Prediction of Esophageal Varices Based on Serum-Ascites Albumin Gradient in Cirrhotic Patients
Abstract
:1. Introduction
2. Material and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Study Process
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Predictive Factors of EVs
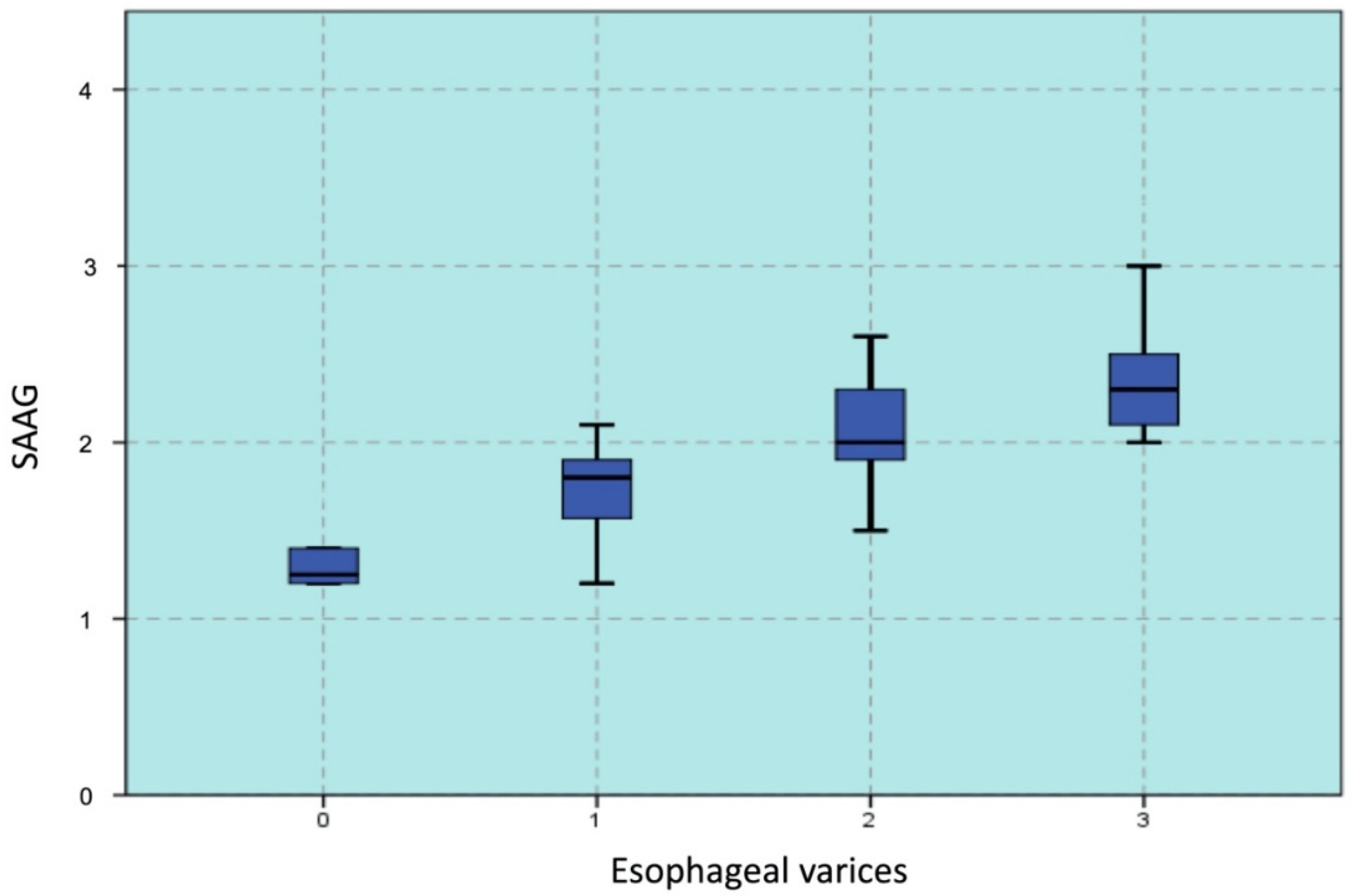
3.3. SAAG to Predict EVs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rye, K.; Scott, R.; Mortimore, G.; Lawson, A.; Austin, A.; Freeman, J. Towards non invasive detection of esophageal varices. Int. J. Hepatol. 2012, 12, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Franchis, R. Revising consensus in portal hypertension: Report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J. Hepatol. 2010, 53, 762–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhasin, D.K.; Malhi, N.J. Variceal bleeding and portal hypertension: Much to learn, much to explore. Endoscopy 2002, 34, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Abraldes, J.G.; Berzigotti, A.; Garcia-Pagan, J.C. Portal hypertension and gastrointestinal bleeding. Semin. Liver Dis. 2008, 28, 3–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalasani, N.; Imperiale, T.F.; Ismail, A.; Sood, G.; Carey, M.; Wilcox, C.M.; Madichetty, H.; Kwo, P.Y.; Boyer, T.D. Predictors of large esophageal varices in patients with cirrhosis. Am. J. Gastroenterol. 1999, 94, 3285–3291. [Google Scholar] [CrossRef] [PubMed]
- Mahassadi, A.K.; Bathaix, F.Y.; Assi, C.; Bangoura, A.D.; Allah-Kouadio, E.; Kissi, H.Y.; Touré, A.; Doffou, S.; Konaté, I.; Attia, A.K.; et al. Usefulness of non invasive predictors of esophageal varices in Black African cirrhotic patients in Côte d’Ivoire. Gastroenterol. Res. Pract. 2012, 2012, 216390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Tsao, G.; Sanyal, A.J.; Grace, N.D.; Carey, W. Practice Guidelines Committee of the American Association for the Study of Liver Diseases, Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007, 46, 922–938. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yang, Z.; Yang, Y.; Gao, F.; Liu, X.; Zhang, Q.; Zhu, B.; Jiang, Y.; Wang, X. Serum-ascites albumin gradient: An independent predictor of esophageal variceal bleeding in cirrhosis patients with ascites. Int. J. Clin. Exp. Med. 2019, 12, 8645–8653. [Google Scholar]
- Lawson-Ananissoh, L.M.; Bagny, A.; Bouglouga, O.; Ganbobo, I.M.S.; Yakoubou, R.E.-H.; Kogoe, L.; Kaaga, L.; Redah, D. Interest of serum-ascites albumin concentration gradient in the diagnosis of portal hypertension in cirrhotic patients. Open J. Gastroenterol. 2019, 09, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Budiyasa, D.G.; Ariawan, Y.; Mariadi, I.K.; Wibawa, I.D.; Purwadi, N.; Suryadarma, I.G. Correlation between serum albumin level and degree of esophageal varices in patients with liver cirrhosis. Indones. J. Gastroenterol. Hepatol. Dig. Endosc. 2011, 12, 23–27. [Google Scholar]
