated. Depending on the solubility of the active ingredient, the continuous phase can be aqueous or hydrophobic [
5,
6].
The production of uni-lamellar liposomes with defined sizes can be achieved in different ways. One possibility is the extrusion process, in which the solution is forced through nano-meter sized pores in a membrane under high pressure. The multilayers are fractured as they enter the nanopores and multilamellar vesicles are converted into uni-lamellar liposomes [
7]. The membrane pore size controls the size of the resulting liposomes [
8]. Another alternative for large production volumes is the use of a homogenizer [
9]. The liposome suspension passes through the homogenizer several times and the liposomes decrease in size with each pass. The minimally achievable sizes are 20 nm [
10,
11].
Based on their mechanism of action all the above methods are reliable regarding size mono-dispersity, but the concentration of active ingredients inside is the same as outside at the moment of vesicle closure. The encapsulation efficiency found in the literature for high molecular weight molecules is only in the low double-digit range and varies from about 2–50% in cases of affinity between active ingredients and liposomal membrane [
12,
13]. Higher efficiencies have not yet been established as so-called ‘remote loading’ is only feasible for small molecules that diffuse through phospholipid bilayers, but not for macromolecular APIs. A “high” encapsulation efficiency is proposed by Xu et al. who use freeze-thaw cycles for entrapment of proteins [
14]. Yet the proposed method does not exceed the 50% level either. Furthermore, the use of solvents is necessary for the majority of the manufacturing methods mentioned. However, even small traces of solvents are undesirable for application as a carrier for active ingredients in the pharmaceutical or food sector as they destabilize and degrade many active ingredients, especially peptides or proteins. A comprehensive removal of possible solvent residues in the production process is therefore extremely costly and time-consuming as is the removal of the API which is not encapsulated and thus, found freely in the solution, needing additional separation and purification steps [
15].
Engineering methods, however, are not common even though they have a great potential for large scale application. Pautot et al. were the first to introduce a centrifugation method where the droplets of a water-in-oil (
w/
o) nano-emulsion transfer to a second aqueous phase to create a bilayer [
16]. While this method works for large uni-lamellar vesicles of between 1 and 10 µm, several limitations exist such as an appropriate size for pharmaceutical applications, the stability of nano-emulsions, and oranogel formation between the three phases (water, oil and phospholipids). A smaller size of liposome was achieved by de Matos et al. [
17]. However, the phase transfer and encapsulation efficiencies were still insufficient. Previous research tackled the above problems and revealed that several advantages regarding the centrifugation process were achieved by using a fluorocarbon as hydrophobic phase instead of a hydrocarbon (such as squalene or dodecane, used previously); hindrance by interfacial tension during phase transfer is compensated for by the much stronger density difference between the hydrophobic and aqueous phase (Δρ ≈1 g/cm³; ρ
water = 0.998 g/cm³; ρ
fluorocarbon = 2.03 g/cm³), enabling transfer. Organogel formation is no longer detectable. Nano-emulsion droplets are stable for several weeks and liposome production is successful [
18]. Because of the heavier hydrophobic phase, the aqueous droplets float up instead of sedimenting during centrifugation, which enables easy removal of the liposomal suspension from the top.
The question remains as to whether a high encapsulation efficiency is possible by applying the centrifugation process. While De Matos et al. used the centrifugation method to produce asymmetric liposomes and encapsulated nucleic acids, they used squalene for the preparation of nano-emulsions leading to a plasmid encapsulation of 10–15% [
17]. Hence, an evaluation of the encapsulation efficiency of the formed liposomes from the water-in-fluorocarbon (w/fc) nano-emulsion remains to be carried out.
The aim of this work is to show the capacity of the process for high encapsulation efficiency by using different fluorocarbons and the phospholipids DPPC, DPPG and DMPC, evaluated with fluorescein-sodium (FS) as a low molecular weight hydrophilic marker, as well as bovine serum albumin (BSA) and fluorescently labelled dextran as high molecular weight protein and polymer, respectively. The sequence of different analytical detection methods was evaluated for its capacity to determine the amount of the encapsulated model API, while being able to trace not only the API surrogates itself but also other compounds such as phospholipids and the fluorocarbon phase.
