3.1. Case Study Settings and Results for Production Planning and Emission Reduction in an Oil Refinery
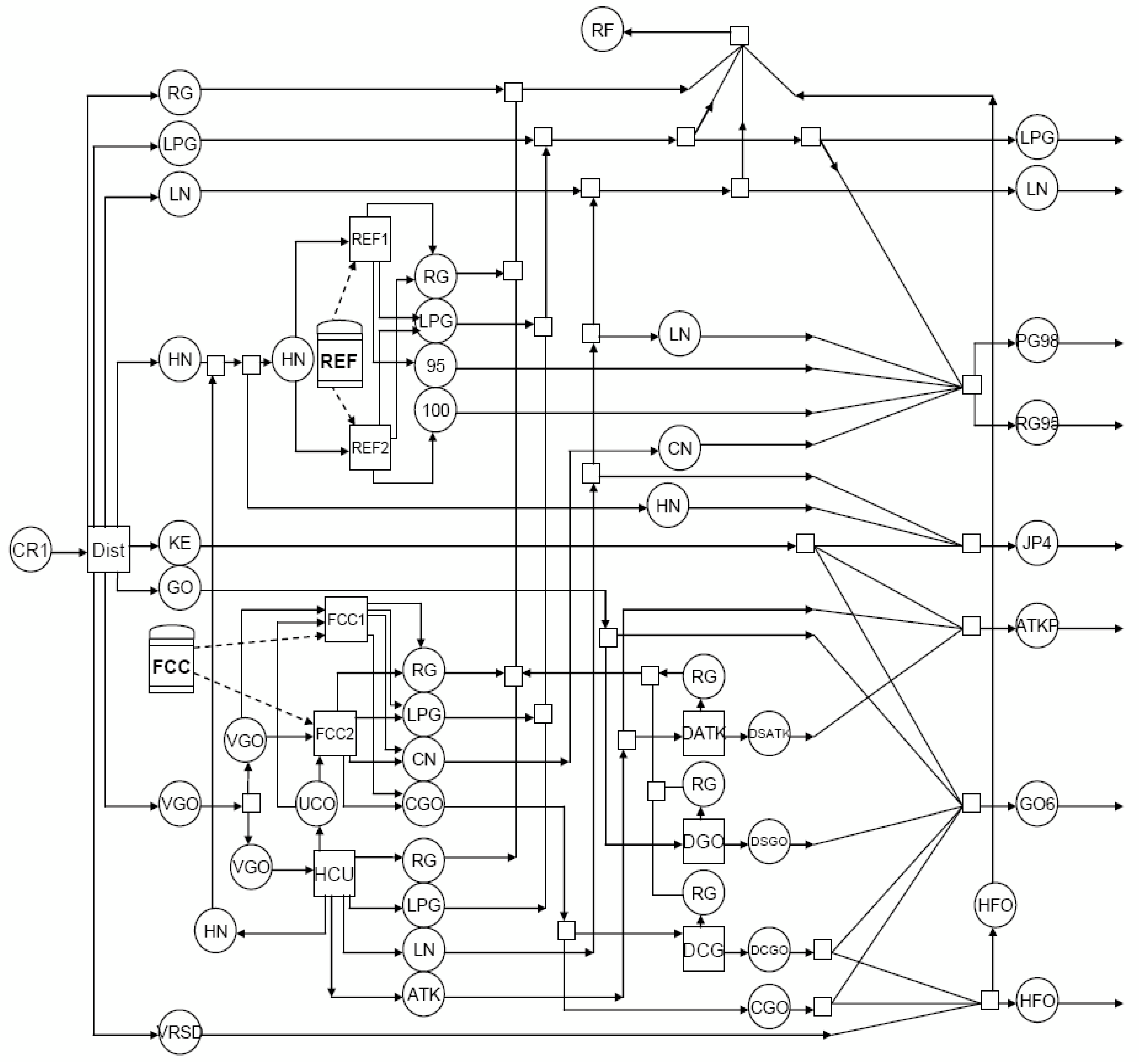
The oil refinery flow diagram considered in the case studies is shown in
Figure 1, and the major unit capacity limits are shown in
Table 1. The planning period considered is one year, and the feedstock to the refinery is of a single type (e.g., Arabian light crude) with a total flow rate of 12,000 kton/y in order to produce different final products. These parameters are inspired by the work of Al-Qahtani [
32], and further details about parametrization and the following results are available in Alnahdi [
31].
