Extracellular Vesicles from M1-Polarized Macrophages Combined with Hyaluronic Acid and a β-Blocker Potentiate Doxorubicin’s Antitumor Activity by Downregulating Tumor-Associated Macrophages in Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Cell Lines and Cell Culture
2.3. Preparation of Single and Modulated EVs and DOX Loading
2.4. Characterization of EVs
2.5. Polarization of RAW 264.7 Cells
2.6. Cell Viability Assays
2.7. Wound-Healing Assay
2.8. Immunofluorescence
2.9. In Vivo Study
2.9.1. Animals
2.9.2. Orthotopic Tumor Induction
2.9.3. qRT-PCR
2.9.4. Immunohistochemistry
2.9.5. Immunofluorescence Microscopy
2.9.6. Statistical Analysis
3. Results and Discussion
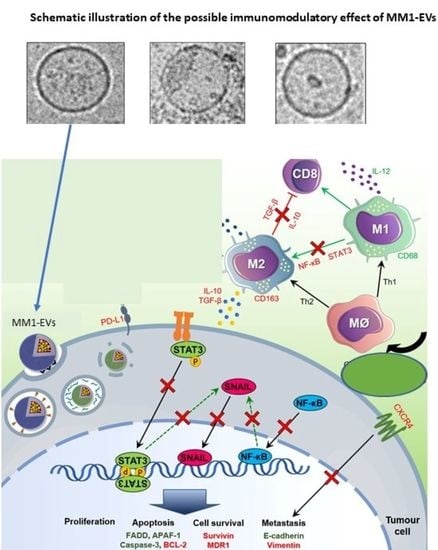
3.1. EVs from Polarized Macrophages
3.2. M1 but Not M2-Derived EVs Have Antitumor Potential in Breast Cancer Cells
3.3. Enhanced Antitumor Properties of M1-Derived EVs by Combination of HA and CV
3.4. The Combination of HA and CV Potentiates In Vivo Anticancer Activity of M1-Derived EVs
3.5. Combined Treatment with HA and CV Increases Antimetastatic Capacity of M1-EVs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [Green Version]
- de Araujo Junior, R.F.; Eich, C.; Jorquera, C.; Schomann, T.; Baldazzi, F.; Chan, A.B.; Cruz, L.J. Ceramide and palmitic acid inhibit macrophage-mediated epithelial-mesenchymal transition in colorectal cancer. Mol. Cell. Biochem. 2020, 468, 153–168. [Google Scholar] [CrossRef] [Green Version]
- Cavalcante, R.S.; Ishikawa, U.; Silva, E.S.; Silva-Júnior, A.A.; Araújo, A.A.; Cruz, L.J.; Chan, A.B.; de Araújo Júnior, R.F. STAT3/NF-κB signalling disruption in M2 tumour-associated macrophages is a major target of PLGA nanocarriers/PD-L1 antibody immunomodulatory therapy in breast cancer. Br. J. Pharmacol. 2021, 178, 2284–2304. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Huang, L. Exosomes from M1-Polarized Macrophages Potentiate the Cancer Vaccine by Creating a Pro-inflammatory Microenvironment in the Lymph Node. Mol. Ther. 2017, 25, 1665–1675. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Li, Y.; Gao, W.; Chen, L.; Xu, W.; Zhu, X. Exosome-Mediated Crosstalk Between Tumor and Tumor-Associated Macrophages. Front. Mol. Biosci. 2021, 8, 764222. [Google Scholar] [CrossRef]
- Wang, D.; Xue, M.; Chen, J.; Chen, H.; Liu, J.; Li, Q.; Xie, Y.; Hu, Y.; Ni, Y.; Zhou, Q. Macrophage-derived implantable vaccine prevents postsurgical tumor recurrence. Biomaterials 2021, 278, 121161. [Google Scholar] [CrossRef]
- Lara, P.; Palma-Florez, S.; Salas-Huenuleo, E.; Polakovicova, I.; Guerrero, S.; Lobos-Gonzalez, L.; Campos, A.; Muñoz, L.; Jorquera-Cordero, C.; Varas-Godoy, M.; et al. Gold nanoparticle based double-labeling of melanoma extracellular vesicles to determine the specificity of uptake by cells and preferential accumulation in small metastatic lung tumors. J. Nanobiotechnol. 2020, 18, 20. [Google Scholar] [CrossRef] [Green Version]
- Lara, P.; Huis in ’t Veld, R.V.; Jorquera-Cordero, C.; Chan, A.B.; Ossendorp, F.; Cruz, L.J. Zinc-Phthalocyanine-Loaded Extracellular Vesicles Increase Efficacy and Selectivity of Photodynamic Therapy in Co-Culture and Preclinical Models of Colon Cancer. Pharmaceutics 2021, 13, 1547. [Google Scholar] [CrossRef] [PubMed]
- Lara, P.; Chan, A.B.; Cruz, L.J.; Quest, A.F.G.; Kogan, M.J. Exploiting the Natural Properties of Extracellular Vesicles in Targeted Delivery towards Specific Cells and Tissues. Pharmaceutics 2020, 12, 1022. [Google Scholar] [CrossRef]