2. Materials and Methods
2.1. Materials
Phospholipids utilized for all experiments were provided by Lipoid (Ludwigshafen, Germany). The synthetic phospholipids 1,2-dimyristoyl-sn-glycero-3-phostphatidylcholine (DMPC), 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC) and 1,2-dipalmitoyl-sn-glycero-3-phospho-rac-glycerol (DPPG) were received in powder form. Cholesterol (chol.) was purchased from Carl Roth (Karlsruhe, Germany). Perfluoro-perhydro-phenanthrene (C14F24) was purchased from F2 Chemicals (Preston, UK) and has a density of 2.03 g/cm³ and a refractive index (RI) of 1.331. For comparison, the fluorocarbons perfluoro-heptane (C7F16, RI = 1.26, ρ = 1.72 g/cm³), perfluoro-1,3-dimethylcyclohexane (C8F16, RI = 1.2895, ρ = 1.83 g/cm³) and perfluoro-methyl-decalin (C11F20, RI = 1.3195, ρ = 1.92 g/cm³) from F2 Chemicals were also tested. Phosphate buffer consisted of a 1:4.2 mixture of sodium di-hydrogen phosphate (NaH2PO4) and di-sodium hydrogen phosphate (Na2HPO4, both Carl Roth, Karlsruhe, Germany). Encapsulation efficiency was measured via fluorescein-sodium (FS, Carl Roth, Karlsruhe, Germany), BSA (VWR International GmbH, Darmstadt, Deutschland) and fluorescein isothiocyanate-dextran (FITC-D, Mw = 70 kDa, Merck, Darmstadt, Germany). For phospholipid quantification, perchloric acid (70%) was purchased from Carl Roth (Karlsruhe, Germany). Further chemicals used for the phosphorus assay were ascorbic acid, hexa-ammonium molybdate and sodium dihydrogen phosphate as a standard phosphate solution (all Carl Roth, Karlsruhe, Germany).
2.2. Preparation of Aqueous Lipid Stock Suspension
Lipid suspensions were prepared with different concentrations of phospholipids in 1 mL with 15 mM phosphate buffer if not stated otherwise. The preparation was performed in micro reaction tubes (Eppendorf, Hamburg, Germany). Ultrasound provided by a 3 mm sonotrode tip (Digital Sonifier 450, Branson Ultrasonic, Danbury, CT, USA) was used for the dispersion of phospholipids with a 100% cycle and an amplitude of 10% for 10 s followed by a 50% cycle and 10% amplitude for 10 min. The temperature was kept constant at 30 °C. To allow the determination of the encapsulation efficiency, fluorescein-sodium was added to the stock suspension at a concentration of 10 g/L, BSA at a concentration of 100 g/L, and FITC-D in a concentration of 50 g/L. For mixtures of phospholipids and cholesterol the molar ratio amounted to 60:40. These aqueous media containing dissolved model active ingredients and dispersed lipids were used as the aqueous phase for the generation of w/fc nano-emulsions as the next step of liposome production.
2.3. Preparation of w/fc Nanoemulsions and Liposomes
The w/fc nano-emulsion contained 1% dispersed phase (aqueous lipid stock suspension, see above) and was used to produce liposomes via centrifugation. The hydrophobic phase consisted of the fluorocarbon perfluoro-perhydro-phenanthrene (C
14F
24), if not stated otherwise. The droplet size averaged around 180 nm and was found to be suitable for liposome production [
18].
Liposomes were prepared by transferring the water droplets of the w/fc nano-emulsion containing the model API to a second aqueous phase via centrifugation (
Figure 1).
Thus, the lipid-coated aqueous cores of the nano-emulsion became surrounded by outer lipid leaflets, incorporating the API. The centrifugation step was performed at 0 °C and 4000×
g for 30 min (Eppendorf Centrifuge 5430 R, Eppendorf, Hamburg, Germany). Because of the higher density of the surrounding fluorocarbon, the lighter water droplets ascend to the upper aqueous phase. A photograph of the transfer including FS as a hydrophilic marker is depicted in
Figure 2a. The produced liposomes show an average size of 60 nm, based on dynamic light scattering and small angle x-ray scattering measurements. A transmission electron microscopy (TEM) picture of DPPC-liposomes (150 mM) evidences the production of vesicles (
Figure 2b, TEM CM12, co. Philips, The Netherlands). Zeta potential of liposomes was measured at a constant voltage of 50 mV with the Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK). For a detailed description of nano-emulsion and liposome preparation, as well as results of droplet and liposome size, please refer to Ullmann et al. [
18].