The pollutants considered in this study are SOx and NOx, whereas CO2 emission is incorporated into the analysis as a greenhouse effect. Several emission mitigation alternatives are considered. Among these, fuel switching represents switching from the current fuel (fuel oil) to natural gas. The SOx capture process considered in this study is wet scrubber (WS) with fuel-gas desulphurization (FGD) technology, and the considered NOx capture process is based on retrofitting current burners with low NOx burner technology (will be denoted as LNB hereafter). For the CO2 capture process, MEA absorption technology is considered.
CO
2 emissions parameters are estimated based on the work by Ritter et al. [
33], whereas emissions data of the pollutants are based on Kassinis [
34]. Cost parameters associated with fuel switching and MEA capture process are estimated based on the work by Elkamel et al. [
35]; while, those associated with SO
x and NO
x capture processes are taken from a report by the World Bank [
36].
Three scenarios are considered in this study in order to illustrate the validity of the model discussed in the previous section. For the first scenario, the process planning model in
Section 2.1 is solved without any emissions mitigation, which is considered as the base case scenario. The second scenario is related to reducing one particular emission at a time (e.g., CO
2) without considering other emissions (e.g., SO
x and NO
x) using a special case of the model in
Section 2.2. That is, each pollutant is reduced in an independent manner. Finally, the third scenario is related to the examination of reducing different emissions together in a general/dependent manner using the general model in
Section 2.2.
3.1.1. Results of Base Case Scenario
The objective of this base case scenario is to minimize the overall annualized cost minus the revenue from export while meeting the internal demand for each product with quality specifications. The market demands and specifications for different products that the refinery has to meet are shown in
Table 2, and this is applied for all case studies. The emission results of the base case scenario, where no emission reduction plans are considered, are shown in
Figure 3. These results imply a total annual production cost minus export revenue of
$3,295,058 for the considered refinery. The total emissions from all the units for SO
x, NO
x, and CO
2 were 8170.6, 2826.9 and 1342.3 kton/y, respectively.
3.1.2. Results for Independent Emission Reduction
In this scenario, the focus is on reducing one emission at a time without considering the other pollutants, in order to analyze the impact of reducing each emission independently and to figure out the overall refinery cost correspondingly. Comparisons of cost increment when reducing SO
x, NO
x, and CO
2 emissions are given in
Table 3,
Table 4 and
Table 5, respectively.
For the SO
x reduction scenario, it has been observed that with only a 6.6% increase in cost, almost 60% of SO
x releases can be alleviated by installing a wet scrubber capture process (WS) and/or through switching of fuels. On the other hand, NO
x discharge may be reduced by retrofitting the existing burners with LNB. There may be a maximum emissions reduction of 60% at a cost increment of 6.9% according to
Table 4. The NO
x release is higher when no fuel switching was selected over the present fuel.
Table 5 shows that a reduction of 60% of CO
2 emissions can be achieved in exchange for a 6.7% increase in cost. Furthermore, it has been observed that a decrease in the CO
2 discharge from 25% to 10% has been achieved without shifting units to the natural gas or by setting up extra MEA capturing practices. This decrease has been attained through load shifting, which is the recommended option for 10% emissions reduction target with a minor increase in cost. These scenarios demonstrate the flexibility of the model for proposing diverse emission reduction actions with different targets.
