Improved Bioavailability of Ebastine through Development of Transfersomal Oral Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
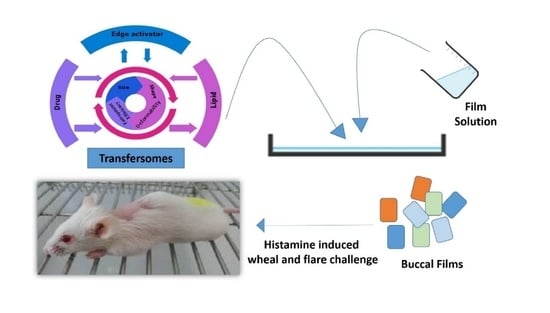
2.2. Preparation of Transfersomes
2.3. Preparation of TOFs
2.4. Characterization of Transfersomes
2.5. Deformability Studies
2.6. Entrapment Efficiency (EE%)
2.7. Characterization of TOFs
2.7.1. Viscosity Measurement of Film Solutions
2.7.2. Film Thickness and Weight
2.7.3. Surface pH
2.7.4. Folding Endurance
2.7.5. Tensile Strength and % of Elongation of TOFs
2.7.6. Moisture Content of TOFs
2.7.7. Content Uniformity
2.7.8. Reconstitution of TOFs
2.7.9. Fourier-Transform Infrared Spectroscopy (FTIR)
2.7.10. Powder X-ray Diffraction (PXRD)
2.7.11. Differential Scanning Calorimetry (DSC)
2.7.12. Scanning Electron Microscopy (SEM)
2.7.13. Atomic Force Microscopy (AFM)
2.7.14. In Vitro Drug Release
2.7.15. Ex Vivo Permeation
2.7.16. Compliance with Ethical Standards
2.7.17. In Vivo Pharmacokinetic Study
2.7.18. Extraction of EBT and Carebestine from Plasma
2.7.19. HPLC Analysis and Pharmacokinetic Parameters
2.7.20. In Vivo Pharmacodynamics (Histamine Induced Wheal and Flare Challenge)
2.8. Statistical Analysis
3. Results
3.1. Physicochemical Characterizations
3.2. Characterization of TOFs
3.3. Reconstitution of TOFs Containing Transfersomes
3.4. Fourier-Transform Infrared Spectroscopy (FTIR)
3.5. Morphological Evaluations Using SEM
3.6. Atomic Force Microscopy (AFM)
3.7. Powder X-ray Diffraction (PXRD)
3.8. Differential Scanning Calorimetry (DSC)
3.9. In Vitro Drug Release Studies
3.10. Ex Vivo Permeation Studies
3.11. In Vivo Pharmacokinetics
3.12. In Vivo Pharmacodynamics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonijoan, R.; García-Gea, C.; Puntes, M.; Pérez, J.; Esbrí, R.; Serra, C.; Fortea, J.; Barbanoj, M.J. Comparison of inhibition of cutaneous histamine reaction of ebastine fast-dissolving tablet (20 mg) versus desloratadine capsule (5 mg): A randomized, double-blind, double-dummy, placebo-controlled, three-period crossover study in healthy, nonatopic adults. Clin. Ther. 2007, 29, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Dierick, B.J.; van der Molen, T.; Flokstra-de Blok, B.M.; Muraro, A.; Postma, M.J.; Kocks, J.W.; van Boven, J.F. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev. Pharm. Outcomes Res. 2020, 20, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J. Ebastine in the treatment of allergic rhinitis and urticaria: 30 years of clinical studies and real-world experience. J. Investig. Allergol. Clin. Immunol. 2020, 30, 156–168. [Google Scholar] [CrossRef] [Green Version]
- Gispert, J.; Antonijoan, R.; Barbanoj, M.; Gich, I.; Garcia, E.; Esbrí, R.; Luria, X. Efficacy of ebastine, cetirizine, and loratadine in histamine cutaneous challenges. Ann. Allergy Asthma Immunol. 2002, 89, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Islam, N.; Zahoor, A.F.; Syed, H.K.; Iqbal, M.S.; Khan, I.U.; Abbas, G.; Mushtaq, M.; Rehman, M.U.; Rasul, A.; Ikram, M. Improvement of solubility and dissolution of ebastine by fabricating phosphatidylcholine/bile salt bilosomes. Pak. J. Pharm. Sci 2020, 33, 2301–2306. [Google Scholar]