- Enas, A.; Dalia, G.; Sara, A.; Wesam, A.I. Correlation between serum-ascites albumin gradient and esophageal varices in patients with portal hypertension. Rep. Opin. 2011, 3, 39–49. [Google Scholar]
- European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 2015, 63, 237–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labrecque, D.; Khan, A.G.; Sarin, S.K.; Le Mair, A.W. Esophageal Varices. In World Gastroenterology Organization Global Guidelines; World Gastroenterology Organization: Milwaukee, WI, USA, 2014; pp. 1–14. [Google Scholar]
- Iqbal, N.; Shah, S.; Hanif, S. Correlation between serum ascites albumin gradient (SAAG) and esophageal varices in patients having chronic liver disease. Pak. Armed Forces Med. J. 2019, 69, 273–278. [Google Scholar]
- Prasad Jagini, S. Correlation of serum-ascites albumin concentration gradient and endoscopic parameters of portal hypertension in chronic liver disease. Int. J. Adv. Med. 2018, 5, 159. [Google Scholar]
- Torres, E.; Calmet, F.; Barrós, P. Endoscopic and clinical parameters in assessing th degree of portal hypertension: The value of the serum-ascitic fluid albumin gradient. Rev. Gastroenterol. Del Peru Organo Soc. Gastroenterol. Del Peru 1996, 16, 20–26. [Google Scholar]
- Shahed, F.H.M.; Mamun-Al-Mahtab, M.; Rahman, S. The Evaluation of Serum ascites albumin Gradient and Portal Hypertensive changes in Cirrhotic Patients with ascites. Euroasian J. Hepatogastroenterol. 2016, 6, 8–9. [Google Scholar] [PubMed] [Green Version]
- Cervantes Pérez, E.; Cervantes Guevara, G.; Cervantes Pérez, G.; Cervantes Cardona, G.A.; Fuentes Orozco, C.; Pintor Belmontes, K.J.; Guzmán Ramírez, B.G.; Reyes Aguirre, L.L.; Barbosa Camacho, F.J.; Bernal Hernández, A.; et al. Diagnostic utility of the serum-ascites albumin gradient in Mexican patients with ascites related to portal hypertension. JGH Open 2020, 4, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, T.M.; Abdel Monem, S.M.; Abdel Wahab, E.; Dawod, H.M.; Ibrahem, M.A. A study on correlation between SAAG and platelet count: Spleen size ratio for the prediction of esophageal varices among chronic liver disease patients. Indian J. Basic Appl. Med. Res. 2015, 7, 502–508. [Google Scholar]
- Gurubacharya, D.L.; Mathura, K.C.; Karki, D.B. Correlation between serum-ascites albumin concentration gradient and endoscopic parameters of portal hypertension. Kathmandu Univ. Med. J. 2005, 3, 327–333. [Google Scholar]

| Variable | Total (n = 80) |
|---|---|
| Age(y) | 54.59 ± 13.23 |
| Sex [No. (%)] | |
| Male | 60 (75.0%) |
| Female | 20 (25.0%) |
| Etiology [No. (%)] | |
| Alcohol intake | 30 (37.5%) |
