An Updated Overview of the Emerging Role of Patch and Film-Based Buccal Delivery Systems
Abstract
:1. Introduction
2. Anatomical and Physiological Features of the Oral Cavity
2.1. Permeability
2.2. Oral Environment
3. Drug Transport Mechanisms
4. Design and Formulation of Buccal Drug-Delivery Systems
4.1. Mucoadhesive Polymers
4.2. Penetration Enhancers
4.3. Enzyme Inhibitors
5. Buccal Patch
6. Buccal Film
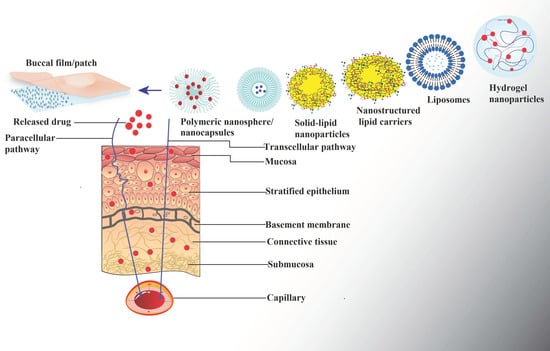
7. Functional Role of Nanoparticles in Buccal Drug-Delivery Systems
7.1. Polymeric Nanoparticles
7.2. Lipid Nanoparticles
7.2.1. Liposomes
7.2.2. Solid-Lipid Nanoparticles
7.2.3. Nanostructured Lipid Carriers (NLCs)
7.3. Nanosuspensions
8. In Vitro Evaluation Techniques
9. Preparation Methods, Scale-Up Process and Manufacturing Considerations
10. Clinical Translation of Buccal Administered Molecules
11. Future Perspectives and Directions
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilhotra, R.M.; Ikram, M.; Srivastava, S.; Gilhotra, N. A clinical perspective on mucoadhesive buccal drug delivery systems. J. Biomed. Res. 2014, 28, 81–97. [Google Scholar] [CrossRef]
- Macedo, A.S.; Castro, P.M.; Roque, L.; Thomé, N.G.; Reis, C.P.; Pintado, M.E.; Fonte, P. Novel and revisited approaches in nanoparticle systems for buccal drug delivery. J. Control Release 2020, 320, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Birudaraj, R.; Mahalingam, R.; Li, X.; Jasti, B.R. Advances in buccal drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2005, 22, 295–330. [Google Scholar] [CrossRef] [PubMed]
- Sankar, V.; Hearnden, V.; Hull, K.; Juras, D.V.; Greenberg, M.S.; Kerr, A.R.; Lockhart, P.B.; Patton, L.L.; Porter, S.; Thornhill, M. Local drug delivery for oral mucosal diseases: Challenges and opportunities. Oral Dis. 2011, 17, 73–84. [Google Scholar] [CrossRef]
- Senel, S.; Hincal, A.A. Drug permeation enhancement via buccal route: Possibilities and limitations. J. Control Release 2001, 72, 133–144. [Google Scholar] [CrossRef]
- Campisi, G.; Paderni, C.; Saccone, R.; Di Fede, O.; Wolff, A.; Giannola, L.I. Human buccal mucosa as an innovative site of drug delivery. Curr. Pharm. Des. 2010, 16, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Ruggeri, M.; Rossi, S.; Bonferoni, M.C.; Vigani, B.; Ferrari, F. Chapter 8—(Trans)buccal drug delivery. In Nanotechnology for Oral Drug Delivery; Martins, J.P., Santos, H.A., Eds.; Academic Press: Cambdrige, MA, USA, 2020; pp. 225–250. [Google Scholar]
- Tran, P.H.L.; Duan, W.; Tran, T.T.D. Recent developments of nanoparticle-delivered dosage forms for buccal delivery. Int. J. Pharm. 2019, 571, 118697. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.; Brogden, K. Human Oral Mucosa: Development, Structure and Function; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Chen, J.; Engelen, L. Food Oral Processing: Fundamentals of Eating and Sensory Perception; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Berkovitz, B.K.; Moxham, B.J.; Linden, R.W.; Sloan, A.J. Master Dentistry Volume 3 Oral Biology E-Book: Oral Anatomy, Histology, Physiology and Biochemistry; Elsevier Health Sciences: London, UK, 2010; Volume 3. [Google Scholar]
- Nelson, S.J. Wheeler’s Dental Anatomy, Physiology and Occlusion-E-Book; Elsevier: London, UK, 2014. [Google Scholar]
- Shojaei, A.H. Buccal mucosa as a route for systemic drug delivery: A review. J. Pharm. Pharm. Sci. 1998, 1, 15–30. [Google Scholar] [PubMed]
- Wertz, P.W.; Squier, C.A. Cellular and molecular basis of barrier function in oral epithelium. Crit. Rev. Ther. Drug Carr. Syst. 1991, 8, 237–269. [Google Scholar]
- Wertz, P.W. Roles of lipids in the permeability barriers of skin and oral mucosa. Int. J. Mol. Sci. 2021, 22, 5229. [Google Scholar] [CrossRef]
- Harris, D.; Robinson, J.R. Drug delivery via the mucous membranes of the oral cavity. J. Pharm. Sci. 1992, 81, 1–10. [Google Scholar] [CrossRef]
- Bierbaumer, L.; Schwarze, U.Y.; Gruber, R.; Neuhaus, W. Cell culture models of oral mucosal barriers: A review with a focus on applications, culture conditions and barrier properties. Tissue Barriers 2018, 6, 1479568. [Google Scholar] [CrossRef]
- Groeger, S.; Meyle, J. Oral mucosal epithelial cells. Front. Immunol. 2019, 10, 208. [Google Scholar] [CrossRef] [Green Version]
- Frenkel, E.S.; Ribbeck, K. Salivary mucins in host defense and disease prevention. J. Oral Microbiol. 2015, 7, 29759. [Google Scholar] [CrossRef] [PubMed]
- Sawada, A.; Wakabayashi, N.; Ona, M.; Suzuki, T. Viscoelasticity of human oral mucosa: Implications for masticatory biomechanics. J. Dent. Res. 2011, 90, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.F.; Liu, F.; Brown, M.B. Advances in oral transmucosal drug delivery. J. Control Release 2011, 153, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Laffleur, F.; Bernkop-Schnürch, A. Strategies for improving mucosal drug delivery. Nanomedicine 2013, 8, 2061–2075. [Google Scholar] [CrossRef] [PubMed]
- Li, L.D.; Crouzier, T.; Sarkar, A.; Dunphy, L.; Han, J.; Ribbeck, K. Spatial configuration and composition of charge modulates transport into a mucin hydrogel barrier. Biophys. J. 2013, 105, 1357–1365. [Google Scholar] [CrossRef] [Green Version]
- Aframian, D.J.; Davidowitz, T.; Benoliel, R. The distribution of oral mucosal pH values in healthy saliva secretors. Oral Dis. 2006, 12, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Shah, J.; Jacob, S.; Al-Dhubiab, B.E.; Patel, V.; Sreeharsha, N.; Shinu, P. Development of mucoadhesive buccal film for rizatriptan: In vitro and in vivo evaluation. Pharmaceutics 2021, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Madhav, N.V.; Shakya, A.K.; Shakya, P.; Singh, K. Orotransmucosal drug delivery systems: A review. J. Control Release 2009, 140, 2–11. [Google Scholar] [CrossRef]
- Smart, J.D. Buccal drug delivery. Expert Opin. Drug Deliv. 2005, 2, 507–517. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Giovino, C.; Ayensu, I.; Tetteh, J.; Boateng, J.S. Development and characterisation of chitosan films impregnated with insulin loaded PEG-b-PLA nanoparticles (NPs): A potential approach for buccal delivery of macromolecules. Int. J. Pharm. 2012, 428, 143–151. [Google Scholar] [CrossRef]
- Davies, A.; Mundin, G.; Vriens, J.; Webber, K.; Buchanan, A.; Waghorn, M. The influence of low salivary flow rates on the absorption of a sublingual fentanyl citrate formulation for breakthrough cancer pain. J. Pain Symptom Manag. 2016, 51, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Sandri, G.; Caramella, C.M. Buccal drug delivery: A challenge already won? Drug Discov. Today. Technol. 2005, 2, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.P.; Santos, H.A. Nanotechnology for Oral Drug Delivery: From Concept to Applications; Academic Press: Cambdrige, MA, USA, 2020. [Google Scholar]
- Hombach, J.; Bernkop-Schnürch, A. Mucoadhesive drug delivery systems. Handb. Exp. Pharmacol. 2010, 197, 251–266. [Google Scholar] [CrossRef]
- Chang, R.-J.; Gent, A.N. Effect of interfacial bonding on the strength of adhesion of elastomers. I. Self-adhesion. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1619–1633. [Google Scholar] [CrossRef]