- Wouters, M.M.; Balemans, D.; Van Wanrooy, S.; Dooley, J.; Cibert-Goton, V.; Alpizar, Y.A.; Valdez-Morales, E.E.; Nasser, Y.; Van Veldhoven, P.P.; Vanbrabant, W. Histamine receptor H1–mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology 2016, 150, 875–887.e9. [Google Scholar] [CrossRef] [Green Version]
- Rico, S.; Antonijoan, R.; Barbanoj, M. Ebastine in the light of CONGA recommendations for the development of third-generation antihistamines. J. Asthma Allergy 2009, 2, 73. [Google Scholar]
- Mahajan, V.R.; Basarkar, G.D. Formulation, characterization, and in vitro-in vivo evaluation of self microemulsifying drug delivery system of Ebastine by spray drying technology using solid carriers. Thai J. Pharm. Sci. 2019, 43, 146–160. [Google Scholar]
- Kamisetti, R.R.; Gupta, V. Solubility enhancement of ebastine by self-nanoemulsifying delivery strategy: Formulation, optimization and characterization. Int. J. Pharmcutical Sci. Nanotechnol. 2017, 10, 3779–3787. [Google Scholar]
- Mehetre, J.; Vimal, K.; Mehta, T.; Gohel, M.; Surti, N. Rationalized Approach for Formulation and Optimization of Ebastine Microemulsion Using Design Expert for Solubility Enhancement. J. Drug Deliv. Ther. 2019, 9, 386–397. [Google Scholar]
- Harmalkar, D.; Godinho, S.; Bhide, P.J.; Kumar, L.; Shirodkar, R.K. New Formulation Technique for Solubility and Dissolution Rate Enhancement of Poorly Soluble Drugs. Pharm. Chem. J. 2019, 53, 720–729. [Google Scholar] [CrossRef]
- Banala, N.; Peddapalli, H.; Dudhipala, N.; Chinnala, K.M. Transmucosal Delivery of Duloxetine Hydrochloride for Prolonged Release: Preparation, in vitro, ex vivo Characteri-zation and in vitro-ex vivo Correlation. Int. J. Pharm. Sci. Nanotechnol. 2018, 11, 4249–4258. [Google Scholar] [CrossRef]
- Singh, R.P.; Gangadharappa, H.; Mruthunjaya, K. Phospholipids: Unique carriers for drug delivery systems. J. Drug Deliv. Sci. Technol. 2017, 39, 166–179. [Google Scholar] [CrossRef]
- Perez, A.P.; Altube, M.J.; Schilrreff, P.; Apezteguia, G.; Celes, F.S.; Zacchino, S.; de Oliveira, C.I.; Romero, E.L.; Morilla, M.J. Topical amphotericin B in ultradeformable liposomes: Formulation, skin penetration study, antifungal and antileishmanial activity in vitro. Colloids Surf. B Biointerfaces 2016, 139, 190–198. [Google Scholar] [CrossRef]
- El Zaafarany, G.M.; Awad, G.A.; Holayel, S.M.; Mortada, N.D. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, M.C.; Cilurzo, F.; Carafa, M.; Paolino, D. Innovative vesicles for dermal and transdermal drug delivery. In Lipid Nanocarriers for Drug Targeting; Elsevier: Amsterdam, The Netherlands, 2018; pp. 175–197. [Google Scholar]
- Wang, J.; Wei, Y.; Fei, Y.-R.; Fang, L.; Zheng, H.-S.; Mu, C.-F.; Li, F.-Z.; Zhang, Y.-S. Preparation of mixed monoterpenes edge activated PEGylated transfersomes to improve the in vivo transdermal delivery efficiency of sinomenine hydrochloride. Int. J. Pharm. 2017, 533, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Pandey, V.; Rai, G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Rev. Exp. 2017, 8, 1325708. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Apostolou, M.; Bnyan, R.; Houacine, C.; Elhissi, A.; Yousaf, S.S. Paclitaxel-loaded micro or nano transfersome formulation into novel tablets for pulmonary drug delivery via nebulization. Int. J. Pharm. 2020, 575, 118919. [Google Scholar] [CrossRef] [PubMed]