2.4. Detection Method
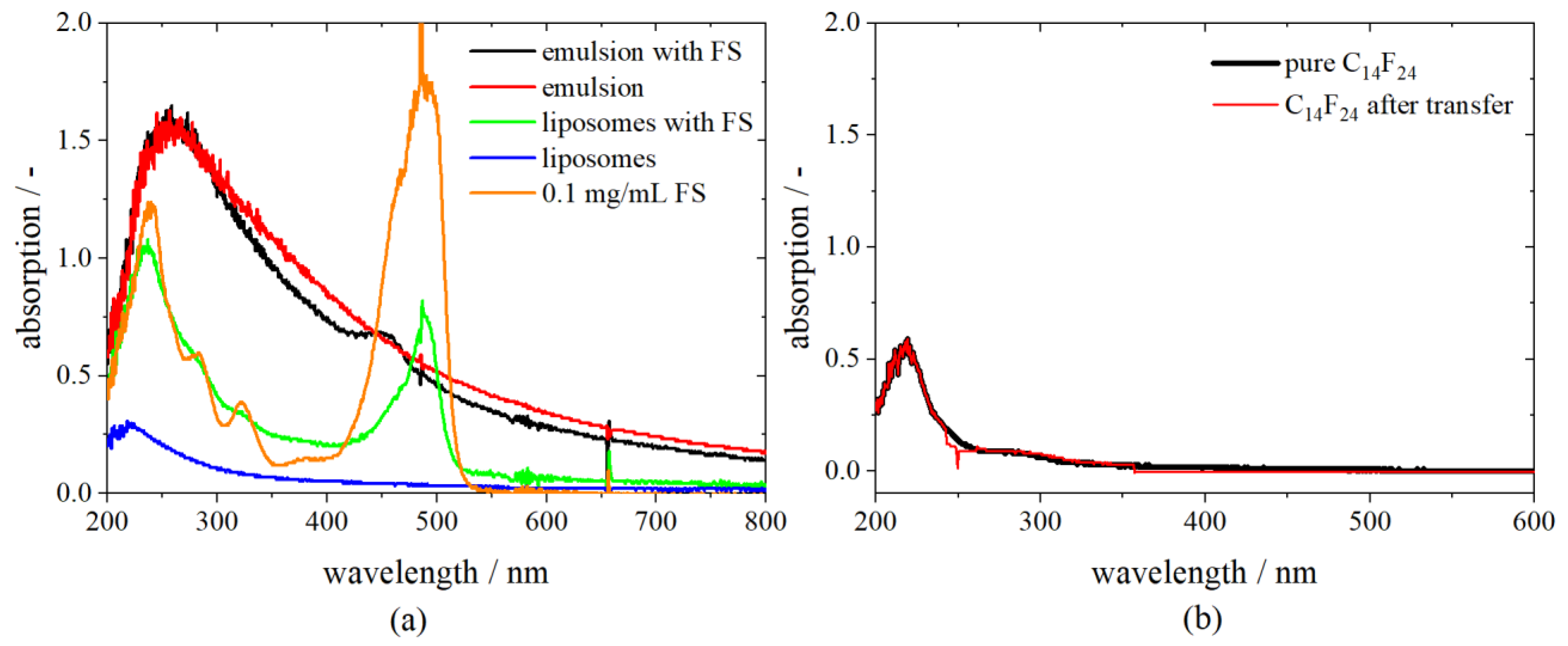
For the detection of the model APIs FS, BSA and FITC-D inside the droplets, UV-Vis spectroscopy (DH-2000 Ocean Optics, Largo, FL, USA and UV-1900 Shimadzu, Kyoto, Japan) was chosen. The spectra of the initial phases—the fluorocarbon, the buffer, the emulsion, the stock solution and the diluted solution of either FS, BSA or FITC-D—were recorded beforehand. The encapsulated model API FS shows a significant absorption peak at 491 nm, BSA at 280 nm and FITC-D at 493 nm. The absorption spectra of different substances applied during the centrifugation process reveal that neither the fluorocarbon, the phosphate buffer, nor different DPPC concentrations of stock solutions expose a significant peak at the same position. PLs absorb at a wavelength of 230 nm.