- Yang, C.; Xing, X.; Li, Z.; Zhang, S. A Comprehensive review on water diffusion in polymers focusing on the polymer-metal interface combination. Polymers 2020, 12, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karoyo, A.H.; Wilson, L.D. A review on the design and hydration properties of natural polymer-based hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Shinkar, D.M.; Dhake, A.S.; Setty, C.M. Drug delivery from the oral cavity: A focus on mucoadhesive buccal drug delivery systems. PDA J. Pharm. Sci. Technol. 2012, 66, 466–500. [Google Scholar] [CrossRef]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.H.; Coakley, R.D.; Button, B.; Henderson, A.G.; Zeman, K.L.; Alexis, N.E.; Peden, D.B.; Lazarowski, E.R.; Davis, C.W.; Bailey, S.; et al. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am. J. Respir. Crit. Care Med. 2015, 192, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Jabbari, E.; Wisniewski, N.; Peppas, N.A. Evidence of mucoadhesion by chain interpenetration at a poly (acrylic acid)/mucin interface using ATR-FTIR spectroscopy. J. Control Release 1993, 26, 99–108. [Google Scholar] [CrossRef]
- Laffleur, F. Mucoadhesive polymers for buccal drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 591–598. [Google Scholar] [CrossRef]
- Bagan, J.; Paderni, C.; Termine, N.; Campisi, G.; Lo Russo, L.; Compilato, D.; Di Fede, O. Mucoadhesive polymers for oral transmucosal drug delivery: A review. Curr. Pharm. Des. 2012, 18, 5497–5514. [Google Scholar] [CrossRef] [Green Version]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Jovanović, M.; Tomić, N.; Cvijić, S.; Stojanović, D.; Ibrić, S.; Uskoković, P. Mucoadhesive gelatin buccal films with propranolol hydrochloride: Evaluation of mechanical, mucoadhesive, and biopharmaceutical properties. Pharmaceutics 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Chaves, P.D.S.; Frank, L.A.; Frank, A.G.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C.R. Mucoadhesive properties of Eudragit®RS100, Eudragit®S100, and poly(ε-caprolactone) nanocapsules: Influence of the vehicle and the mucosal surface. AAPS PharmSciTech 2018, 19, 1637–1646. [Google Scholar] [CrossRef]
- Collado-González, M.; González Espinosa, Y.; Goycoolea, F.M. Interaction between chitosan and mucin: Fundamentals and applications. Biomimetics 2019, 4, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puri, V.; Sharma, A.; Kumar, P.; Singh, I. Thiolation of biopolymers for developing drug delivery systems with enhanced mechanical and mucoadhesive properties: A review. Polymers 2020, 12, 1803. [Google Scholar] [CrossRef]
- Schmitz, T.; Grabovac, V.; Palmberger, T.F.; Hoffer, M.H.; Bernkop-Schnürch, A. Synthesis and characterization of a chitosan-N-acetyl cysteine conjugate. Int. J. Pharm. 2008, 347, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Catron, N.D.; Lee, H.; Messersmith, P.B. Enhancement of poly(ethylene glycol) mucoadsorption by biomimetic end group functionalization. Biointerphases 2006, 1, 134–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Soliman, G.M.; Barralet, J.; Cerruti, M. Mollusk glue inspired mucoadhesives for biomedical applications. Langmuir ACS J. Surf. Colloids 2012, 28, 14010–14017. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.; Ryu, J.H.; Lee, H. Chitosan-catechol: A polymer with long-lasting mucoadhesive properties. Biomaterials 2015, 52, 161–170. [Google Scholar] [CrossRef]
- Hu, S.; Pei, X.; Duan, L.; Zhu, Z.; Liu, Y.; Chen, J.; Chen, T.; Ji, P.; Wan, Q.; Wang, J. A mussel-inspired film for adhesion to wet buccal tissue and efficient buccal drug delivery. Nat. Commun. 2021, 12, 1689. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Morales, J.O.; Huang, S.; Williams, R.O., 3rd; McConville, J.T. Films loaded with insulin-coated nanoparticles (ICNP) as potential platforms for peptide buccal delivery. Colloids Surf. B Biointerfaces 2014, 122, 38–45. [Google Scholar] [CrossRef]
- Aungst, B.J. Absorption enhancers: Applications and advances. AAPS J. 2012, 14, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, H.; Khedkar, A.; Verma, M. Oral insulin—A review of current status. Diabetes Obes. Metab. 2010, 12, 179–185. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2021. [Google Scholar] [CrossRef]
- Park, K.; Kwon, I.C.; Park, K. Oral protein delivery: Current status and future prospect. React. Funct. Polym. 2011, 71, 280–287. [Google Scholar] [CrossRef]
- Nicolazzo, J.A.; Reed, B.L.; Finnin, B.C. Buccal penetration enhancers—How do they really work? J. Control Release 2005, 105, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Som, I.; Bhatia, K.; Yasir, M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2–9. [Google Scholar] [CrossRef]
- Hassan, N.; Ahad, A.; Ali, M.; Ali, J. Chemical permeation enhancers for transbuccal drug delivery. Expert Opin. Drug Deliv. 2010, 7, 97–112. [Google Scholar] [CrossRef]
- Padula, C.; Pescina, S.; Nicoli, S.; Santi, P. New insights on the mechanism of fatty acids as buccal permeation enhancers. Pharmaceutics 2018, 10, 201. [Google Scholar] [CrossRef] [Green Version]
- Jampilek, J.; Brychtova, K. Azone analogues: Classification, design, and transdermal penetration principles. Med. Res. Rev. 2012, 32, 907–947. [Google Scholar] [CrossRef]
- Palem, C.R.; Kumar Battu, S.; Gannu, R.; Yamsani, V.V.; Repka, M.A.; Yamsani, M.R. Role of cyclodextrin complexation in felodipine-sustained release matrix tablets intended for oral transmucosal delivery: In vitro and ex vivo characterization. Pharm. Dev. Technol. 2012, 17, 321–332. [Google Scholar] [CrossRef]
- Senel, S.; Kremer, M.J.; Kaş, S.; Wertz, P.W.; Hincal, A.A.; Squier, C.A. Enhancing effect of chitosan on peptide drug delivery across buccal mucosa. Biomaterials 2000, 21, 2067–2071. [Google Scholar] [CrossRef]
- Sood, A.; Panchagnula, R. Peroral route: An opportunity for protein and peptide drug delivery. Chem. Rev. 2001, 101, 3275–3303. [Google Scholar] [CrossRef]
- Hou, S.Y.; Flynn, G.L. Influences of 1-dodecylazacycloheptan-2-one on permeation of membranes by weak electrolytes. 1. Theoretical analysis of weak electrolyte diffusion through membranes and studies involving silicone rubber membranes. J. Pharm. Sci. 1997, 86, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Sonaje, K.; Chuang, E.Y.; Lin, K.J.; Yen, T.C.; Su, F.Y.; Tseng, M.T.; Sung, H.W. Opening of epithelial tight junctions and enhancement of paracellular permeation by chitosan: Microscopic, ultrastructural, and computed-tomographic observations. Mol. Pharm. 2012, 9, 1271–1279. [Google Scholar] [CrossRef]
- Zubareva, A.; Shagdarova, B.; Varlamov, V.; Kashirina, E.; Svirshchevskaya, E. Penetration and toxicity of chitosan and its derivatives. Eur. Polym. J. 2017, 93, 743–749. [Google Scholar] [CrossRef]
- Sandri, G.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Muzzarelli, C.; Caramella, C. Assessment of chitosan derivatives as buccal and vaginal penetration enhancers. Eur. J. Pharm. Sci. 2004, 21, 351–359. [Google Scholar] [CrossRef]
- Caon, T.; Jin, L.; Simões, C.M.; Norton, R.S.; Nicolazzo, J.A. Enhancing the buccal mucosal delivery of peptide and protein therapeutics. Pharm. Res. 2015, 32, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Renukuntla, J.; Vadlapudi, A.D.; Patel, A.; Boddu, S.H.; Mitra, A.K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013, 447, 75–93. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Heng, P.W. Buccal delivery systems. Drug Dev. Ind. Pharm. 2003, 29, 821–832. [Google Scholar] [CrossRef]
- Veuillez, F.; Kalia, Y.N.; Jacques, Y.; Deshusses, J.; Buri, P. Factors and strategies for improving buccal absorption of peptides. Eur. J. Pharm. Biopharm. 2001, 51, 93–109. [Google Scholar] [CrossRef]
