Radiological Parameters Review for Choanal Atresia
Abstract
:1. Introduction
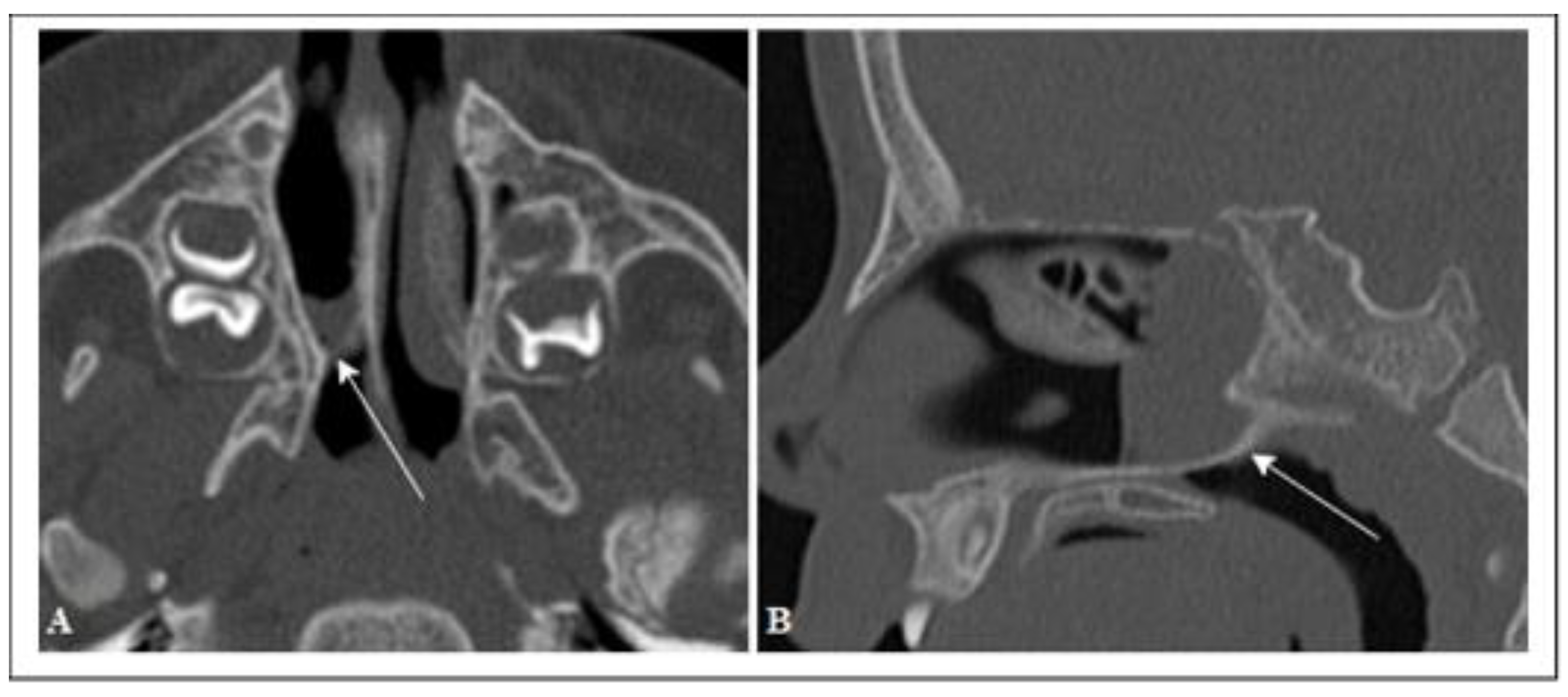
2. Materials and Methods
- −
- Children with an age range <1 year;
- −
- Children with an age range of 1–3 years.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHARGE | coloboma of the eye: heart defects: atresia of the nasal choanae, retardation of growth and development, genital and urinary abnormalities, and ear abnormalities, and deafness syndrome |
| CT | computer tomography |
| HRCT | High-resolution computer tomography |
| MPR | multiplanar reconstruction |
| CH | Choanal height |
| RW | Rostrum width |
| RH | Rostrum height |
| REF | reference |
References
- Kwong, K.M. Current Updates on Choanal Atresia. Front. Pediatrics 2015, 3, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, J.R.; Harmon, P.; Shirley, W.P.; Hill, J.S.; Woolley, A.L.; Wiatrak, B.J. Operative management of choanal atresia: A 15-year experience. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 71–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wormald, P.J.; Zhao, Y.C.; Valdes, C.J.; Pacheco, A.E.; Ha, T.N.; Tewfik, M.A.; Wabnitz, D.; Shaw, C.K. The endoscopic transseptal approach for choanal atresia repair. Int. Forum Allergy Rhinol. 2016, 6, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Samadi, D.S.; Shah, U.K.; Handler, S.D. Choanal atresia: A twenty-year review of medical comorbidities and surgical outcomes. Laryngoscope 2003, 113, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Teissier, N.; Kaguelidou, F.; Couloigner, V.; François, M.; Van Den Abbeele, T. Predictive factors for success after transnasal endoscopic treatment of choanal atresia. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 57–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hengerer, A.S.; Brickman, T.M.; Jeyakumar, A. Choanal atresia: Embryologic analysis and evolution of treatment, a 30-year experience. Laryngoscope 2008, 118, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Brown, O.E.; Pownell, P.; Manning, S.C. Choanal atresia: A new anatomic classification and clinical management applications. Laryngoscope 1996, 106, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Brown, O.E.; Smith, T.; Armstrong, E.; Grundfast, K. The evaluation of choanal atresia by computed tomography. Int. J. Pediatric Otorhinolaryngol. 1986, 12, 85–98. [Google Scholar] [CrossRef]