- Kumria, R.; Nair, A.B.; Goomber, G.; Gupta, S. Buccal films of prednisolone with enhanced bioavailability. Drug Deliv. 2016, 23, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Montenegro-Nicolini, M.; Morales, J.O. Overview and future potential of buccal mucoadhesive films as drug delivery systems for biologics. AAPS Pharmscitech 2017, 18, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Bawazir, A.O.; Alharbi, W.S.; Safo, M.K. Enhancement of Simvastatin ex vivo Permeation from Mucoadhesive Buccal Films Loaded with Dual Drug Release Carriers. Int. J. Nanomed. 2020, 15, 4001–4020. [Google Scholar] [CrossRef]
- Zaman, M.; Hanif, M.; Shaheryar, Z.A. Development of Tizanidine HCl-Meloxicam loaded mucoadhesive buccal films: In-vitro and in-vivo evaluation. PLoS ONE 2018, 13, e0194410. [Google Scholar] [CrossRef]
- Kraisit, P.; Limmatvapirat, S.; Luangtana-Anan, M.; Sriamornsak, P. Buccal administration of mucoadhesive blend films saturated with propranolol loaded nanoparticles. Asian J. Pharm. Sci. 2018, 13, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Irfan, M.; Abbas, N.; Syed, H.K.; Iqbal, M.S.; ullah Khan, I.; Rasul, A.; Inam, S.; Hussain, A.; Arshad, M.S. Enhancement of solubility and dissolution rate of ebastine fast-disintegrating tablets by solid dispersion method. Trop. J. Pharm. Res. 2020, 19, 1797–1805. [Google Scholar] [CrossRef]
- Haraguchi, T.; Yoshida, M.; Uchida, T. Evaluation of ebastine-loaded orally disintegrating tablets using new apparatus of detecting disintegration time and e-tongue system. J. Drug Deliv. Sci. Technol. 2014, 24, 684–688. [Google Scholar] [CrossRef]
- Roger, A.; Fortea, J.; Mora, S.; Artés, M. Ebastine fast-dissolving tablets versus regular tablets: Acceptability and preference in patients with allergic rhinitis. Expert Rev. Clin. Pharmacol. 2008, 1, 381–389. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Koshti, A.R.; Bari, M.; Barhate, S. Formulation optimization and Evaluation of Transdermal patch of losartan potassium containing DMSO as permeation enhancer. Asian J. Pharm. Technol. 2019, 9, 220–227. [Google Scholar] [CrossRef]
- Elkomy, M.H.; El Menshawe, S.F.; Abou-Taleb, H.A.; Elkarmalawy, M.H. Loratadine bioavailability via buccal transferosomal gel: Formulation, statistical optimization, in vitro/in vivo characterization, and pharmacokinetics in human volunteers. Drug Deliv. 2017, 24, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Speer, I.; Preis, M.; Breitkreutz, J. Dissolution testing of oral film preparations: Experimental comparison of compendial and non-compendial methods. Int. J. Pharm. 2019, 561, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.M.; Hasan, O.A.; El Sisi, A.M. Preparation and optimization of lidocaine transferosomal gel containing permeation enhancers: A promising approach for enhancement of skin permeation. Int. J. Nanomed. 2019, 14, 1551. [Google Scholar] [CrossRef] [Green Version]
- Otoni, C.G.; Lorevice, M.V.; de Moura, M.R.; Mattoso, L.H. On the effects of hydroxyl substitution degree and molecular weight on mechanical and water barrier properties of hydroxypropyl methylcellulose films. Carbohydr. Polym. 2018, 185, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Leonyza, A.; Surini, S. Optimization of sodium deoxycholate-based transfersomes for percutaneous delivery of peptides and proteins. Int. J. Appl. Pharm. 2019, 11, 329–332. [Google Scholar] [CrossRef] [Green Version]