- Netsomboon, K.; Suchaoin, W.; Laffleur, F.; Prüfert, F.; Bernkop-Schnürch, A. Multifunctional adhesive polymers: Preactivated thiolated chitosan-EDTA conjugates. Eur. J. Pharm. Biopharm. 2017, 111, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Krauland, A.H.; Leitner, V.M.; Palmberger, T. Thiomers: Potential excipients for non-invasive peptide delivery systems. Eur. J. Pharm. Biopharm. 2004, 58, 253–263. [Google Scholar] [CrossRef]
- Langoth, N.; Kalbe, J.; Bernkop-Schnürch, A. Development of buccal drug delivery systems based on a thiolated polymer. Int. J. Pharm. 2003, 252, 141–148. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Guggi, D.; Pinter, Y. Thiolated chitosans: Development and in vitro evaluation of a mucoadhesive, permeation enhancing oral drug delivery system. J. Control Release 2004, 94, 177–186. [Google Scholar] [CrossRef]
- Guggi, D.; Krauland, A.H.; Bernkop-Schnürch, A. Systemic peptide delivery via the stomach: In vivo evaluation of an oral dosage form for salmon calcitonin. J. Control Release 2003, 92, 125–135. [Google Scholar] [CrossRef]
- Marschütz, M.K.; Caliceti, P.; Bernkop-Schnürch, A. Design and in vivo evaluation of an oral delivery system for insulin. Pharm. Res. 2000, 17, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Targhotra, M.; Chauhan, M.K. An overview on various approaches and recent patents on buccal drug delivery systems. Curr. Pharm. Des. 2020, 26, 5030–5039. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, A.; Katona, B.; Módra, S.; Aigner, Z.; Sebe, I.; Pintye-Hódi, K.; Zelkó, R.; Regdon, G.J.; Kristó, K. Effects of sucrose palmitate on the physico-chemical and mucoadhesive properties of buccal films. Molecules 2020, 25, 5248. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, C.; Fini, A.; Ospitali, F. Mucoadhesive multiparticulate patch for the intrabuccal controlled delivery of lidocaine. Eur. J. Pharm. Biopharm. 2013, 83, 405–414. [Google Scholar] [CrossRef]
- Adhikari, S.N.; Nayak, B.S.; Nayak, A.K.; Mohanty, B. Formulation and evaluation of buccal patches for delivery of atenolol. AAPS PharmSciTech 2010, 11, 1038–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiledar, R.R.; Tagalpallewar, A.A.; Kokare, C.R. Formulation and in vitro evaluation of xanthan gum-based bilayered mucoadhesive buccal patches of zolmitriptan. Carbohydr. Polym. 2014, 101, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, P.; Kesavan, B.R.; Narasimha, J.K. Formulation of unidirectional release buccal patches of carbamazepine and study of permeation through porcine buccal mucosa. Asian Pac. J. Trop. Biomed. 2013, 3, 995–1002. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, A.; Mitragotri, S. Ionic liquid-mediated delivery of insulin to buccal mucosa. J. Control Release 2020, 327, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Chun, M.K.; Cho, C.S.; Choi, H.K. A novel mucoadhesive polymer prepared by template polymerization of acrylic acid in the presence of poloxamer. J. Appl. Polym. Sci. 2001, 79, 1525–1530. [Google Scholar] [CrossRef]
- Escalona-Rayo, C.F.; Serrano-Castañeda, P.; López-Cervantes, M.; Escobar-Chávez, J.J. Optimization of unidirectional mucoadhesive buccal patches based on chitosan and pluronic® F-127 for metoprolol controlled release: In vitro and ex vivo evaluations. J. Pharm. Innov. 2020, 15, 556–568. [Google Scholar] [CrossRef]
- Saxena, A.; Tewari, G.; Saraf, S.A. Formulation and evaluation of mucoadhesive buccal patch of acyclovir utilizing inclusion phenomenon. Braz. J. Pharm. Sci. 2011, 47, 887–897. [Google Scholar] [CrossRef] [Green Version]
- Vishnu, Y.V.; Chandrasekhar, K.; Ramesh, G.; Rao, Y.M. Development of mucoadhesive patches for buccal administration of carvedilol. Curr. Drug Deliv. 2007, 4, 27–39. [Google Scholar] [CrossRef]
- Kraisit, P.; Limmatvapirat, S.; Nunthanid, J.; Sriamornsak, P.; Luangtana-Anan, M. Preparation and characterization of hydroxypropyl methylcellulose/polycarbophil mucoadhesive blend films using a mixture design approach. Chem. Pharm. Bull. 2017, 65, 284–294. [Google Scholar] [CrossRef] [Green Version]
- Montero-Padilla, S.; Velaga, S.; Morales, J.O. Buccal dosage forms: General considerations for pediatric patients. AAPS PharmSciTech 2017, 18, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Patel, V.; Shah, J. 3D printing technologies: Recent development and emerging applications in various drug delivery systems. AAPS PharmSciTech 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Nicoletta, F.P.; Dalena, F.; Cerchiara, T.; Luppi, B.; Bigucci, F. Bilayered buccal films as child-appropriate dosage form for systemic administration of propranolol. Int. J. Pharm. 2017, 531, 257–265. [Google Scholar] [CrossRef]
- Serrano, D.R.; Fernandez-Garcia, R.; Mele, M.; Healy, A.M.; Lalatsa, A. Designing fast-dissolving orodispersible films of amphotericin B for oropharyngeal candidiasis. Pharmaceutics 2019, 11, 369. [Google Scholar] [CrossRef] [Green Version]
- Rogawski, M.A.; Heller, A.H. Diazepam buccal film for the treatment of acute seizures. Epilepsy Behav. 2019, 101, 106537. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Liu, Y.; Li, W.; Gao, P.; Xiang, D.; Ren, X.; Liu, D. Mucoadhesive buccal film containing ornidazole and dexamethasone for oral ulcers: In vitro and in vivo studies. Pharm. Dev. Technol. 2019, 24, 118–126. [Google Scholar] [CrossRef]
- Alves, T.F.R.; Rios, A.C.; da Silva Pontes, K.; Portella, D.L.; Aranha, N.; Severino, P.; Souto, E.B.; Gonsalves, J.K.M.; de Souza Nunes, R.; Chaud, M.V. Bilayer mucoadhesive buccal film for mucosal ulcers treatment: Development, characterization, and single study case. Pharmaceutics 2020, 12, 657. [Google Scholar] [CrossRef]
- Ammar, H.O.; Ghorab, M.M.; Mahmoud, A.A.; Shahin, H.I. Design and in vitro/in vivo evaluation of ultra-thin mucoadhesive buccal film containing fluticasone propionate. AAPS PharmSciTech 2017, 18, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Bala, R.; Pawar, P.; Khanna, S.; Arora, S. Orally dissolving strips: A new approach to oral drug delivery system. Int. J. Pharm. Investig. 2013, 3, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Montenegro-Nicolini, M.; Miranda, V.; Morales, J.O. Inkjet printing of proteins: An experimental approach. The AAPS journal 2017, 19, 234–243. [Google Scholar] [CrossRef]
- Palem, C.R.; Dudhipala, N.R.; Battu, S.K.; Repka, M.A.; Rao Yamsani, M. Development, optimization and in vivo characterization of domperidone-controlled release hot-melt-extruded films for buccal delivery. Drug Dev. Ind. Pharm. 2016, 42, 473–484. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Alomari, M.; Dodoo, C.C.; Trenfield, S.J.; Velaga, S.; Basit, A.W.; Gaisford, S. Personalisation of warfarin therapy using thermal ink-jet printing. Eur. J. Pharm. Sci. 2018, 117, 80–87. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Selmin, F.; Ortenzi, M.A.; Mohammed, G.K.; Franzé, S.; Minghetti, P.; Cilurzo, F. Personalized orodispersible films by hot melt ram extrusion 3D printing. Int. J. Pharm. 2018, 551, 52–59. [Google Scholar] [CrossRef]
- Ehtezazi, T.; Algellay, M.; Islam, Y.; Roberts, M.; Dempster, N.M.; Sarker, S.D. The application of 3D printing in the formulation of multilayered fast dissolving oral films. J. Pharm. Sci. 2018, 107, 1076–1085. [Google Scholar] [CrossRef]
- Salehi, S.; Boddohi, S. Design and optimization of kollicoat ® IR based mucoadhesive buccal film for co-delivery of rizatriptan benzoate and propranolol hydrochloride. Mater. Sci. Engineering. C Mater. Biol. Appl. 2019, 97, 230–244. [Google Scholar] [CrossRef]
