The present work led to the development of a strategy for conversion of nutrients in milk to various valuable products via a two-stage fed-batch cultivation with N. intermedia and A. oryzae in membrane bioreactors. During the first stage, A. oryzae degrades fat and protein producing biomass, thus easing medium filtration into a second membrane bioreactor, where N. intermedia converts the nutrients into additional biomass and ethanol. These products add to the released and unconsumed fatty acids, amino acids, and glycerol due to the hydrolysis reactions in the first bioreactor. Preliminary studies were conducted in both semi-synthetic medium and expired milk to gain insights in fungal growth for further reactor cultivation development.
3.1. Cultivation in Semi-Synthetic Medium
The fungus
N. intermedia has been previously reported [
19] to hydrolyze lactose and assimilate its degradation monomers glucose and galactose. Additionally, there are other nutrients in dairy waste, namely lactic acid, which is a product of bacterial metabolism, fat that releases glycerol and fatty acids when degraded, proteins, and minerals. Therefore, the influence of several factors, including glycerol and lactic acid concentration, pH, and medium supplementation with copper sulphate, trace metals and yeast extract on the growth of
N. intermedia were studied in semi-synthetic medium. Ultimately, a performance comparison was carried out between
N. intermedia growth in semi-synthetic medium and expired milk. Glycerol was included to study the potential of using the biological conversion to both low- and high-fat dairy substrates and show the potentially wide application of the final developed strategy. Cultivations in semi-synthetic medium were carried out with only
N. intermedia in view of the composition of the medium reaching the second bioreactor as part of the integrated strategy developed within this work. Understanding which conditions and substrate preference is of utmost importance in order to reach complete assimilation of nutrients in the second cultivation leading to a clear effluent easy to treat.
From single-carbon cultivations using glycerol at 10, 20, and 30 g/L and lactose at 50 g/L, a similar consumption of glycerol of 7–8 g/L and 11 g/L of lactose was obtained after 96 h of cultivation (
Figure 2a). Consumption rates were 0.04–0.08 and 0.09–0.012 g/L/h when the carbon source was glycerol or lactose, respectively. Finally, glycerol consumption and maximum consumption rates were not statistically different among the conditions tested here (
p = 0.603). The biomass obtained (4 g/L) was similar for all conditions studied. When both carbon sources were added to the semi-synthetic medium (lactose at 50 g/L and glycerol at 20 g/L),
N. intermedia consumed similar amounts (3–4 g/L) of both carbon sources. Substrate consumption maximum rates were significantly lower than those during single-carbon cultivations (0.02–0.03 (
p = 0.001) and 0.02–0.05 g/L/h (
p = 0.01) of glycerol and lactose, respectively).
When lactic acid (at 5, 10, 15 and 20 g/L) was added to the medium containing lactose and glycerol, carbon substrate consumption was inhibited at lactic acid concentrations above 10 g/L. A similar observation for lower lactic acid concentrations was that
N. intermedia first depleted lactic acid before it started to consume lactose and glycerol, highlighting most probably the need of the fungus to relieve the inhibitory effect of the acid (
Figure 2b). Only 1.3 g/L of lactose and 3.1 g/L of glycerol were consumed after 6 days of cultivation at 5 g/L of lactic acid, whereas only 1 g/L of glycerol was consumed when the acid was added at 10 g/L. Therefore, the results show that
N. intermedia can grow in media containing ≤10 g/L of lactic acid; however, longer lag phases are obtained.
In a further set of experiments, the addition of copper sulphate as a strategy to add the lactase cofactor, trace metals, or a higher amount of yeast extract did not lead to further statistically significant improvements in the assimilation of carbon sources (
p = 0.227) (
Figure 2c). Controlling the pH during cultivation at 4.0, 4.5, and 5.0 did not lead to differences regarding carbon assimilation. On the other hand, inoculation of the medium with pre-grown biomass instead of spores significantly increased lactose consumption (18 vs. 5.5 g/L;
p = 0.030) and
N. intermedia preference for lactose instead of glycerol became clearer (
Figure 2d). Altogether, the components present in the semi-synthetic medium used in this study did not seem to support a satisfactory consumption of lactose; or when the disaccharide was present either as the sole carbon source or together with glycerol, suggesting the lack of key nutrients in the recipe.
3.3. MBR Fungal Cultivation in Sterile and Non-Sterile Milk Medium
Considering the results of shake flask fungal cultivations in sterile (S-) and non-sterile (NS-) milk and its fractions, MBR cultivations were performed to evaluate the filtration performance and the compatibility of the results in larger scale. In order to have an understanding of the filtration behavior of milk using synthetic polymer membrane, initially 850 mL of 2- and 10- times diluted NS-whole milk were subjected to filtration using 0.3 and 1 µm pore sizes PES IPC membrane. It was observed that, as expected due to the complex lipid and protein content of milk [
25], the membrane surface totally fouled in both media (figure not presented). As reported in the literature, this filtration failure in milk media is mainly due to the presence of lipids [
25,
26]. This deterioration in filtration performance was demonstrated by the sharp drop in the permeate flow. The best filtration performance was obtained for the 10-times diluted sample, with an average 13 mL/h permeate withdrawal, running for 3 h before complete fouling.