- Chulpanova, D.S.; Kitaeva, K.V.; James, V.; Rizvanov, A.A.; Solovyeva, V.V. Therapeutic Prospects of Extracellular Vesicles in Cancer Treatment. Front. Immunol. 2018, 9, 1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raimondo, S.; Giavaresi, G.; Lorico, A.; Alessandro, R. Extracellular Vesicles as Biological Shuttles for Targeted Therapies. Int. J. Mol. Sci. 2019, 20, 1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Yin, Y.; Lai, R.C.; Lim, S.K. Immunotherapeutic potential of extracellular vesicles. Front. Immunol. 2014, 5, 518. [Google Scholar] [CrossRef] [Green Version]
- Taghikhani, A.; Farzaneh, F.; Sharifzad, F.; Mardpour, S.; Ebrahimi, M.; Hassan, Z.M. Engineered Tumor-Derived Extracellular Vesicles: Potentials in Cancer Immunotherapy. Front. Immunol. 2020, 11, 221. [Google Scholar] [CrossRef]
- Goughnour, P.C.; Park, M.C.; Kim, S.B.; Jun, S.; Yang, W.S.; Chae, S.; Cho, S.; Song, C.; Lee, J.H.; Hyun, J.K.; et al. Extracellular vesicles derived from macrophages display glycyl-tRNA synthetase 1 and exhibit anti-cancer activity. J. Extracell. Vesicles 2020, 10, e12029. [Google Scholar] [CrossRef]
- Bao, Q.; Huang, Q.; Chen, Y.; Wang, Q.; Sang, R.; Wang, L.; Xie, Y.; Chen, W. Tumor-Derived Extracellular Vesicles Regulate Cancer Progression in the Tumor Microenvironment. Front. Mol. Biosci. 2021, 8, 796385. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Huang, Q.; Peng, C.; Yao, L.; Chen, H.; Qiu, Z.; Wu, Y.; Wang, L.; Chen, W. Exosomes from M1-Polarized Macrophages Enhance Paclitaxel Antitumor Activity by Activating Macrophages-Mediated Inflammation. Theranostics 2019, 9, 1714–1727. [Google Scholar] [CrossRef]
- Sladek, Z.; Rysanek, D. Expression of macrophage CD44 receptor in the course of experimental inflammatory response of bovine mammary gland induced by lipopolysaccharide and muramyl dipeptide. Res. Vet. Sci. 2009, 86, 235–240. [Google Scholar] [CrossRef]
- Rios de la Rosa, J.M.; Tirella, A.; Gennari, A.; Stratford, I.J.; Tirelli, N. The CD44-Mediated Uptake of Hyaluronic Acid-Based Carriers in Macrophages. Adv. Healthc. Mater. 2017, 6, 1601012. [Google Scholar] [CrossRef] [PubMed]
- Rangasami, V.K.; Samanta, S.; Parihar, V.S.; Asawa, K.; Zhu, K.; Varghese, O.P.; Teramura, Y.; Nilsson, B.; Hilborn, J.; Harris, R.A.; et al. Harnessing hyaluronic acid-based nanoparticles for combination therapy: A novel approach for suppressing systemic inflammation and to promote antitumor macrophage polarization. Carbohydr. Polym. 2021, 254, 117291. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.Y.; Xiao, R.P. β-Adrenergic receptor subtype signaling in heart: From bench to bedside. Acta Pharmacol. Sin. 2012, 33, 335–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.F.; Jin, F.J.; Li, N.; Guan, H.T.; Lan, L.; Ni, H.; Wang, Y. Adrenergic receptor β2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015, 48, 295–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Eynden, G.G.; Bird, N.C.; Majeed, A.W.; Van Laere, S.; Dirix, L.Y.; Vermeulen, P.B. The histological growth pattern of colorectal cancer liver metastases has prognostic value. Clin. Exp. Metastasis 2012, 29, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Araújo Júnior, R.F.; Garcia, V.B.; Leitão, R.F.; Brito, G.A.; Miguel Ede, C.; Guedes, P.M.; de Araújo, A.A. Carvedilol Improves Inflammatory Response, Oxidative Stress and Fibrosis in the Alcohol-Induced Liver Injury in Rats by Regulating Kuppfer Cells and Hepatic Stellate Cells. PLoS ONE 2016, 11, e0148868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Z.Z.; Li, H.Y.; Li, C.H.; Sheng, C.L.; Zhao, X.N. M1 Macrophage-Derived Exosomal MicroRNA-326 Suppresses Hepatocellular Carcinoma Cell Progression Via Mediating NF-κB Signaling Pathway. Nanoscale Res. Lett. 2020, 15, 221. [Google Scholar] [CrossRef]