| HBV | 20 (25.0%) |
| HCV | 12 (15.0%) |
| NAFLD | 5 (5.25%) |
| Wilson’s disease | 1 (1.25%) |
| Other | 12 (15.0%) |
| Serum albumin (g/dL) | 2.49 ± 0.52 |
| Ascitic fluid albumin (g/dL) | 0.53 ± 0.54 |
| Total bilirubin (mg/dL) | 5.39 ± 7.12 |
| Platelet count (G/L) | 138.35 ± 86.54 |
| Ascites protein/fluid (g/dL) | 1.15 ± 1.05 |
| Child–Pugh Score | |
| Child–Pugh A | 3 (3.75%) |
| Child–Pugh B | 19 (23.75%) |
| Child–Pugh C | 58 (72.50%) |
| Esophageal varices | |
| Absent | 6 (7.5%) |
| I | 28 (35.0%) |
| II | 33 (41.2%) |
| III | 13 (16.3) |
| SAAG (g/dL) | |
| 1.10–1.49 | 10 (12.5%) |
| 1.50–1.99 | 34 (42.5%) |
| ≥2 | 36 (45.0%) |
| SAAG (g/dL) | Absence of Esophageal Varices | Presence of Esophageal Varices | p Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| 1.1–1.49 | 5 | 6.25 | 5 | 6.25 | |
| 1.5–1.99 | 1 | 1.25 | 33 | 41.25 | <0.0001 ++ |
| ≥2 | 0 | 0 | 36 | 45.0% | |
| Absence of Esophageal Varices | Presence of Esophageal Varices | p Value | r Value | |
|---|---|---|---|---|
| Age (y) | 46.50 | 55.24 | 0.120 | 0.175 |
| Platelets (G/L) | 233.33 | 130.65 | 0.004 | −0.314 |
| Prothrombin | 19.23 | 21.99 | 0.367 | 0.102 |
| INR | 1.53 | 1.81 | 0.215 | 0.140 |
| Serum albumin (g/dL) | 2.55 | 2.49 | 0.769 | 0.033 |
| Ascites albumin (g/dL) | 1.22 | 0.47 | 0.001 | 0.362 |
| Total bilirubin (mg/dL) | 1.07 | 5.74 | 0.123 | 0.174 |
| Child–Pugh score | 8.17 | 10.66 | <0.001 | 0.388 |
| SAAG | 1.33 | 2.012 | <0.001 | 0.429 |
| OR | 95% CI | p Value | |
|---|---|---|---|
| Platelets (G/L) <150 vs. ≥150 | 1.011 | 0.995–1.027 | 0.1875 |
| Child C vs. A and B | 2.407 | 0.831–6.971 | 0.1056 |
| SAAG (g/dL) ≥1.1 vs. <1.1 | 22.778 | 1.628–318.7109 | 0.0104 |
| Cut-Off SAAG | Se | Spe | PPV (+) | NPV (−) | AUC | |
|---|---|---|---|---|---|---|
| Presence of EVs | 1.75 | 78.38% | 83.33% | 98.31% | 23.81% | 0.952 |
| EVs grade II | 1.8 | 88.24% | 50.79% | 74.14% | 86.36% | 0.704 |
| EVs grade III | 1.9 | 100% | 63.77% | 23.91% | 88.23% | 0.856 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thong, V.D.; Anh, H.T.V. Prediction of Esophageal Varices Based on Serum-Ascites Albumin Gradient in Cirrhotic Patients. Gastroenterol. Insights 2021, 12, 270-277. https://0-doi-org.brum.beds.ac.uk/10.3390/gastroent12020023
Thong VD, Anh HTV. Prediction of Esophageal Varices Based on Serum-Ascites Albumin Gradient in Cirrhotic Patients. Gastroenterology Insights. 2021; 12(2):270-277. https://0-doi-org.brum.beds.ac.uk/10.3390/gastroent12020023
Chicago/Turabian StyleThong, Vo Duy, and Ho Thi Van Anh. 2021. "Prediction of Esophageal Varices Based on Serum-Ascites Albumin Gradient in Cirrhotic Patients" Gastroenterology Insights 12, no. 2: 270-277. https://0-doi-org.brum.beds.ac.uk/10.3390/gastroent12020023