- Aslan, S.; Yilmazer, C.; Yildirim, T.; Akkuzu, B.; Yilmaz, I. Comparison of nasal region dimensions in bilateral choanal atresia patients and normal controls: A computed tomographic analysis with clinical implications. Int. J. Pediatric Otorhinolaryngol. 2009, 73, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Likus, W.; Bajor, G.; Gruszczyńska, K.; Baron, J.; Markowski, J. Nasal region dimensions in children: A CT study and clinical implications. BioMed Res. Int. 2014, 2014, 125810. [Google Scholar] [CrossRef] [PubMed]
- Waitzman, A.A.; Posnick, J.C.; Armstrong, D.C.; Pron, G.E. Craniofacial skeletal measurements based on computed tomography: Part II. Typical values and growth trends. Cleft Palate Craniofac. J. Off. Publ. Am. Cleft Palate Craniofac. Assoc. 1992, 29, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Waitzman, A.A.; Posnick, J.C.; Armstrong, D.C.; Pron, G.E. Craniofacial skeletal measurements based on computed tomography: Part I. Accuracy and reproducibility. Cleft Palate Craniofac. J. Off. Publ. Am. Cleft Palate Craniofac. Assoc. 1992, 29, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Corrales, C.E.; Koltai, P.J. Choanal atresia: Current concepts and controversies. Curr. Opin. Otolaryngol. Head Neck Surg. 2009, 17, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Slovis, T.L.; Renfro, B.; Watts, F.B.; Kuhns, L.R.; Belenky, W.; Spoylar, J. Choanal atresia: Precise CT evaluation. Radiology 1985, 155, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.; Prince, M. Comparison of craniofacial skeletal characteristics of infants with bilateral choanal atresia and an age-matched normative population: Computed tomography analysis. J. Otolaryngol. 2001, 30, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, N.S.; Bartley, A.C.; Bekhit, E.; Berkowitz, R.G. Skull base anatomy and surgical safety in isolated and CHARGE-associated bilateral choanal atresia. Int. J. Pediatric Otorhinolaryngol. 2018, 115, 61–64. [Google Scholar] [CrossRef] [PubMed]



| Age Ranges | Average Age (Days) | Females | Males | Total | |
|---|---|---|---|---|---|
| Study group | ≤1 year | 46.79 ± 98.39 | 8 | 6 | 14 |
| 1–3 year | 657.20 ± 354.09 | 2 | 3 | 5 | |
| Total | 207.42 ± 333.34 | 10 | 9 | 19 | |
| Control group | ≤1 year | 151.94 ± 82.20 | 8 | 8 | 16 |
| 1–3 years | 806.05 ± 344.35 | 6 | 13 | 19 | |
| Total | 507.03 ± 418.40 | 14 | 21 | 35 |
| Measurements | Age | Study Group | Control Group | p-Value | REF. | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Media (mm) | Std. Dev. | N | Media (mm) | Std. Dev. | ||||