- Sanyakamdhorn, S.; Agudelo, D.; Tajmir-Riahi, H.A. Encapsulation of antitumor drug Doxorubicin and its analogue by chitosan nanoparticles. Biomacromolecules 2013, 14, 557–563. [Google Scholar] [CrossRef]
- Shao, M.; Zhu, W.; Lv, X.; Yang, Q.; Liu, X.; Xie, Y.; Tang, P.; Sun, L. Encapsulation of chloroquine and doxorubicin by MPEG-PLA to enhance anticancer effects by lysosomes inhibition in ovarian cancer. Int. J. Nanomed. 2018, 13, 8231–8245. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, R.K.; Kim, B.J.; Shah, P.D.; Carver, J.; Ky, B.; Ryeom, S. Damage to cardiac vasculature may be associated with breast cancer treatment-induced cardiotoxicity. Cardiooncology 2021, 7, 15. [Google Scholar] [CrossRef]
- Xing, Y.; Sun, X.; Dou, Y.; Wang, M.; Zhao, Y.; Yang, Q.; Zhao, Y. The Immuno-Modulation Effect of Macrophage-Derived Extracellular Vesicles in Chronic Inflammatory Diseases. Front. Immunol. 2021, 12, 785728. [Google Scholar] [CrossRef] [PubMed]
- Reed, T.; Schorey, J.; D’Souza-Schorey, C. Tumor-Derived Extracellular Vesicles: A Means of Co-opting Macrophage Polarization in the Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 746432. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Lin, A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002, 3, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Bravo, S.B.; Pampín, S.; Cameselle-Teijeiro, J.; Carneiro, C.; Domínguez, F.; Barreiro, F.; Alvarez, C.V. TGF-beta-induced apoptosis in human thyrocytes is mediated by p27kip1 reduction and is overridden in neoplastic thyrocytes by NF-kappaB activation. Oncogene 2003, 22, 7819–7830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.H.; Hong, J.T. Roles of NF-κB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef]
- Lalle, G.; Twardowski, J.; Grinberg-Bleyer, Y. NF-κB in Cancer Immunity: Friend or Foe? Cells 2021, 10, 355. [Google Scholar] [CrossRef]
- Gunderson, A.J.; Yamazaki, T.; McCarty, K.; Fox, N.; Phillips, M.; Alice, A.; Blair, T.; Whiteford, M.; O’Brien, D.; Ahmad, R.; et al. TGFβ suppresses CD8(+) T cell expression of CXCR3 and tumor trafficking. Nat. Commun. 2020, 11, 1749. [Google Scholar] [CrossRef] [Green Version]
- Erguven, M.; Yazihan, N.; Aktas, E.; Sabanci, A.; Li, C.J.; Oktem, G.; Bilir, A. Carvedilol in glioma treatment alone and with imatinib in vitro. Int. J. Oncol. 2010, 36, 857–866. [Google Scholar] [CrossRef] [Green Version]
- De Araújo, R.F., Jr.; Pessoa, J.B.; Cruz, L.J.; Chan, A.B.; De Castro Miguel, E.; Cavalcante, R.S.; Brito, G.A.C.; Silva, H.F.O.; Gasparotto, L.H.S.; Guedes, P.M.M.; et al. Apoptosis in human liver carcinoma caused by gold nanoparticles in combination with carvedilol is mediated via modulation of MAPK/Akt/mTOR pathway and EGFR/FAAD proteins. Int. J. Oncol. 2018, 52, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Ma, Z.; Fu, Q.; Yu, Z. Carvedilol suppresses migration and invasion of malignant breast cells by inactivating Src involving cAMP/PKA and PKCδ signaling pathway. J. Cancer Res. Ther. 2014, 10, 998–1003. [Google Scholar] [CrossRef]