- Repka, M.A.; Prodduturi, S.; Stodghill, S.P. Production and characterization of hot-melt extruded films containing clotrimazole. Drug Dev. Ind. Pharm. 2003, 29, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Alhijjaj, M.; Bouman, J.; Wellner, N.; Belton, P.; Qi, S. Creating drug solubilization compartments via phase separation in multicomponent buccal patches prepared by direct hot melt extrusion-injection molding. Mol. Pharm. 2015, 12, 4349–4362. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Nicolini, M.; Morales, J.O. Overview and future potential of buccal mucoadhesive films as drug delivery systems for biologics. AAPS PharmSciTech 2017, 18, 3–14. [Google Scholar] [CrossRef]
- Mortazavian, E.; Dorkoosh, F.A.; Rafiee-Tehrani, M. Design, characterization and ex vivo evaluation of chitosan film integrating of insulin nanoparticles composed of thiolated chitosan derivative for buccal delivery of insulin. Drug Dev. Ind. Pharm. 2014, 40, 691–698. [Google Scholar] [CrossRef]
- de Barros, J.M.S.; Scherer, T.; Charalampopoulos, D.; Khutoryanskiy, V.V.; Edwards, A.D. A laminated polymer film formulation for enteric delivery of live vaccine and probiotic bacteria. J. Pharm. Sci. 2014, 103, 2022–2032. [Google Scholar] [CrossRef]
- Borges, O.; Borchard, G.; Verhoef, J.C.; de Sousa, A.; Junginger, H.E. Preparation of coated nanoparticles for a new mucosal vaccine delivery system. Int. J. Pharm. 2005, 299, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.N.; Allon, A.; Roni, M.A.; Kouzi, S. Overview and future potential of fast dissolving buccal films as drug delivery system for vaccines. J. Pharm. Pharm. Sci. 2019, 22, 388–406. [Google Scholar] [CrossRef]
- Kraan, H.; Vrieling, H.; Czerkinsky, C.; Jiskoot, W.; Kersten, G.; Amorij, J.P. Buccal and sublingual vaccine delivery. J. Control Release 2014, 190, 580–592. [Google Scholar] [CrossRef] [Green Version]
- Song, J.H.; Nguyen, H.H.; Cuburu, N.; Horimoto, T.; Ko, S.Y.; Park, S.H.; Czerkinsky, C.; Kweon, M.N. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc. Natl. Acad. Sci. USA 2008, 105, 1644–1649. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Zhao, D.; Li, D.; Wang, X.; Jin, Z.; Zhao, K. Polymer-based nanomaterials and applications for vaccines and drugs. Polymers 2018, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Mašek, J.; Lubasová, D.; Lukáč, R.; Turánek-Knotigová, P.; Kulich, P.; Plocková, J.; Mašková, E.; Procházka, L.; Koudelka, Š.; Sasithorn, N.; et al. Multi-layered nanofibrous mucoadhesive films for buccal and sublingual administration of drug-delivery and vaccination nanoparticles—Important step towards effective mucosal vaccines. J. Control Release 2017, 249, 183–195. [Google Scholar] [CrossRef]
- Sun, B.; Xia, T. Nanomaterial-based vaccine adjuvants. J. Mater. Chem. B 2016, 4, 5496–5509. [Google Scholar] [CrossRef]
- Hu, Y.; Hoerle, R.; Ehrich, M.; Zhang, C. Engineering the lipid layer of lipid-PLGA hybrid nanoparticles for enhanced in vitro cellular uptake and improved stability. Acta Biomater. 2015, 28, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Hensley, C.; Zhou, P.; Schnur, S.; Mahsoub, H.M.; Liang, Y.; Wang, M.X.; Page, C.; Yuan, L.; Bronshtein, V. Thermostable, dissolvable buccal film rotavirus vaccine is highly effective in neonatal gnotobiotic pig challenge model. Vaccines 2021, 9, 437. [Google Scholar] [CrossRef]
- Amorij, J.P.; Huckriede, A.; Wilschut, J.; Frijlink, H.W.; Hinrichs, W.L. Development of stable influenza vaccine powder formulations: Challenges and possibilities. Pharm. Res. 2008, 25, 1256–1273. [Google Scholar] [CrossRef] [Green Version]
- Kraisit, P.; Limmatvapirat, S.; Luangtana-Anan, M.; Sriamornsak, P. Buccal administration of mucoadhesive blend films saturated with propranolol loaded nanoparticles. Asian J. Pharm. Sci. 2018, 13, 34–43. [Google Scholar] [CrossRef]
- Al-Dhubiab, B.E.; Nair, A.B.; Kumria, R.; Attimarad, M.; Harsha, S. Development and evaluation of buccal films impregnated with selegiline-loaded nanospheres. Drug Deliv. 2016, 23, 2154–2162. [Google Scholar] [CrossRef] [Green Version]
- Santos, T.C.D.; Rescignano, N.; Boff, L.; Reginatto, F.H.; Simões, C.M.O.; de Campos, A.M.; Mijangos, C.U. Manufacture and characterization of chitosan/PLGA nanoparticles nanocomposite buccal films. Carbohydr. Polym. 2017, 173, 638–644. [Google Scholar] [CrossRef]
- Tzanova, M.M.; Hagesaether, E.; Tho, I. Solid lipid nanoparticle-loaded mucoadhesive buccal films—Critical quality attributes and in vitro safety & efficacy. Int. J. Pharm. 2021, 592, 120100. [Google Scholar] [CrossRef]
- Chen, J.; Duan, H.; Pan, H.; Yang, X.; Pan, W. Two types of core/shell fibers based on carboxymethyl chitosan and sodium carboxymethyl cellulose with self-assembled liposome for buccal delivery of carvedilol across TR146 cell culture and porcine buccal mucosa. Int. J. Biol. Macromol. 2019, 128, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Basahih, T.S.; Alamoudi, A.A.; El-Say, K.M.; Alhakamy, N.A.; Ahmed, O.A.A. Improved transmucosal delivery of glimepiride via unidirectional release buccal film loaded with vitamin E TPGS-based nanocarrier. Dose Response Publ. Int. Hormesis Soc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Zaman, M.; Chaurasiya, V. Polymers used in buccal film: A review. Des. Monomers. Polym. 2015, 18, 105–111. [Google Scholar] [CrossRef]
- Leal, J.; Smyth, H.D.C.; Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 2017, 532, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Hoogstraate, A.; Senel, S.; Cullander, C.; Verhoef, J.; Junginger, H.; Bodde, H. Effects of bile salts on transport rates and routes of FITC-labelled compounds across porcine buccal epithelium in vitro. J. Control Release 1996, 40, 211–221. [Google Scholar] [CrossRef]
- Jacob, S.; Shirwaikar, A.; Srinivasan, K.; Alex, J.; Prabu, S.; Mahalaxmi, R.; Kumar, R. Stability of proteins in aqueous solution and solid state. Indian J. Pharm. Sci. 2006, 68, 154–163. [Google Scholar]
- Al-Nemrawi, N.K.; Alsharif, S.S.; Alzoubi, K.H.; Alkhatib, R.Q. Preparation and characterization of insulin chitosan-nanoparticles loaded in buccal films. Pharm. Dev. Technol. 2019, 24, 967–974. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Alwani, S.; Badea, I. Polymeric nanoparticles in gene therapy: New avenues of design and optimization for delivery applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Mok, H.; Jeong, J.H.; Kim, S.W.; Park, T.G. Comparative evaluation of target-specific GFP gene silencing efficiencies for antisense ODN, synthetic siRNA, and siRNA plasmid complexed with PEI-PEG-FOL conjugate. Bioconjugate Chem. 2006, 17, 241–244. [Google Scholar] [CrossRef]
- Akbari, V.; Rezazadeh, M.; Minayian, M.; Amirian, M.; Moghadas, A.; Talebi, A. Effect of freeze drying on stability, thermo-responsive characteristics, and in vivo wound healing of erythropoietin-loaded trimethyl chitosan/glycerophosphate hydrogel. Res. Pharm. Sci. 2018, 13, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, M.; Jafari, N.; Akbari, V.; Amirian, M.; Tabbakhian, M.; Minaiyan, M.; Rostami, M. A mucoadhesive thermosensitive hydrogel containing erythropoietin as a potential treatment in oral mucositis: In vitro and in vivo studies. Drug Deliv. Transl. Res. 2018, 8, 1226–1237. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Chorilli, M. An overview of polymeric dosage forms in buccal drug delivery: State of art, design of formulations and their in vivo performance evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 86, 129–143. [Google Scholar] [CrossRef] [Green Version]