It has previously been reported that the fungal strains
A. oryzae and
N. intermedia applied in this research work are capable of secreting a variety of enzymes such as lactases for lactose hydrolysis and assimilation, and proteases and lipases that assist denaturation and degradation of protein and fat components of dairy products, respectively [
10,
27]. Therefore, in order to examine fungal utilization of nutrients to facilitate membrane filtration of the products, bioreactor cultivations of
N. intermedia in lactose-rich (liquid supernatant after centrifugation) and
A. oryzae in protein- and lipid-rich fractions of milk were performed. The protein- and lipid-rich fractions of milk consisted of the remaining solids after centrifugation of milk containing fat and thermally denaturized proteins. As
N. intermedia was cultivated in the liquid fraction of milk, similar lactose assimilation compared to that of shake flask cultivation was obtained for both S- (20 g/L) and NS-media (29 g/L). Moreover, after 120 h of cultivation, the initially turbid liquid fraction had turned into a clear medium containing suspended fungal biomass. Due to the presence of lactic acid bacteria (LAB) in the NS-fraction, 8 g/L of lactic acid were built up in the medium from 48 to 72 h (
Figure 6a). Not only did the presence of this level of lactic acid in the media not disturb fungal activity, but
N. intermedia was even capable of utilizing and reducing the acid level until 120 h. Production of nearly 3 g/L ethanol was observed in the NS-cultivation, which could be attributed to the activity of the heterofermentative LAB [
28] and/or
N. intermedia due to the favorable low pH as the result of lactic acid production [
10].
The same approach was taken in cultivation of
A. oryzae in the solid fraction of milk. In parallel, the same S- and NS-media were used for shake flask fungal cultivation. The glycerol released in both conditions, due to fat degradation, was ca 0.04–0.05 g/L (
Figure 6b).
Aspergillus oryzae can degrade high contents of dairy lipid to glycerol and fatty acids in fat-rich dairy media [
11]. However, regarding the physical characteristics of the media, it was observed that about 8 g/L of biomass was produced after 120 h of cultivation in S- and NS-media, and a clear and transparent liquid remained in both cases.
As the main goal of this research work was to treat whole milk using an MBR to have an integrated process with reduced processing stages benefiting from the different metabolic activities of the two fungal strains, MBR cultivation of both fungal strains in whole milk was investigated. In this regard, fungi were cultivated in whole milk in batch mode for 72 h prior to the start of filtration. However, due to excessive casein denaturation and separation, severe foam formation as the result of aeration and casein build-up on the reactor head space, and also considerable membrane fouling, filtration could not be preceded effectively.
In order to alleviate the issues related to excessive foaming and casein separation, remediate membrane fouling and ease of the filtration of the medium, and to reduce the conversion time needed for fungal treatment, milk samples were diluted using sterile milli-Q water. The whole milk was diluted up to ~70% based on the aforementioned filtration results of the diluted milk with no acting microorganism. Fungal strains of
A. oryzae and
N. intermedia were cultivated in separate MBRs for a period of 120 h (
Figure 6c). In accordance to the earlier made membrane filtration experiments, after 72 h, both media were subjected to filtration for the removal of fungal metabolites while retaining the fungal mycelia in the reactor. In order to keep the liquid level constant, permeate obtained through filtration was recirculated back to the media. It was observed that although the treated medium with
A. oryzae could easily be filtrated without any substantial membrane fouling and reduction in permeate flux (stable at 6.12 mL/h.cm
2) for several hours, the medium used for cultivation of
N. intermedia fouled the membrane in early stages of filtration (flux dropped to 0.26 mL/h.cm
2). This 96% difference in filterability of the same media inoculated with different fungal strain proves that, unlike
N. intermedia, A. oryzae has been capable of degrading and/or consuming milk lipids and proteins that were previously problematic regarding membrane fouling. The final biomass obtained from cultivation
N. intermedia (6 g/L) in this condition was nearly half of that of
A. oryzae (11 g/L). However,
N. intermedia proved to be a better strain in controlling the cultivation conditions by utilizing lactic acid resulting from bacterial activity and controlling the pH. As the pH in the preparation with
N. intermedia dropped from 6.3 to about 5.4 throughout 144 h cultivation, that of
A. oryzae was substantially lower (pH = 4.3). Therefore, in order to have full conversion of milk nutrient by fungal activity using a stepwise treatment with
A. oryzae prior to cultivation of
N. intermedia for lactose removal in an MBR is essential. As it is illustrated in
Figure 6c and as it has been corroborated in shake flask scale experiments,
N. intermedia more successfully assimilates lactose reaching 64% consumption of its initial concentration in comparison to 27% by
A. oryzae after 120 h. Although the initial fat content of the diluted milk media was low (9 g/L), the release of 0.3 g/L of glycerol during
A. oryzae (48 h) cultivation dominates fat degradation. The glycerol released was later consumed by the fungal strain. Moreover, during cultivation of
A. oryzae, after 12 h of cultivation, casein content of the medium totally coagulated most probably due to the secretion of aspartic proteases by the fungal strain [
27,
29]. However, this separated casein was further dispersed as suspended particles by 72 h of cultivation.