As an example, the spectra of different compounds needed for the preparation of liposomes are shown in
Figure 3a. A comparison between the absorption spectrum of an emulsion with FS and an emulsion without FS reveals that the fluorocarbon has a shielding effect on the dye. However, detection, as well as distinction, is possible as there is a small peak visible at 458 nm for the emulsion, including FS.
After centrifuging the w/fc nano-emulsion and transferring of droplets to the upper aqueous phase, the lower hydrophobic phase (C
14F
24) was measured again. The fluorocarbon shows the same absorbance after centrifugation as in its pure condition, indicating that no FS nor phospholipids remain in the hydrophobic phase and the transfer is completed entirely (
Figure 3b).
2.5. Encapsulation Efficiency
To determine the encapsulation efficiency of the produced liposomes, FS was added at a concentration of 10 g/L to the lipid stock suspension, BSA at a concentration of 100 g/L and FITC-D at a concentration of 50 g/L. The final concentration of the model APIs in the nano-emulsion was 1:100 of the initial concentration as the emulsions were prepared with 1% (v/v) of the dispersed phase.
After producing the nano-emulsion and centrifuging the droplets through the interface, the upper aqueous phase containing the liposomes and the model API is collected, and non-encapsulated FS is separated by Vivaspin 500® (Sartorius, Göttingen, Germany). The Vivaspin 500® has a capacity of 500 µL and a separation limit of 50 kDa. For BSA and FITC-D, a Vivaspin 