- SreeHarsha, N.; Hiremath, J.G.; Sarudkar, S.; Attimarad, M.; Al-Dhubiab, B.; Nair, A.B.; Venugopala, K.N.; Asif, A.H. Spray dried amorphous form of simvastatin: Preparation and evaluation of the buccal tablet. Indian J. Pharm. Educ. Res. 2019, 54, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Panda, S. Formulation and evaluation by appling 32 (three squire) factorial design of lercanidipine hydrochloride buccal tablets with mucoadhesive polymers. Indian J. Pharm. Educ. Res. 2020, 54, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Zeng, N.; Seguin, J.; Destruel, P.L.; Dumortier, G.; Maury, M.; Dhotel, H.; Bessodes, M.; Scherman, D.; Mignet, N.; Boudy, V. Cyanine derivative as a suitable marker for thermosensitive in situ gelling delivery systems: In vitro and in vivo validation of a sustained buccal drug delivery. Int. J. Pharm. 2017, 534, 128–135. [Google Scholar] [CrossRef]
- Sharpe, L.A.; Daily, A.M.; Horava, S.D.; Peppas, N.A. Therapeutic applications of hydrogels in oral drug delivery. Expert Opin. Drug Deliv. 2014, 11, 901–915. [Google Scholar] [CrossRef] [Green Version]
- Hibbins, A.R.; Kumar, P.; Choonara, Y.E.; Kondiah, P.P.D.; Marimuthu, T.; Du Toit, L.C.; Pillay, V. Design of a versatile pH-responsive hydrogel for potential oral delivery of gastric-sensitive bioactives. Polymers 2017, 9, 474. [Google Scholar] [CrossRef] [Green Version]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Al-Dhubiab, B.E.; Nair, A.B.; Kumria, R.; Attimarad, M.; Harsha, S. Formulation and evaluation of nano based drug delivery system for the buccal delivery of acyclovir. Colloids Surf. B Biointerfaces 2015, 136, 878–884. [Google Scholar] [CrossRef]
- Nair, A.B.; Shah, J.; Al-Dhubiab, B.E.; Jacob, S.; Patel, S.S.; Venugopala, K.N.; Morsy, M.A.; Gupta, S.; Attimarad, M.; Sreeharsha, N.; et al. Clarithromycin solid lipid nanoparticles for topical ocular therapy: Optimization, evaluation and in vivo studies. Pharmaceutics 2021, 13, 523. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid lipid nanoparticles and nanostructured lipid carriers: Structure, preparation and application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd El Azim, H.; Nafee, N.; Ramadan, A.; Khalafallah, N. Liposomal buccal mucoadhesive film for improved delivery and permeation of water-soluble vitamins. Int. J. Pharm. 2015, 488, 78–85. [Google Scholar] [CrossRef]
- Chen, J.; Pan, H.; Yang, Y.; Xiong, S.; Duan, H.; Yang, X.; Pan, W. Self-assembled liposome from multi-layered fibrous mucoadhesive membrane for buccal delivery of drugs having high first-pass metabolism. Int. J. Pharm. 2018, 547, 303–314. [Google Scholar] [CrossRef]
- El-Samaligy, M.S.; Afifi, N.N.; Mahmoud, E.A. Increasing bioavailability of silymarin using a buccal liposomal delivery system: Preparation and experimental design investigation. Int. J. Pharm. 2006, 308, 140–148. [Google Scholar] [CrossRef]
- Bashyal, S.; Seo, J.E.; Keum, T.; Noh, G.; Choi, Y.W.; Lee, S. Facilitated permeation of insulin across TR146 cells by cholic acid derivatives-modified elastic bilosomes. Int. J. Nanomed. 2018, 13, 5173–5186. [Google Scholar] [CrossRef] [Green Version]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [Green Version]
- Hazzah, H.A.; Farid, R.M.; Nasra, M.M.; Zakaria, M.; Gawish, Y.; El-Massik, M.A.; Abdallah, O.Y. A new approach for treatment of precancerous lesions with curcumin solid-lipid nanoparticle-loaded gels: In vitro and clinical evaluation. Drug Deliv. 2016, 23, 1409–1419. [Google Scholar] [CrossRef]
- Hazzah, H.A.; Farid, R.M.; Nasra, M.M.; El-Massik, M.A.; Abdallah, O.Y. Lyophilized sponges loaded with curcumin solid lipid nanoparticles for buccal delivery: Development and characterization. Int. J. Pharm. 2015, 492, 248–257. [Google Scholar] [CrossRef]
- Portero, A.; Teijeiro-Osorio, D.; Alonso, M.J.; Remuñán-López, C. Development of chitosan sponges for buccal administration of insulin. Carbohydr. Polym. 2007, 68, 617–625. [Google Scholar] [CrossRef]
- Kassem, M.A.; ElMeshad, A.N.; Fares, A.R. Lyophilized sustained release mucoadhesive chitosan sponges for buccal buspirone hydrochloride delivery: Formulation and in vitro evaluation. AAPS PharmSciTech 2015, 16, 537–547. [Google Scholar] [CrossRef]
- Lv, Q.; Shen, C.; Li, X.; Shen, B.; Yu, C.; Xu, P.; Xu, H.; Han, J.; Yuan, H. Mucoadhesive buccal films containing phospholipid-bile salts-mixed micelles as an effective carrier for cucurbitacin B delivery. Drug Deliv. 2015, 22, 351–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, E.; Ojewole, E.; Kalhapure, R.; Govender, T. In vitro comparative evaluation of monolayered multipolymeric films embedded with didanosine-loaded solid lipid nanoparticles: A potential buccal drug delivery system for ARV therapy. Drug Dev. Ind. Pharm. 2014, 40, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Kraisit, P.; Hirun, N.; Mahadlek, J.; Limmatvapirat, S. Fluconazole-loaded solid lipid nanoparticles (SLNs) as a potential carrier for buccal drug delivery of oral candidiasis treatment using the Box-Behnken design. J. Drug Deliv. Sci. Technol. 2021, 63, 102437. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; D’Ambrosio, M.; Luceri, C.; Cirri, M. Evaluation and comparison of solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) as vectors to develop hydrochlorothiazide effective and safe pediatric oral liquid formulations. Pharmaceutics 2021, 13, 437. [Google Scholar] [CrossRef]
- Kraisit, P.; Sarisuta, N. Development of triamcinolone acetonide-loaded nanostructured lipid carriers (NLCs) for buccal drug delivery using the box-behnken design. Molecules 2018, 23, 982. [Google Scholar] [CrossRef] [Green Version]
- Tetyczka, C.; Griesbacher, M.; Absenger-Novak, M.; Fröhlich, E.; Roblegg, E. Development of nanostructured lipid carriers for intraoral delivery of Domperidone. Int. J. Pharm. 2017, 526, 188–198. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Qu, L.; Wu, H.; Kong, H.; Yang, Z.; Chen, D.; Mäkilä, E.; Salonen, J.; Santos, H.A.; et al. Gold nanorods conjugated porous silicon nanoparticles encapsulated in calcium alginate nano hydrogels using microemulsion templates. Nano Lett. 2018, 18, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Richter, K.; Nguyen, T.H.; Boyd, B.J.; Porter, C.J.; Tan, A.; Prestidge, C.A. Pluronic-functionalized silica-lipid hybrid microparticles: Improving the oral delivery of poorly water-soluble weak bases. Mol. Pharm. 2015, 12, 4424–4433. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 2020, 24, 3. [Google Scholar] [CrossRef] [Green Version]
- Rana, P.; Murthy, R.S. Formulation and evaluation of mucoadhesive buccal films impregnated with carvedilol nanosuspension: A potential approach for delivery of drugs having high first-pass metabolism. Drug Deliv. 2013, 20, 224–235. [Google Scholar] [CrossRef] [Green Version]
- Pornpitchanarong, C.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T.; Patrojanasophon, P. Clotrimazole nanosuspensions-loaded hyaluronic acid-catechol/polyvinyl alcohol mucoadhesive films for oral candidiasis treatment. J. Drug Deliv. Sci. Technol. 2020, 60, 101927. [Google Scholar] [CrossRef]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al-Dhubiab, B.E.; Alhaider, I.A. In vitro techniques to evaluate buccal films. J. Control Release 2013, 166, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Morsy, M.A.; Jacob, S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev. Res. 2018, 79, 373–382. [Google Scholar] [CrossRef]