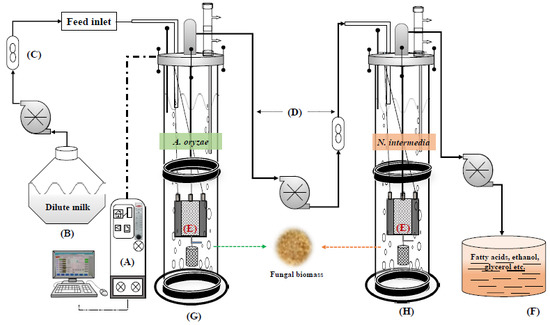
Based on the acquired results, in order to build up a robust fungal bioconversion of expired milk into value-added products and to reduce the COD of the treatment effluent, a double-stage iMBR was developed. As presented in
Figure 1, the double-stage MBR included an iMBR for cultivation of
A. oryzae in diluted milk, a buffer tank, and a second iMBR for cultivation of
N. intermedia in the permeate from the first reactor. The interconnected MBR system was set in a semi-continuous mode.
A. oryzae was cultivated in the first MBR containing 3.5 l of diluted milk for 72 h followed by filtration and removal of 1 l of the medium to the connected buffer tank. This 1 l was replaced by fresh milk medium. After 48 h of cultivation the second filtration cycle, another 1 l was withdrawn and fed to the tank. The second MBR was then fed with the 2 l permeate (starting volume) and inoculated with
N. intermedia. From this stage onwards, every 72 h, 0.5 l of the medium in the second MBR was removed and directly replaced by 0.5 l of permeate from 48 h treated medium from the first MBR.
The changes in the concentration of different compounds during cultivation of
A. oryzae in semi-continuous mode with periodical filtration and feeding are presented in
Figure 7a. During the start-up phase, prior to filtration, 41% of the initial lactose content of the medium was converted to biomass and metabolites by
A. oryzae. This lactose consumption in between every feeding interval was around 50% during 48 h. This better lactose consumption of
A. oryzae in the MBR compared to shake flask cultivations could be due to better fungal growth condition in the highly aerated reactor. Although the production of lactic acid as a result of LAB activity was not detected in the 1st stage MBR, bacteria are likely to be present in the non-sterile milk medium; acetic acid producing bacteria actively produced the acid from 72 h to the end of the cultivation (4.2 g/L). The semi-continuous iMBR cultivation proved to be satisfyingly robust in pH control, not to be dramatically affected by the changes in the acetic acid content of the medium, as the filtration and feeding regime plus metabolic activity of
A. oryzae could sustain the pH of the medium within 4.6–6.4 throughout the cultivation (
Figure 7a).
The same trend of changes in the lactose content of the medium was observed while filtration and feeding was applied from 72 to 124 h, followed by a further decrease in lactose concentration resulting in a final biomass of ca 11 g/L. As
A. oryzae has degraded compounds (proteins and lipids) problematic for filtration, the clear permeate medium is withdrawn at each stage without excessive membrane fouling and reserved in the buffer tank. The medium kept in the connecting tank for 48 h, containing bacteria and bacterial metabolites (lactic and acetic acids), comprised the starting feed to the 2nd stage MBR. In addition to lactose,
N. intermedia has been capable of assimilating acids fed to the culture as a result of bacterial activity in expired milk (
Figure 7b). The ability of
N. intermedia to simultaneously consume lactic acid and milk lactose was observed previously in shake flask experiments and also in literature [
11]. This metabolic activity was accompanied with an increase in the initial pH from 5.1 to 7.1 by the end of the cultivation (
Figure 7b). By the end of the 2nd stage MBR cultivation (144 h), full conversion of the feed substrate (lactose and acids) was successfully achieved resulting in a biomass content of ca 7 g/L.
This semi-continuous process has the potential to efficiently convert expired milk to valuable fungal biomass and other metabolites while lowering the organic load of the effluent (clear permeate where 83% of the initial COD has been reduced) and corresponding environmental impact. It can be hypothesized that this process can be applied to more concentrated milk and dairy media if the duration of conversion at each stage is taken into consideration. In addition, such integration has various advantages including its continuous character that hampers the need to restart batch after batch, the possibility to produce biomass for feed, the control of bacterial dominance over the cultivation which circumvents the need of a sterilization step and no pH adjustment required since the bacterial metabolites are lowered through fungal activity. In order to benefit more from the highly valuable by-products of this process, by integration of an ultrafiltration unit in the general picture of a waste biorefinery, enzymes such as proteases, namely keratinase, pectin lyase, and lipases secreted by fungi throughout the process, can be isolated.