- Mima, K.; Beppu, T.; Ishiko, T.; Chikamoto, A.; Nakagawa, S.; Hayashi, H.; Watanabe, M.; Sakamaki, K.; Baba, H. Preoperative serum hyaluronic acid level as a prognostic factor in patients undergoing hepatic resection for hepatocellular carcinoma. Br. J. Surg. 2014, 101, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.; Modi, S.; McGinn, O.; Zhao, X.; Dudeja, V.; Ramakrishnan, S.; Saluja, A.K. Impaired Synthesis of Stromal Components in Response to Minnelide Improves Vascular Function, Drug Delivery, and Survival in Pancreatic Cancer. Clin. Cancer Res. 2016, 22, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yamada, O.; Kida, S.; Matsushita, Y.; Yamaoka, S.; Chagan-Yasutan, H.; Hattori, T. Identification of CD44 as a downstream target of noncanonical NF-κB pathway activated by human T-cell leukemia virus type 1-encoded Tax protein. Virology 2011, 413, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Lyu, Y.L.; Cai, L. NF-κB affects proliferation and invasiveness of breast cancer cells by regulating CD44 expression. PLoS ONE 2014, 9, e106966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wald, O.; Shapira, O.M.; Izhar, U. CXCR4/CXCL12 axis in non small cell lung cancer (NSCLC) pathologic roles and therapeutic potential. Theranostics 2013, 3, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Mao, X.; Fan, C.; Liu, C.; Guo, A.; Guan, S.; Jin, Q.; Li, B.; Yao, F.; Jin, F. CXCL12-CXCR4 axis promotes the natural selection of breast cancer cell metastasis. Tumour Biol. 2014, 35, 7765–7773. [Google Scholar] [CrossRef] [Green Version]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef]
- Gomes, L.R.; Terra, L.F.; Wailemann, R.A.; Labriola, L.; Sogayar, M.C. TGF-β1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer 2012, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mott, J.D.; Werb, Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 2004, 16, 558–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGowan, P.M.; Duffy, M.J. Matrix metalloproteinase expression and outcome in patients with breast cancer: Analysis of a published database. Ann. Oncol. 2008, 19, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, M.; Zhang, Y.; Ge, S.; Zhong, F.; Xia, G.; Sun, C. Tumor-Associated Macrophages: A Potential Target for Cancer Therapy. Front. Oncol. 2021, 11, 693517. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorquera-Cordero, C.; Lara, P.; Cruz, L.J.; Schomann, T.; van Hofslot, A.; de Carvalho, T.G.; Guedes, P.M.D.M.; Creemers, L.; Koning, R.I.; Chan, A.B.; et al. Extracellular Vesicles from M1-Polarized Macrophages Combined with Hyaluronic Acid and a β-Blocker Potentiate Doxorubicin’s Antitumor Activity by Downregulating Tumor-Associated Macrophages in Breast Cancer. Pharmaceutics 2022, 14, 1068. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14051068
Jorquera-Cordero C, Lara P, Cruz LJ, Schomann T, van Hofslot A, de Carvalho TG, Guedes PMDM, Creemers L, Koning RI, Chan AB, et al. Extracellular Vesicles from M1-Polarized Macrophages Combined with Hyaluronic Acid and a β-Blocker Potentiate Doxorubicin’s Antitumor Activity by Downregulating Tumor-Associated Macrophages in Breast Cancer. Pharmaceutics. 2022; 14(5):1068. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14051068
Chicago/Turabian StyleJorquera-Cordero, Carla, Pablo Lara, Luis J. Cruz, Timo Schomann, Anna van Hofslot, Thaís Gomes de Carvalho, Paulo Marcos Da Matta Guedes, Laura Creemers, Roman I. Koning, Alan B. Chan, and et al. 2022. "Extracellular Vesicles from M1-Polarized Macrophages Combined with Hyaluronic Acid and a β-Blocker Potentiate Doxorubicin’s Antitumor Activity by Downregulating Tumor-Associated Macrophages in Breast Cancer" Pharmaceutics 14, no. 5: 1068. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14051068