| Choanal height (CH) | ≤1 year | 14 | 8.1 | 1.6 | 16 | 12.0 | 1.9 | <0.001 | [1] |
| 1–3 years | 5 | 14.2 | 1.3 | 19 | 16.2 | 2.5 | 0.103 | [1] | |
| Rostrum width (RW) | ≤di 1 anno | 14 | 6.3 | 1.2 | 16 | 6.3 | 1.1 | 0.932 | [1] |
| 1–3 years | 5 | 8.2 | 2.8 | 19 | 7.9 | 0.9 | 0.731 | [1] | |
| Rostrum height (RH) | ≤1 year | 14 | 3.6 | 0.7 | 16 | 5.1 | 1.0 | <0.001 | [1] |
| 1–3 years | 5 | 7.0 | 2.3 | 19 | 6.6 | 1.3 | 0.640 | [1] | |
| Anterior interorbital distance (AID) | ≤1 year | 14 | 11.3 | 2.1 | 104 | 17.8 | 1.4 | <0.001 | [2] |
| 1–3 years | 5 | 14.9 | 3.7 | 65 | 18.3 | 1.8 | <0.001 | [4] | |
| Inter-orbital distance average (MID) | ≤1 year | 14 | 14.8 | 2.2 | 104 | 16.8 | 1.5 | <0.001 | [2] |
| 1–3 years | 5 | 20.6 | 2.1 | 65 | 19.2 | 1.9 | 0.118 | [4] | |
| Bone septum thickness (VW) | ≤1 year | 14 | 4.9 | 1.2 | 104 | 2.2 | 0.6 | <0.001 | [2] |
| 1–3 years | 5 | 3.5 | 0.9 | 69 | 3.7 | 0.8 | 0.665 | [3] | |
| Septum length (LS) | ≤1 year | 14 | 28.4 | 2.2 | 104 | 27.6 | 4.5 | 0.500 | [2] |
| 1–3 years | 5 | 38.4 | 3.9 | 69 | 38.2 | 2.9 | 0.923 | [3] | |
| Maximum septum length (MLS) | ≤1 year | 14 | 40.1 | 2.8 | 111 | 45.4 | 5.4 | 0.001 | [3] |
| 1–3 years | 5 | 47.5 | 11.4 | 69 | 53.2 | 4.5 | 0.018 | [3] | |
| Anterior bone width (ABW) | ≤1 year | 14 | 13.9 | 1.9 | 104 | 14.3 | 1.6 | 0.343 | [2] |
| 1–3 years | 5 | 17.2 | 3.8 | 69 | 18.6 | 1.3 | 0.055 | [3] | |
| Posterior right bone width (RPBW) | ≤1 year | 14 | 2.1 | 1.4 | 111 | 7.3 | 0.7 | <0.001 | [3] |
| 1–3 years | 5 | 4.6 | 1.8 | 69 | 8.6 | 0.9 | <0.001 | [3] | |
| Posterior left bone width (LPBW) | ≤1 year | 14 | 2.3 | 1.4 | 111 | 7.2 | 0.9 | <0.001 | [3] |
| 1–3 years | 5 | 8.0 | 2.7 | 69 | 8.5 | 0.6 | 0.193 | [3] | |
| Bone width of choana (BCAW) | ≤1 year | 14 | 9.4 | 2.3 | 104 | 13.2 | 1.4 | <0.001 | [2] |
| 1–3 years | 5 | 16.6 | 1.5 | 69 | 19.8 | 1.5 | <0.001 | [3] | |
| Vertical distance nasopharynx (NVD) | ≤1 year | 14 | 16.9 | 4.0 | 104 | 20.7 | 3.2 | <0.001 | [2] |
| 1–3 years | 5 | 25.8 | 5.3 | nd | |||||
| Horizontal nasopharynx distance (NHD) | ≤1 year | 13 | 10.5 | 1.7 | 104 | 11.6 | 2.0 | 0.046 | [2] |
| 1–3 years | 5 | 16.2 | 1.3 | nd | |||||
| Measurements | Description |
|---|---|
| Choanal height (CH) | The distance between the horizontal lamina of the palatine bone and the body of the sphenoid |
| Rostrum width (RW) | The maximum width of the sphenoid triangular bone spine. |
| Rostrum height (RH) | The distance between the body of the sphenoid and the junction point of the vomer wings |