- Shuwaili, A.H.A.; Rasool, B.K.A.; Abdulrasool, A.A. Optimization of elastic transfersomes formulations for transdermal delivery of pentoxifylline. Eur. J. Pharm. Biopharm. 2016, 102, 101–114. [Google Scholar] [CrossRef]
- Wang, L.-L.; He, D.-D.; Wang, S.-X.; Dai, Y.-H.; Ju, J.-M.; Zhao, C.-L. Preparation and evaluation of curcumin-loaded self-assembled micelles. Drug Dev. Ind. Pharm. 2018, 44, 563–569. [Google Scholar] [CrossRef]
- Prateepchanachai, S.; Thakhiew, W.; Devahastin, S.; Soponronnarit, S. Mechanical properties improvement of chitosan films via the use of plasticizer, charge modifying agent and film solution homogenization. Carbohydr. Polym. 2017, 174, 253–261. [Google Scholar] [CrossRef]
- Speer, I.; Steiner, D.; Thabet, Y.; Breitkreutz, J.; Kwade, A. Comparative study on disintegration methods for oral film preparations. Eur. J. Pharm. Biopharm. 2018, 132, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Zaman, M. Thiolation of arabinoxylan and its application in the fabrication of controlled release mucoadhesive oral films. DARU J. Pharm. Sci. 2017, 25, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, Y.; Ikeda, N.; Tahara, K.; Takeuchi, H. Mechanical characteristics of orally disintegrating films: Comparison of folding endurance and tensile properties. Int. J. Pharm. 2020, 589, 119876. [Google Scholar] [CrossRef]
- Talekar, S.D.; Haware, R.V.; Dave, R.H. Evaluation of self-nanoemulsifying drug delivery systems using multivariate methods to optimize permeability of captopril oral films. Eur. J. Pharm. Sci. 2019, 130, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.; Pavurala, N.; Manda, P.; Xu, X.; Cruz, C.N.; Krishnaiah, Y.S. Quality by Design approach for studying the impact of formulation and process variables on product quality of oral disintegrating films. Int. J. Pharm. 2017, 527, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; Gupta, A.K.; Parashar, A.K. Formulation and Evaluation of fast dissolving films of Granisetron Hydrochloride. J. Drug Deliv. Ther. 2019, 9, 36–38. [Google Scholar]
- Islam, N.; Irfan, M.; Khan, S.-U.-D.; Syed, H.K.; Iqbal, M.S.; Khan, I.U.; Mahdy, A.; Raafat, M.; Hossain, M.A.; Inam, S. Poloxamer-188 and d-α-Tocopheryl Polyethylene Glycol Succinate (TPGS-1000) Mixed Micelles Integrated Orodispersible Sublingual Films to Improve Oral Bioavailability of Ebastine; In Vitro and In Vivo Characterization. Pharmaceutics 2021, 13, 54. [Google Scholar] [CrossRef]
- Shamma, R.N.; Elsayed, I. Transfersomal lyophilized gel of buspirone HCl: Formulation, evaluation and statistical optimization. J. Liposome Res. 2013, 23, 244–254. [Google Scholar] [CrossRef]
- Balguri, S.P.; Adelli, G.R.; Majumdar, S. Topical ophthalmic lipid nanoparticle formulations (SLN, NLC) of indomethacin for delivery to the posterior segment ocular tissues. Eur. J. Pharm. Biopharm. 2016, 109, 224–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Z.-y.; Jia, X.-W.; Liu, Q.; Kong, B.-h.; Wang, H. Fast dissolving oral films for drug delivery prepared from chitosan/pullulan electrospinning nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Pitta, S.K.; Dudhipala, N.; Narala, A.; Veerabrahma, K. Development of zolmitriptan transfersomes by Box–Behnken design for nasal delivery: In vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2018, 44, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.M.; Baptista, P.; Zuccheri, G.