- Berben, P.; Bauer-Brandl, A.; Brandl, M.; Faller, B.; Flaten, G.E.; Jacobsen, A.C.; Brouwers, J.; Augustijns, P. Drug permeability profiling using cell-free permeation tools: Overview and applications. Eur. J. Pharm. Sci. 2018, 119, 219–233. [Google Scholar] [CrossRef]
- Bodini, R.B.; Guimarães, J.d.G.L.; Monaco-Lourenço, C.A.; Aparecida de Carvalho, R. Effect of starch and hydroxypropyl methylcellulose polymers on the properties of orally disintegrating films. J. Drug Deliv. Sci. Technol. 2019, 51, 403–410. [Google Scholar] [CrossRef]
- Preis, M.; Knop, K.; Breitkreutz, J. Mechanical strength test for orodispersible and buccal films. Int. J. Pharm. 2014, 461, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Walicová, V.; Gajdziok, J.; Pavloková, S.; Vetchý, D. Design and evaluation of mucoadhesive oral films containing sodium hyaluronate using multivariate data analysis. Pharm. Dev. Technol. 2017, 22, 229–236. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Ritzoulis, C.; Bouropoulos, N.; Tzetzis, D.; Andreadis, D.A.; Boetker, J.; Rantanen, J.; Fatouros, D.G. Unidirectional drug release from 3D printed mucoadhesive buccal films using FDM technology: In vitro and ex vivo evaluation. Eur. J. Pharm. Biopharm. 2019, 144, 180–192. [Google Scholar] [CrossRef]
- Kumria, R.; Nair, A.B.; Al-Dhubiab, B.E. Loratidine buccal films for allergic rhinitis: Development and evaluation. Drug Dev. Ind. Pharm. 2014, 40, 625–631. [Google Scholar] [CrossRef]
- Kumria, R.; Nair, A.B.; Goomber, G.; Gupta, S. Buccal films of prednisolone with enhanced bioavailability. Drug Deliv. 2016, 23, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Al-Dhubiab, B.E.; Nair, A.B.; Kumria, R.; Attimarad, M.; Harsha, S. Development and evaluation of nebivolol hydrochloride nanocrystals impregnated buccal film. Farmacia 2019, 67, 282–289. [Google Scholar] [CrossRef]
- Nair, A.B.; Al-ghannam, A.A.; Al-Dhubiab, B.E.; Hasan, A.A. Mucoadhesive film embedded with acyclovir loaded biopolymeric nanoparticles: In vitro studies. J. Young Pharm. 2017, 9, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Maher, E.M.; Ali, A.M.; Salem, H.F.; Abdelrahman, A.A. In vitro/in vivo evaluation of an optimized fast dissolving oral film containing olanzapine co-amorphous dispersion with selected carboxylic acids. Drug Deliv. 2016, 23, 3088–3100. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Jacob, S.; Saraiya, V.; Attimarad, M.; SreeHarsha, N.; Akrawi, S.H.; Shehata, T.M. Mucoadhesive buccal film of almotriptan improved therapeutic delivery in rabbit model. Saudi Pharm. J. SPJ 2020, 28, 201–209. [Google Scholar] [CrossRef]
- Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Vimal, P.; Attimarad, M.; Harsha, S. Development and evaluation of palonosetron loaded mucoadhesive buccal films. J. Drug Deliv. Sci. Technol. 2018, 47, 351–358. [Google Scholar] [CrossRef]
- Kumria, R.; Al-Dhubiab, B.E.; Shah, J.; Nair, A.B. Formulation and evaluation of chitosan-based buccal bioadhesive films of Zolmitriptan. J. Pharm. Innov. 2018, 13, 133–143. [Google Scholar] [CrossRef]
- Jug, M.; Hafner, A.; Lovrić, J.; Kregar, M.L.; Pepić, I.; Vanić, Ž.; Cetina-Čižmek, B.; Filipović-Grčić, J. An overview of in vitro dissolution/release methods for novel mucosal drug delivery systems. J. Pharm. Biomed. Anal. 2018, 147, 350–366. [Google Scholar] [CrossRef]
- Lin, G.C.; Leitgeb, T.; Vladetic, A.; Friedl, H.P.; Rhodes, N.; Rossi, A.; Roblegg, E.; Neuhaus, W. Optimization of an oral mucosa in vitro model based on cell line TR146. Tissue Barriers 2020, 8, 1748459. [Google Scholar] [CrossRef] [Green Version]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Bhagurkar, A.M.; Darji, M.; Lakhani, P.; Thipsay, P.; Bandari, S.; Repka, M.A. Effects of formulation composition on the characteristics of mucoadhesive films prepared by hot-melt extrusion technology. J. Pharm. Pharmacol. 2019, 71, 293–305. [Google Scholar] [CrossRef]
- Montenegro-Nicolini, M.; Reyes, P.E.; Jara, M.O.; Vuddanda, P.R.; Neira-Carrillo, A.; Butto, N.; Velaga, S.; Morales, J.O. The effect of inkjet printing over polymeric films as potential buccal biologics delivery systems. AAPS PharmSciTech 2018, 19, 3376–3387. [Google Scholar] [CrossRef]
- Morales, J.O.; McConville, J.T. Manufacture and characterization of mucoadhesive buccal films. Eur. J. Pharm. Biopharm. 2011, 77, 187–199. [Google Scholar] [CrossRef]
- Irfan, M.; Rabel, S.; Bukhtar, Q.; Qadir, M.I.; Jabeen, F.; Khan, A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm. J. SPJ 2016, 24, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Perumal, V.A.; Govender, T.; Lutchman, D.; Mackraj, I. Investigating a new approach to film casting for enhanced drug content uniformity in polymeric films. Drug Dev. Ind. Pharm. 2008, 34, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.C.; Morales, J.O. Buccal delivery of nanoparticles. Mucosal Deliv. Drugs Biol. Nanoparticles 2020, 41, 107. [Google Scholar]
- Barnhart, S.D. Thin film oral dosage forms. In Modified-Release Drug Delivery Technology; CRC Press: Boca Raton, FL, USA, 2008; pp. 235–256. [Google Scholar]
- Morales, J.O.; Brayden, D.J. Buccal delivery of small molecules and biologics: Of mucoadhesive polymers, films, and nanoparticles. Curr. Opin. Pharmacol. 2017, 36, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Caffarel-Salvador, E.; Kim, S.; Soares, V.; Tian, R.Y.; Stern, S.R.; Minahan, D.; Yona, R.; Lu, X.; Zakaria, F.R.; Collins, J.; et al. A microneedle platform for buccal macromolecule delivery. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef]
- Oh, Y.J.; Cha, H.R.; Hwang, S.J.; Kim, D.S.; Choi, Y.J.; Kim, Y.S.; Shin, Y.R.; Nguyen, T.T.; Choi, S.O.; Lee, J.M.; et al. Ovalbumin and cholera toxin delivery to buccal mucus for immunization using microneedles and comparison of immunological response to transmucosal delivery. Drug Deliv. Transl. Res. 2021, 11, 1390–1400. [Google Scholar] [CrossRef]






| Category | Examples | Transport Mechanism | Key Findings | References |
|---|---|---|---|---|
| Surfactants | Anionic: Sodium lauryl sulfate, sodium dodecanoate Cationic: Cetylpyridinium chloride | Disruption of intercellular lipids and integrity of protein Increase water solubility of drugs | Mucosal lipids might be extracted above critical micelle concentration therefore reducing the barrier properties of buccal mucosa | [58,59,60,61] |
| Non-ionic: Polyoxyethylene-9-lauryl ether, nonylphenoxy poly oxyethylene, polysorbates (Tweens), sorbitan fatty acid esters (Spans), macrogol ethers (Brijs), macrogol esters (Myrjs) | Hydrophobic interaction between surfactant and keratin fibrils causes swelling of epithelium | |||
| Bile salts: Sodium taurocholate, sodium cholate, sodium deoxycholate, sodium taurodihydrofusidate, sodium taurodeoxycholate | Penetration into intercellular regions, increase fluidity, solubilization and extraction of lipids Interaction with keratin leads to disruption of corneocytes | |||
| Fatty acids and their esters | Capric acid, caprylic acid, lauric acid, linoleic acid, linolenic acid, oleic acid, 2-octyldodecyl myristate, 1-[(N,N-dimethylamino)propan-2-yl]dodecanoate) | Interact with phospholipid domain and increase the membrane fluidity | A parabolic correlation observed between fatty acid lipophilicity and permeation enhancement Ability to diffuse through mucosa and interact with the lipid region is determined by fatty acid chain length Improve paracellular bioabsorption through transient opening of tight junctions | [62,63] |