| Anterior interorbital distance (AID) | The distance between a point on each tear bone that represents the anterior end of the medial orbital wall |
| Mid Interorbital Distance (MID) | The distance between a point on each medial wall of the bone orbit (ethmoid bone) halfway between the torn bone and the base of the optical pillar |
| Septum thickness (VW) | The maximum width of the vomer |
| Septum length (LS) | The distance from the pyriform opening to the rear end of the vomer |
| Maximum septum length (MLS) | The maximum septum length from the most anterior part of the nasal septum to the rear end of the vomer |
| Anterior bone width (ABW) | The distance between the two ridges protruding from the anterior wall of the maxillary bones |
| Bone width of choana (BCAW) | The distance between the two pterygoid processes |
| Posterior right bone width (RPBW) | The distance between the lateral bone wall of the right nasal cavity and the mucosa of the septum |
| Posterior left bone width (LPBW) | The distance between the lateral bone wall of the left nasal cavity and the mucosa of the septum |
| The vertical distance of the nasopharynx (NVD) | The distance between the rear vomer and the base of the skull |
| Horizontal nasopharynx distance (NHD) | The distance between the middle third of the nasopharyngeal sidewalls |
| Coeff. Correlation R2 | Correlation with Age (in Days) | |
|---|---|---|
| Study Group | Control Group | |
| Choanal height (CH) | 0.77 | 0.69 |
| Rostrum width (RW) | 0.31 | 0.47 |
| Rostrum height (RH) | 0.60 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messineo, D.; Chernikava, M.; Pasquali, V.; Bertin, S.; Ciotti, M.; de Soccio, G.; Savastano, V.; Catalano, C. Radiological Parameters Review for Choanal Atresia. Pediatr. Rep. 2021, 13, 302-311. https://0-doi-org.brum.beds.ac.uk/10.3390/pediatric13020038
Messineo D, Chernikava M, Pasquali V, Bertin S, Ciotti M, de Soccio G, Savastano V, Catalano C. Radiological Parameters Review for Choanal Atresia. Pediatric Reports. 2021; 13(2):302-311. https://0-doi-org.brum.beds.ac.uk/10.3390/pediatric13020038
Chicago/Turabian StyleMessineo, Daniela, Maryia Chernikava, Valeria Pasquali, Serena Bertin, Mario Ciotti, Giulia de Soccio, Vincenzo Savastano, and Carlo Catalano. 2021. "Radiological Parameters Review for Choanal Atresia" Pediatric Reports 13, no. 2: 302-311. https://0-doi-org.brum.beds.ac.uk/10.3390/pediatric13020038