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Film-nanoparticle composite for enhanced oral delivery of alpha-casozepine. Colloids Surf. B Biointerfaces 2019, 181, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahallawi, A.M.; Khowessah, O.M.; Shoukri, R.A. Enhanced non invasive trans-tympanic delivery of ciprofloxacin through encapsulation into nano-spanlastic vesicles: Fabrication, in-vitro characterization, and comparative ex-vivo permeation studies. Int. J. Pharm. 2017, 522, 157–164. [Google Scholar] [CrossRef]
- Rahmi, A.D.; Pangesti, D.M. Comparison of the Characteristics of Transfersomes and Protransfersomes Containing Azelaic Acid. J. Young Pharm. 2018, 10, S11. [Google Scholar]
- Abdel-Hafez, S.M.; Hathout, R.M.; Sammour, O.A. Curcumin-loaded ultradeformable nanovesicles as a potential delivery system for breast cancer therapy. Colloids Surf. B Biointerfaces 2018, 167, 63–72. [Google Scholar] [CrossRef]
- Ingallina, C.; Rinaldi, F.; Bogni, A.; Ponti, J.; Passeri, D.; Reggente, M.; Rossi, M.; Kinsner-Ovaskainen, A.; Mehn, D.; Rossi, F. Niosomal approach to brain delivery: Development, characterization and in vitro toxicological studies. Int. J. Pharm. 2016, 511, 969–982. [Google Scholar] [CrossRef]
- Garg, V.; Singh, H.; Bimbrawh, S.; Kumar Singh, S.; Gulati, M.; Vaidya, Y.; Kaur, P. Ethosomes and transfersomes: Principles, perspectives and practices. Curr. Drug Deliv. 2017, 14, 613–633. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, H.; Nik, M.E.; Mashreghi, M.; Saberi, Z.; Jaafari, M.R.; Teymouri, M. A study on the role of cholesterol and phosphatidylcholine in various features of liposomal doxorubicin: From liposomal preparation to therapy. Int. J. Pharm. 2018, 551, 300–308. [Google Scholar] [CrossRef]
- Shreya, A.; Managuli, R.S.; Menon, J.; Kondapalli, L.; Hegde, A.R.; Avadhani, K.; Shetty, P.K.; Amirthalingam, M.; Kalthur, G.; Mutalik, S. Nano-transfersomal formulations for transdermal delivery of asenapine maleate: In vitro and in vivo performance evaluations. J. Liposome Res. 2016, 26, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Dudhipala, N.; Phasha Mohammed, R.; Adel Ali Youssef, A.; Banala, N. Effect of lipid and edge activator concentration on development of aceclofenac-loaded transfersomes gel for transdermal application: In vitro and ex vivo skin permeation. Drug Dev. Ind. Pharm. 2020, 46, 1334–1344. [Google Scholar] [CrossRef]
- Fernández-García, R.; Lalatsa, A.; Statts, L.; Bolás-Fernández, F.; Ballesteros, M.P.; Serrano, D.R. Transferosomes as nanocarriers for drugs across the skin: Quality by design from lab to industrial scale. Int. J. Pharm. 2020, 573, 118817. [Google Scholar] [CrossRef]
- Moawad, F.A.; Ali, A.A.; Salem, H.F. Nanotransfersomes-loaded thermosensitive in situ gel as a rectal delivery system of tizanidine HCl: Preparation, in vitro and in vivo performance. Drug Deliv. 2017, 24, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Zeb, A.; Qureshi, O.S.; Kim, H.-S.; Cha, J.-H.; Kim, H.-S.; Kim, J.-K. Improved skin permeation of methotrexate via nanosized ultradeformable liposomes. Int. J. Nanomed. 2016, 11, 3813. [Google Scholar]