| Cyclodextrins | α,β,γ cyclodextrins, methylated cyclodextrins | Disruption of intercellular lipids and integrity of protein | Molecular inclusion complex resulting in solubilization, lipid extraction and increasing buccal absorption | [64] |
| Polymers | Cationic: Chitosan, trimethyl chitosan, poly-L-arginine, L-lysine | Ionic interaction with negatively charged carboxyl and sulfate groups on mucin | Enhancement effect may be due to increasing the retention of the drug at the mucosal surface, which decrease the clearance of the drug by salivary flow Cationic cell penetrating peptide permit its interaction with anionic motifs on the mucin by a receptor-independent mechanism thus overcoming cell membrane impermeability and cellular internalization of actives | [65] |
| Chelating agents | Ethylenediaminetetraacetic acid, polyacrylate, citric acid, salicylates | The chelators form complexes with Ca2+ ions | Probably widen the gap between the cells and consequently facilitate paracellular transport of particularly, hydrophilic drugs | [66] |
| Miscellaneous | Azone (1-dodecylazacycloheptan-2-one) | Disrupts the lipid bilayers and increases the fluidity and permeation in the lipid regions of the biological barrier | Efficacy strongly dependent on its concentration (1–5%) and is also influenced by the choice of vehicle from which it is applied Effective for both hydrophilic and lipophilic drugs in polar medium | [67] |
| Type | Polymer Constituents | Drugs Used | Manufacturing Method | Highlights | References |
|---|---|---|---|---|---|
| Controlled release | Carbopol, hydroxypropyl methylcellulose (HPMC), poloxamer and compritol 888 ATO | Lidocaine | Solvent casting | Free lidocaine and/or microspheres loaded patch fabricated using HPMC/carbopol and poloxamer Lidocaine microspheres prepared from Compritol 888 ATO employing spray congealing technique Change in formulation composition demonstrated to change the drug release mechanisms and able to provide either rapid, delayed or prolonged local anesthetic activity | [83] |
| Sustained release | Sodium alginate, HPMC, sodium carboxymethyl cellulose (NaCMC) and carbopol | Atenolol | Solvent casting | Patch prepared from sodium alginate Ex vivo permeation studies across goat buccal mucosa revealed 70.17 ± 2.28% release over a period of 24 h with maximum permeation flux (30.83 ± 1.23 μg/cm2/h) and minimum lag time (0.95 ± 0.22 h) Polymers used could provide sustained release of atenolol across porcine buccal mucosa for 24 h | [84] |
| Modified release | Xanthan gum, polyvinyl alcohol (PVA) and HPMC E-15 | Zolmitriptan | Solvent casting | Bilayer patch prepared from xanthan gum In vitro drug release studies showed rapid drug release; 43.15% within 15 min, followed by sustained release rate over 5 h Incorporation of 4% dimethyl sulfoxide demonstrated 3.29-fold drug permeation, transported 29.10% of drug after 5 h | [85] |
| Immediate release | HPMC, PVA, polyvinylpyrrolidone and ethyl cellulose | Carbamazepine | Solvent casting | Water impermeable polypropylene backing layer provided unidirectional drug release Due to high water uptake, PEG 400 containing batches showed maximum in vitro release and increased mucoadhesion Drug release was controlled by either diffusion or non-Fickian diffusion | [86] |
| Peptide delivery | Chitosan, choline and geranic acid | Insulin | Solvent casting | Viscous gel made of choline and geranic acid sandwiched between two layers of chitosan Significant increase (7-fold) in the cumulative insulin transport across the ex vivo porcine buccal tissue was demonstrated (~26% of loaded insulin) In vivo studies in rat buccal pouch lowered blood glucose levels up to 50% in a dose dependent manner Serum insulin plateaued after 3 h for the duration of the study | [87] |
| Therapeutic Classification | Polymer/Plasticizer | Active Ingredient | Manufacturing Method | Comments | References |
|---|---|---|---|---|---|
| Antihypertensive | Chitosan, polyvinylpyrrolidone, PVA, gelatin/propylene glycol | Propranolol HCl | Solvent casting | Personalized bilayered buccal film useful for pediatric population | [95] |
| Antifungal | Dextran, maltodextrin, HPMC, HPC/PEG 400 and glycerol | Amphotericin B | Solvent casting | Mechanical strength of the film was contributed by Avicel 200 and Avicel CL611 Physically stable orodispersible film was effective in oropharyngeal candidiasis | [96] |
| Antiepileptic | HPMC | Diazepam | * | Soluble film formulation of diazepam (Libervant™) effective in acute seizure emergencies Dose can be adjusted by cutting the film of suitable size | [97] |
| Antiprotozoal/anti-inflammatory | HPMC, PVA, chitosan/glycerin | Ornidazole and dexamethasone sodium phosphate | Solvent casting | Double layered film demonstrated >95% drug release in 4 h Significant effect on mucosal repair and reduced ulcer inflammation | [98] |
| Anesthetic/analgesic and anti-inflammatory/ mucolytic | HPMC, NaCMC, Chitosan/propylene glycol and sorbitol | Lidocaine HCl, benzydamine HCl, N-acetyl-cysteine | Solvent casting | Biocompatible bilayered mucoadhesive film stimulates cell proliferation and demonstrated therapeutic effect in buccal mucositis | [99] |
| Anti-inflammatory | HPMC, ethyl cellulose, chitosan, NaCMC, carbopol 971P/propylene glycol, PEG 8000 | Fluticasone propionate | Solvent casting | Optimized formulation exhibited sustained drug release for 10 h Enhanced pharmacokinetic parameters was demonstrated compared to equivalent dose of mouthwash | [100] |
| Types of Nanoparticles | Nanoparticle Composition | Method | Polymers/Drug | Outcome | Key Points | References |
|---|---|---|---|---|---|---|
| Nanospheres | Poly (lactic-co-glycolic acid) | Double-emulsion solvent evaporation | HPMC K15 and Eudragit RS 100/selegiline | Potential to prolong retention, provide controlled release, enhance bioavailability | Buccal film fabricated from HPMC and eudragit embedded with poly (lac-tic-co-glycolic acid) nanospheres Permeation rate of selegiline mainly influenced by the film composition used The overall mean value of AUC0-α (2935.65 ± 194.24 ng.h/mL) from buccal film was found to be ~3 fold higher (p < 0.0001) as compared to oral solution | [124] |
| Nanoparticles | Poly (lactic-co-glycolic acid) | Double-emulsion solvent evaporation | Chitosan/ C-glycosyl flavonoid fraction of Cecropia glaziovii | Capacity to overcome low bioavailability of flavonoid extract | Dynamic mechanical analysis tests indicated that increasing of nanoparticles concentration caused decreased stiffness and an increased glass transition temperature Cytotoxic assay results indicated that these systems showed no cytotoxicity | [125] |
| Solid-lipid nanoparticles | Lipoid S100 and polysorbate 80 | Solvent injection | HPMC/coumarin 6 | Could be used for poorly aqueous soluble drugs | Lipid nanoparticles improved the cellular permeability through mucosal epithelial cells The quality of the solid-lipid nanoparticles loaded film and placebo mucoadhesive film were same | [126] |
| Liposomes | Polyvinylpyrrolidone | Electron spinning | Na CMC and chitosan/carvedilol | Initial burst release avoided with positive effect on permeation | Coaxial fibers-based self-assembling liposomes formed Demonstrated significant permeation across porcine TR146 cell culture and porcine buccal mucosa Cytotoxicity assay indicated absence of any toxicity caused by the fibers | [127] |