2® with a 100 kDa cut off was chosen. To separate liposomes from free FS, BSA, or FITC-D, the samples were centrifuged for 3 h with 4000× g at 0 °C. Liposomes remain in the filter module (retentate) while the non-encapsulated model API passes through the membrane and is quantified via the UV-Vis spectrometer (filtrate).
The encapsulation efficiency considering the initial concentration
is called
, and its value is provided in % and is determined by
where
is the amount of free model API in the filtrate and
is the initial concentration of API in the emulsion.
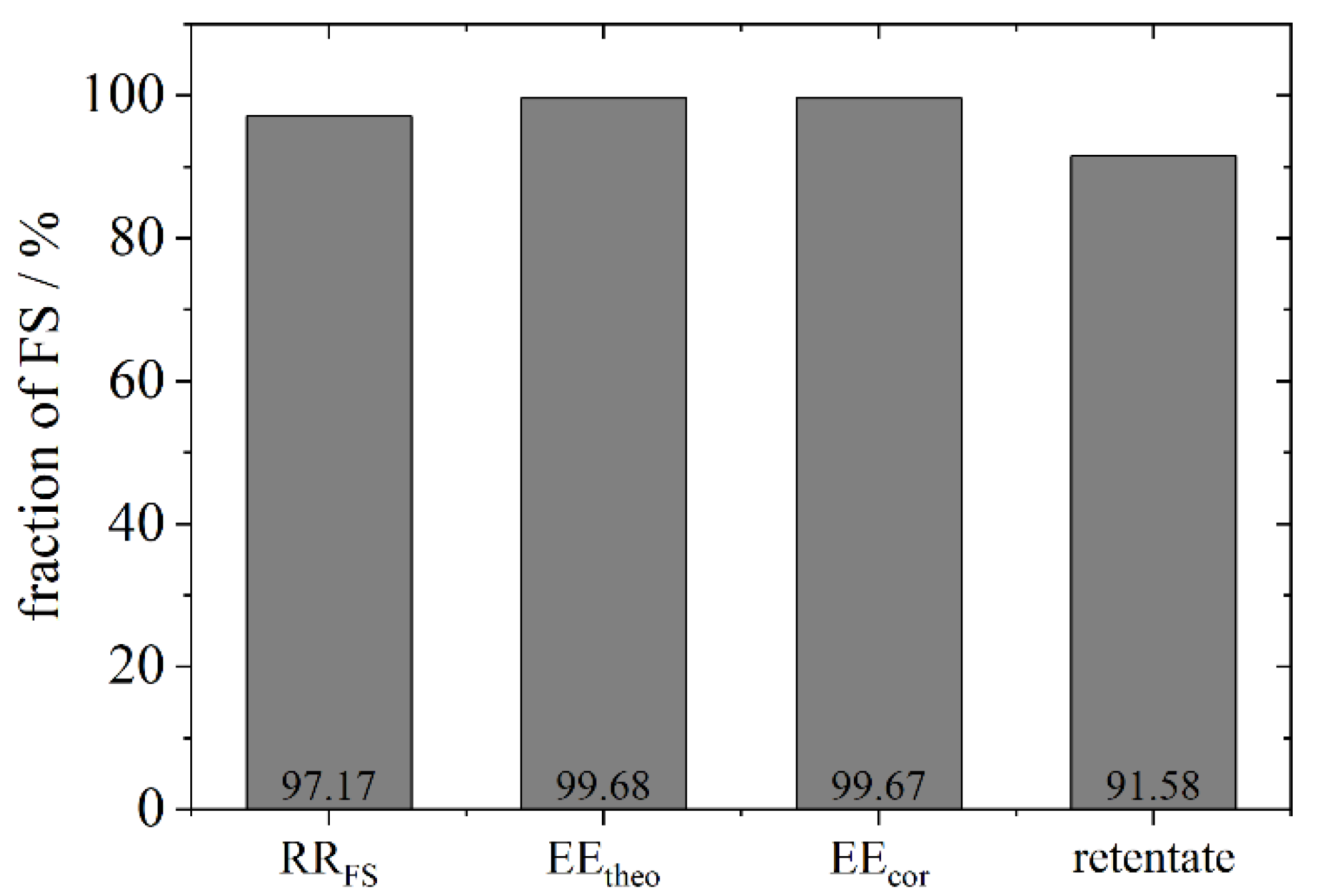
2.6. Recovery Rate (RR)
In addition to the measurement of the free model API after the separation step, the amount of the marker in the complete aqueous phase was detected, e.g., shown here for FS (RR
FS). Perchloric acid destroys the liposomes and allows the measurement of the total amount of FS which should add up to 100% of the initial amount. To determine the total amount of FS compared to the initial amount, the liposome suspension was treated with 70% perchloric acid at a volume ratio of 1:1 for solubilisation of liposomes and quantified via UV-Vis spectroscopy at 435 nm according to a calibration curve with perchloric acid, respectively. Treatment took place for 10 min and 50 °C on a heated shaker. In addition, possible losses of FS at the wall of the reaction tube were taken into account by washing the tubes with perchloric acid and quantification by UV-Vis spectroscopy. The retentate (liposome remains on the Vivaspin membrane) was treated likewise with perchloric acid to specify the amount of FS. The quantification is summed up as follows and was performed for FS, dextran and phospholipids:
where the index x stands for either FS, D (dextran) or PL (phospholipid),
cx,total is the total amount of model API or phospholipids found in the upper aqueous phase after transfer, and c
0 is the initial amount from the stock suspension. When referring to RR as a concentration, e.g., the RR of FS, it is defined as c
RR in mM.
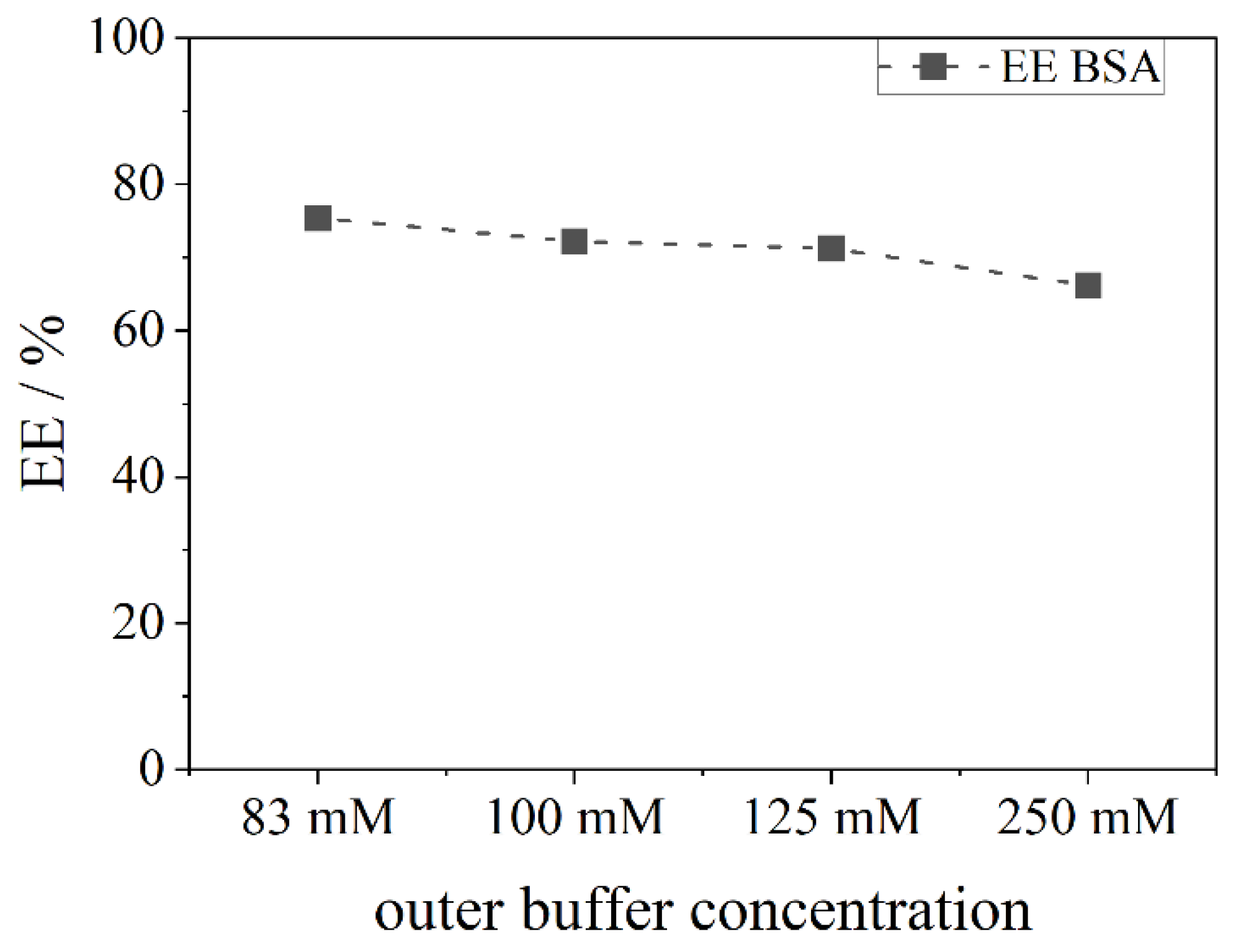
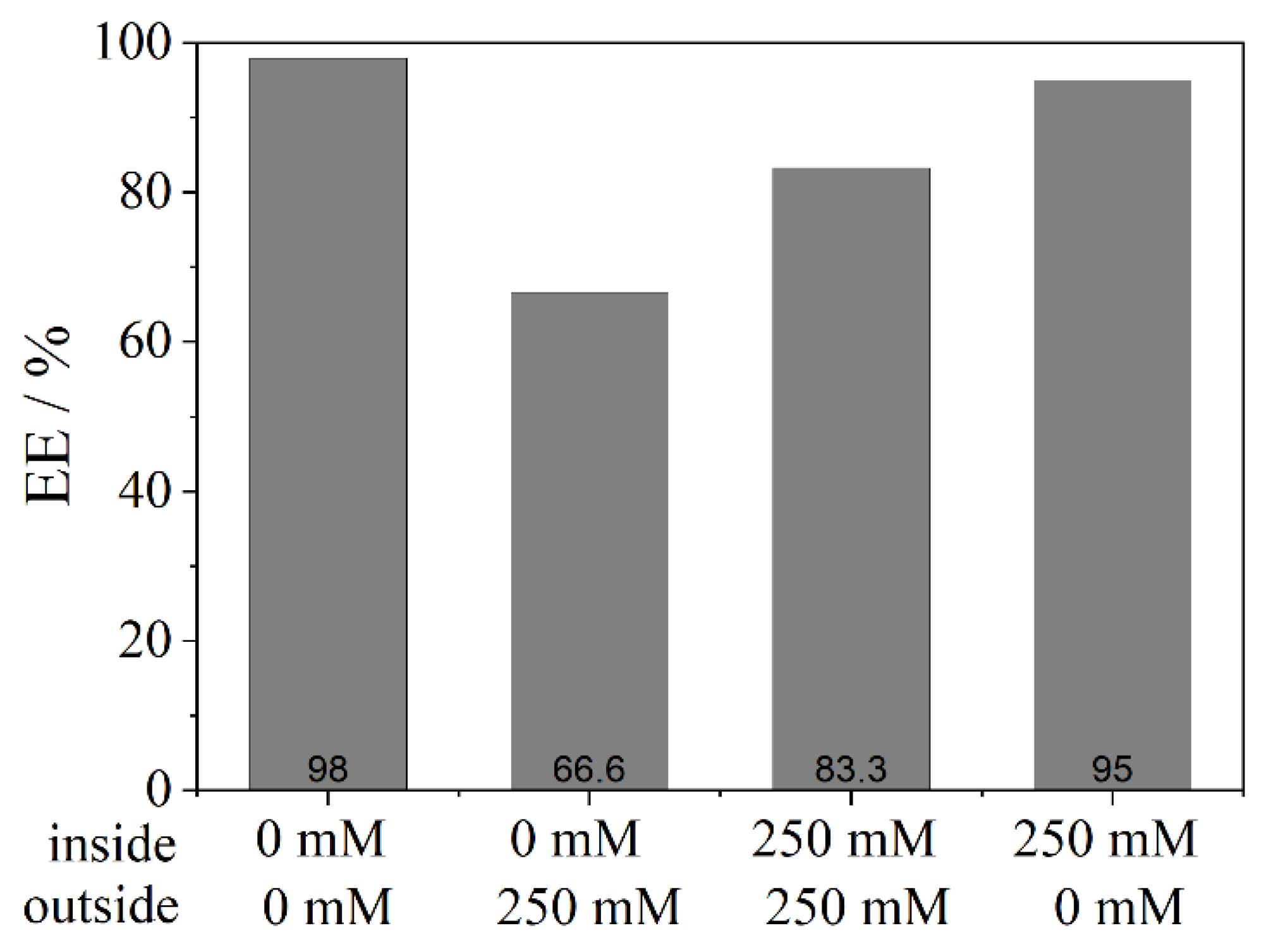
2.7. Encapsulation under Different Osmotic Conditions
The efficiency of the encapsulation was monitored under different osmotic conditions inside and outside the liposomes. For this purpose, the phosphate buffer concentration was changed from 15 mM to a range between 83 mM and 250 mM, and no salt ions (0 mM).
2.8. Determination of Phospholipids
The concentration of phospholipids in the filtrate as well as possible losses and the concentration of phospholipids that are completely transferred were determined by phosphorus assay according to Fiske [
19]. Perchloric acid (70%) destroys the phospholipid molecules while ascorbic acid and ammonium molybdate lead to a change of blue color under the presence of phosphorus.
4. Conclusions
The encapsulation not only of low molecular weight substances (FS), but also of macromolecules (BSA and dextrane) with > 60 kDa and without a high affinity to the bilayer, which could enhance the encapsulation, was successfully achieved. The encapsulation efficiency was determined and found to be between 48–98% for the fluorocarbon C14F24 depending on the used phospholipid. Other fluorocarbons showed a lower EE (20–60%) which revealed that not all fluorocarbons encapsulate the model APIs equally well. A higher density of the fluorocarbon is to be favoured for the process. These achievements were possible due to the novel preparation method of liposomes by using a centrifuge to transfer droplets from a w/fc nano-emulsion. The results show that compounds which stabilize a membrane, such as cholesterol or charged phospholipids, have a negative effect on the encapsulation efficiency. This effect is likely to be due to the greater bending elasticity of the membrane which leads to a slower closure of the vesicle after the detachment of the liposome from the interface. Hence, the leakage increases. For further stabilization of liposomes and for mimicking a future application in the blood, osmotic conditions were imitated. An efflux negatively affects the encapsulation efficiency, an influx leads to a better encapsulation than under efflux conditions, while best results were found for pure water inside and outside.
Thus, the results and the liposome preparation method show not only the capacity for large-scale application, but the method of analysis is also applicable for a scale-up. These data demonstrate a promising alternative for industry in producing liposomes for pharmaceutical application, which additionally hold the capacity to encapsulate high amounts of macromolecules.