- Bodini, R.B.; das Graças Lapa Guimarães, J.; Monaco-Lourenço, C.A.; de Carvalho, R.A. Effect of starch and hydroxypropyl methylcellulose polymers on the properties of orally disintegrating films. J. Drug Deliv. Sci. Technol. 2019, 51, 403–410. [Google Scholar] [CrossRef]
- Yildiz Pekoz, A.; Sedef Erdal, M.; Okyar, A.; Ocak, M.; Tekeli, F.; Kaptan, E.; Sagirli, O.; Araman, A. Preparation and in-vivo evaluation of dimenhydrinate buccal mucoadhesive films with enhanced bioavailability. Drug Dev. Ind. Pharm. 2016, 42, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Gajera, B.Y.; Shah, D.A.; Dave, R.H. Development of an amorphous nanosuspension by sonoprecipitation-formulation and process optimization using design of experiment methodology. Int. J. Pharm. 2019, 559, 348–359. [Google Scholar] [CrossRef]
- Morsi, N.M.; Aboelwafa, A.A.; Dawoud, M.H. Enhancement of the bioavailability of an antihypertensive drug by transdermal protransfersomal system: Formulation and in vivo study. J. Liposome Res. 2018, 28, 137–148. [Google Scholar] [CrossRef]
- Sundralingam, U.; Muniyandy, S.; Radhakrishnan, A.K.; Palanisamy, U.D. Ratite oils for local transdermal therapy of 4-OH Tamoxifen: Development, Characterization and Ex-vivo Evaluation. J. Liposome Res. 2020, 31, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.P.; Omase, S.B.; Mute, V.M. A chitosan film containing quercetin-loaded transfersomes for treatment of secondary osteoporosis. Drug Deliv. Transl. Res. 2020, 10, 1495–1506. [Google Scholar] [CrossRef]
- Tamai, I.; Kido, Y.; Yamashita, J.; Sai, Y.; Tsuji, A. Blood-brain barrier transport of H1-antagonist ebastine and its metabolite carebastine. J. Drug Target. 2000, 8, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-H.; Kim, M.-G.; Lee, D.-J.; Yoon, Y.-J.; Kim, M.-J.; Shon, J.-H.; Choi, C.S.; Choi, Y.K.; Desta, Z.; Shin, J.-G. Characterization of ebastine, hydroxyebastine, and carebastine metabolism by human liver microsomes and expressed cytochrome P450 enzymes: Major roles for CYP2J2 and CYP3A. Drug Metab. Dispos. 2006, 34, 1793–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senna, T.D.; Mata dos Santos, H.A.; Kibwila, D.M.; Leitao, A.C.; Dos Santos Pyrrho, A.; de Padula, M.; Rosas, E.C.; Padua, T.A.; Lara, M.G.; Riemma Pierre, M.B. In vitro and in vivo evaluation of DMSO and azone as penetration enhancers for cutaneous application of celecoxib. Curr. Drug Deliv. 2017, 14, 992–1004. [Google Scholar] [CrossRef]
- Yang, T.-Z.; Wang, X.-T.; Yan, X.-Y.; Zhang, Q. Phospholipid deformable vesicles for buccal delivery of insulin. Chem. Pharm. Bull. 2002, 50, 749–753. [Google Scholar] [CrossRef] [Green Version]
- Badr-Eldin, S.M.; Ahmed, O.A. Optimized nano-transfersomal films for enhanced sildenafil citrate transdermal delivery: Ex vivo and in vivo evaluation. Drug Des. Devel. Ther. 2016, 10, 1323. [Google Scholar] [CrossRef] [Green Version]
- Ahad, A.; Al-Saleh, A.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I.; Raish, M.; Yassin, A.E.B.; Alam, M.A. Formulation and characterization of Phospholipon 90 G and tween 80 based transfersomes for transdermal delivery of eprosartan mesylate. Pharm. Dev. Technol. 2018, 23, 787–793. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Antonijoan, R.; Coimbra, J.; García-Gea, C.; Puntes, M.; Gich, I.; Campo, C.; Valiente, R.; Labeaga, L. Comparative efficacy of bilastine, desloratadine and rupatadine in the suppression of wheal and flare response induced by intradermal histamine in healthy volunteers. Curr. Med. Res. Opin. 2017, 33, 129–136. [Google Scholar] [CrossRef] [PubMed]