| Nanolipid structures | D-α-tocopherol PEG 1000 succinate, almond oil, compritol, phosphatidylcholine, gelucire 44/14 | Hot emulsification–ultrasonication technique | Carbopol 934 and HPMC/glimepiride | Suitability to transport across buccal mucosa in sustained release manner | Selected concentration of micelles to nanostructured lipid carriers, carbopol and sodium cholate were 100%, 0.05% and 1.8%, respectively using a Box-Behnken design Optimized mucoadhesive film with a backing layer of ethyl cellulose demonstrated unidirectional glimepiride release of 93.9% at 6 h | [128] |
| Technique | Principle | Evaluation Parameters | Ranges Units | References |
|---|---|---|---|---|
| Tensile test | The resistance of the thin strip of film against a dragging force is determined using a texture analyzer or modified balance method. Young modulus measures the deformation tendency of the film | Tensile strength = breaking force (N)/cross-sectional area (cm2) of the film The slope value from stress strain curve measures the Young modulus Percentage at the break, strain energy, energy to break can be calculated | 16.6–24.3 MPa | [169,172] |
| Puncture test | The resistance of the thin film against the compression force until it breaks, cracks, or a desired loss in the force resisting the probe movement occurs | Toughness | 0.2–13 mJ | [173,174] |
| Indentation test | Measure load as a function of penetration depth | Hardness and elastic modulus | 1 mPa and ~100 mPa | [175] |
| Folding endurance | Repeatedly fold the film at 180° angle of the plane at the same plane until it breaks or folded to 300 times without breaking. The number of times the film is folded without breaking is computed as the folding endurance value | Flexibility | ~300 count | [176] |
| Water absorption capacity | Swelling capacity assess bioadhesion behavior and drug release from the film | Percentage hydration is calculated by the equation [(W2 − W1) × 100/W1], where W1 weight of the film, W2 weight of the film after swelling in simulated saliva after predetermined time | 5–25% | [177] |
| Thickness and weight variation | Thickness is determined using electronic digital micrometer, screw gauge, vernier caliper or by scanning electron microscopy images. Weight variation is calculated by subtracting weight of individual film from average weight and then divided by average weight of the film | Uniformity of the dose in the film | 50–1000 μm and <50 mg | [169] |
| Surface morphology | Fixing the films on stubs, sputter coated with gold in an inert environment and imaged | Surface texture, pores, crystallinity, uniformity of drug distribution, thickness | - | [178] |
| Surface pH | Allowing it to swell by contact with distilled water for a short time (<2 h) at room temperature (25 °C) | pH at the area of application | 6.0–7.5 | [179] |
| Crystallinity | Place the sample in the sample holder of X-ray diffractometer and scan | Presence of crystalline or amorphous form of the sample | % | [180] |
| Thermal analysis | Heating the sample in aluminum pan at elevated temperature at uniform heating rate | Identify the existence of phase transition, recrystallization or molecular interaction of drug within the film | °C | [181] |
| Fourier-transform infrared spectroscopy | Specific ratio of drug and potassium bromide compressed at particular pressure and scanned | Drug-polymer interaction | cm−1 | [182] |
| Mucoadhesive strength | Buccal film is attached to the probe of the texture analyzer using cyanoacrylate adhesive. Buccal epithelium of rabbit is fixed on the stationary platform of a texture analyzer. The probe of the texture analyzer was brought down gradually till the probe touch the mucosa | Adhesion strength is evaluated using shear stress, peel strength and tensile strength depending on the direction in which the mucoadhesive material is detached from the biological surface | 6–7 N | [183] |
| In vitro drug release | Paddle over disc method using USPXXIV Type 2 apparatus | Release of drug from the prepared film using simulated saliva (pH 6.2) | % | [184] |
| Ex vivo permeation | Freshly excised buccal mucosa of rabbit using Franz diffusion cell, continuous flow diffusion cell, Ussing chamber, human buccal cell line (TR146), cell culture model | Establishing the absorption of drug across buccal epithelium by means of flux (J) and permeability coefficient (P) | J = μg/cm2/h P = cm/h | [17,181,185] |
| Clinical Trials | Indication | Phase | Enrolment | Identifier |
|---|---|---|---|---|
| Buccal prochlorperazine (6 mg) plus 2 cc normal saline versus intravenous prochlorperazine (10 mg) 2 cc plus two saccharin absorbable placebo tablets | Migraine disorders | Phase III | 80 | NCT02779959 |
| Diazepam buccal film (10 mg–17.5 mg based on body weight) administered on inner aspect of the following a low or high fatty meal versus diastat rectal gel (10 mg–20 mg based on body weight) following a moderate fatty meal | Epilepsy | Phase I and Phase II | 31 | NCT03953820 |
| Palonosetron hydrochloride buccal film (0.25 mg and 0.5 mg) versus palonosetron hydrochloride, 0.25 mg/5 mL intravenous solution | Nausea with vomiting chemotherapy-induced | Phase II | 22 | NCT04592198 |
| Montelukast buccal film, administered 10 mg once or 30 mg twice daily versus placebo buccal film administered once or twice daily | Alzheimer’s disease | Phase II | 70 | NCT03402503 |
| A comparison of sublingual and buccal misoprostol regimens after mifepristone for mid-trimester abortion | Legally induced abortion | Phase IV | 320 | NCT02708446 |
| Pharmacokinetic and pharmacodynamic study of three different doses (0.5 µg/kg, 0.75 µg/kg, and 1 µg/kg) of oral transmucosal dexmedetomidine | Sedation | Phase II and Phase III | 36 | NCT03120247 |
| Single dose crossover study to compare the respiratory drive after administration of belbuca (300 μg, 600 μg and 900 μg), oxycodone (30 mg and 60 mg) and placebo | Respiratory depression | Phase 1 | 19 | NCT03996694 |
| Safety and efficacy study of NH004 films (intra oral) with tropicamide at different dose (0.3 mg,1 mg and 3 mg) for relief of sialorrhea symptoms in Parkinson’s disease patients versus placebo | Sialorrhea in Parkinson’s disease | Phase II | 19 | NCT00761137 |
| A double-blind, placebo-controlled evaluation of the efficacy, safety and tolerability of BEMA™ fentanyl (bioerodible mucoadhesive soluble fentanyl citrate film) in the treatment of breakthrough pain in cancer subjects | Breakthrough pain in cancer | Phase III | 152 | NCT00293033 |
| Long-term open-label safety study to evaluate EN3409 (BEMA® Buprenorphine buccal film) at doses 300–900 μg | Low back pain, osteoarthritis, neuropathic pain | Phase III | 303 | NCT01755546 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacob, S.; Nair, A.B.; Boddu, S.H.S.; Gorain, B.; Sreeharsha, N.; Shah, J. An Updated Overview of the Emerging Role of Patch and Film-Based Buccal Delivery Systems. Pharmaceutics 2021, 13, 1206. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13081206
Jacob S, Nair AB, Boddu SHS, Gorain B, Sreeharsha N, Shah J. An Updated Overview of the Emerging Role of Patch and Film-Based Buccal Delivery Systems. Pharmaceutics. 2021; 13(8):1206. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13081206
Chicago/Turabian StyleJacob, Shery, Anroop B. Nair, Sai H. S. Boddu, Bapi Gorain, Nagaraja Sreeharsha, and Jigar Shah. 2021. "An Updated Overview of the Emerging Role of Patch and Film-Based Buccal Delivery Systems" Pharmaceutics 13, no. 8: 1206. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13081206