| Formulation Code | PC (%) | Edge Activator (%) | |
|---|---|---|---|
| Tween 80® | Span 20® | ||
| VT-1 | 95 | 05 | - |
| VT-2 | 90 | 10 | - |
| VT-3 | 85 | 15 | - |
| VT-4 | 80 | 20 | - |
| VS-1 | 95 | - | 05 |
| VS-2 | 90 | - | 10 |
| VS-3 | 85 | - | 15 |
| VS-4 | 80 | - | 20 |
| TOFs Formulation | HPMC-K15M (%) | Glycerol (%) |
|---|---|---|
| ETF-1 | 2.0 | 4.0 |
| ETF-2 | 2.5 | 6.0 |
| ETF-3 | 3.0 | 8.0 |
| ETF-4 | 2.0 | 4.0 |
| ETF-5 | 2.5 | 6.0 |
| ETF-6 | 3.0 | 8.0 |
| ETF-7 | 2.0 | 4.0 |
| ETF-8 | 2.5 | 6.0 |
| ETF-9 | 3.0 | 8.0 |
| TOF Formulations | Thickness (mm) ± SD | Weight (mg) ± SD | Folding Endurance (n) ± SD | Tensile Strength (Mpa) ± SD | Elongation (%) ± SD |
|---|---|---|---|---|---|
| ETF-1 | 0.35 ± 0.21 | 57.4 ± 2.64 | 21 ± 2.73 | 43 ± 1.24 | 4.1 ± 0.16 |
| ETF-2 | 0.25 ± 0.11 | 69.5 ± 2.55 | 27 ±1.42 | 56 ± 1.53 | 5.4 ± 0.31 |
| ETF-3 | 0.26 ± 0.06 | 87.1 ± 3.60 | 29 ± 2.51 | 67 ± 2.29 | 6.8 ± 0.12 |
| ETF-4 | 0.24 ± 0.24 | 61.4 ± 1.42 | 34 ± 1.18 | 82 ± 3.22 | 8.2 ± 0.26 |
| ETF-5 | 0.27 ± 0.04 | 76.2 ± 1.27 | 38 ± 1.45 | 96 ± 1.32 | 9.5 ± 0.12 |
| ETF-6 | 0.36 ± 0.06 | 88.0 ± 3.30 | 38 ± 4.54 | 107 ± 2.14 | 10.9 ± 0.18 |
| ETF-7 | 0.34 ± 0.12 | 62.3 ± 1.37 | 39 ± 3.93 | 76 ± 3.51 | 12.7 ± 0.28 |
| ETF-8 | 0.36 ± 0.34 | 77.9 ± 1.24 | 41 ± 2.67 | 118 ± 1.04 | 14.8 ± 0.21 |
| ETF-9 | 0.39 ± 0.26 | 92.1 ± 1.15 | 46 ± 4.88 | 137 ± 0.29 | 17.9 ± 0.31 |
| Pharmacokinetic Parameters | EBT Suspension | Plain Film | ETF-5 Transferosomal Film |
|---|---|---|---|
| Group E | Group P | Group T | |
| Cmax (ng/mL) | 78.4 ± 2.31 | 114.8 ± 4.01 | 152.3 ± 2.18 |
| Tmax (h) | 4.6 | 4.1 | 7.8 |
| t1/2 (h) | 19.12 | 18.64 | 22.6 |
| AUC0–72 (ng/mL/h) | 2116 ± 32.51 | 3697 ± 12.04 | 6249 ± 21.7 |
| Ke (1/h) | 0.0372 | 0.0382 | 0.028 |
| Wheal Area (mm2) | ||||
|---|---|---|---|---|
| Group | 0 h (Baseline) | 4 h | 8 h | 24 h |
| Placebo | 215.4 ± 21.4 | 221.2 ± 18.6 | 224.1 ± 71.5 | 193.4 ± 13.2 |
| Pure drug | 219.5 ± 18.5 | 114.5 ± 12.1 | 90.43 ± 21.2 | 55.3 ± 3.14 |
| ETF-5 | 211.8 ± 11.8 | 62.12 ± 10.4 | 12.42 ± 1.4 | 2.84 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, N.; Irfan, M.; Zahoor, A.F.; Iqbal, M.S.; Syed, H.K.; Khan, I.U.; Rasul, A.; Khan, S.-U.-D.; Alqahtani, A.M.; Ikram, M.; et al. Improved Bioavailability of Ebastine through Development of Transfersomal Oral Films. Pharmaceutics 2021, 13, 1315. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13081315
Islam N, Irfan M, Zahoor AF, Iqbal MS, Syed HK, Khan IU, Rasul A, Khan S-U-D, Alqahtani AM, Ikram M, et al. Improved Bioavailability of Ebastine through Development of Transfersomal Oral Films. Pharmaceutics. 2021; 13(8):1315. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13081315
Chicago/Turabian StyleIslam, Nayyer, Muhammad Irfan, Ameer Fawad Zahoor, Muhammad Shahid Iqbal, Haroon Khalid Syed, Ikram Ullah Khan, Akhtar Rasul, Salah-Ud-Din Khan, Alaa M. Alqahtani, Muzzamil Ikram, and et al. 2021. "Improved Bioavailability of Ebastine through Development of Transfersomal Oral Films" Pharmaceutics 13, no. 8: 1315. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13081315