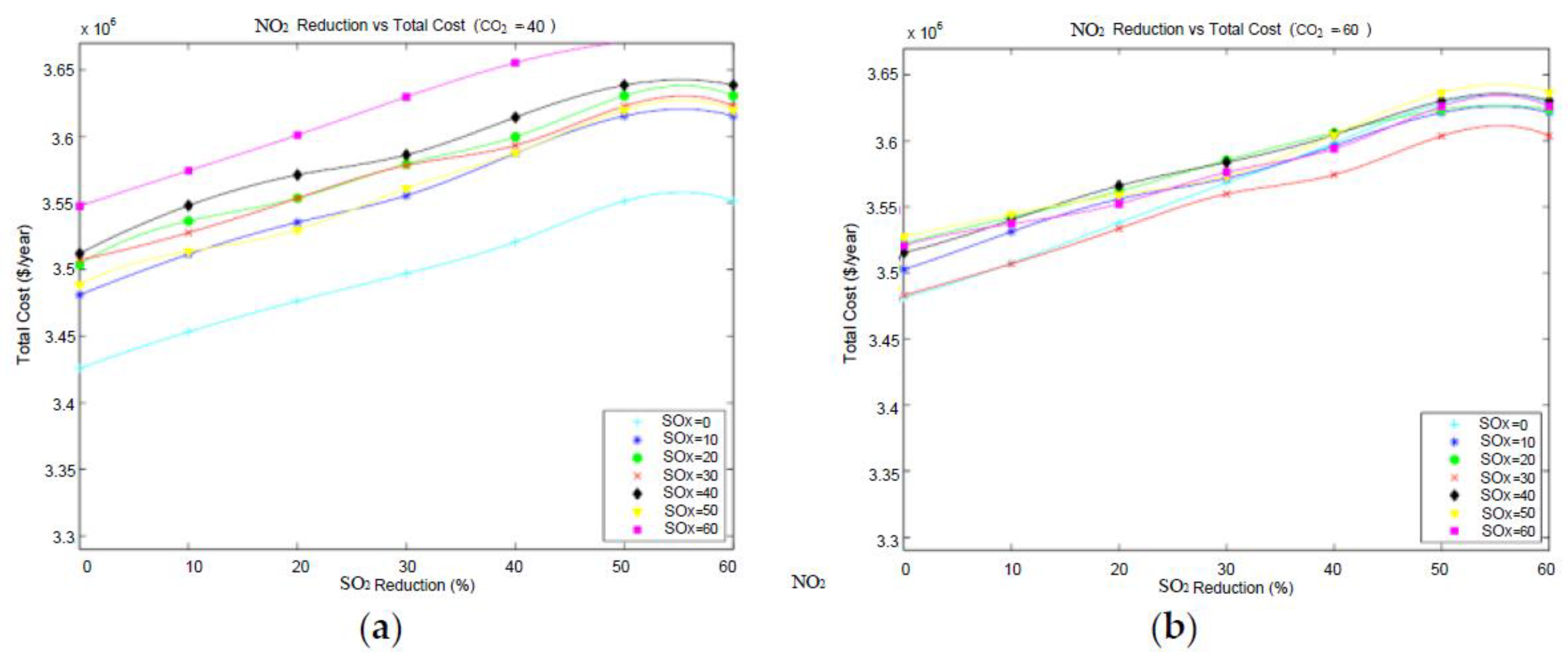
3.1.3. Results for Simultaneous Emissions Reduction
The goal here is to mitigate SO
2, NO
2, and CO
2 emissions simultaneously to various levels, and to compare the overall costs of the resulting mitigation plan.
Table 6 provides a summary of selected scenarios when varying reduction targets for all emissions including SO
2, NO
2, and CO
2 and the corresponding annual cost for each plan. For instance, reducing SO
2, NO
2, and CO
2 by 10%, 30%, and 60% each, results in increasing the annual cost by 3.2%, 9.2%, and 10.7%, respectively.
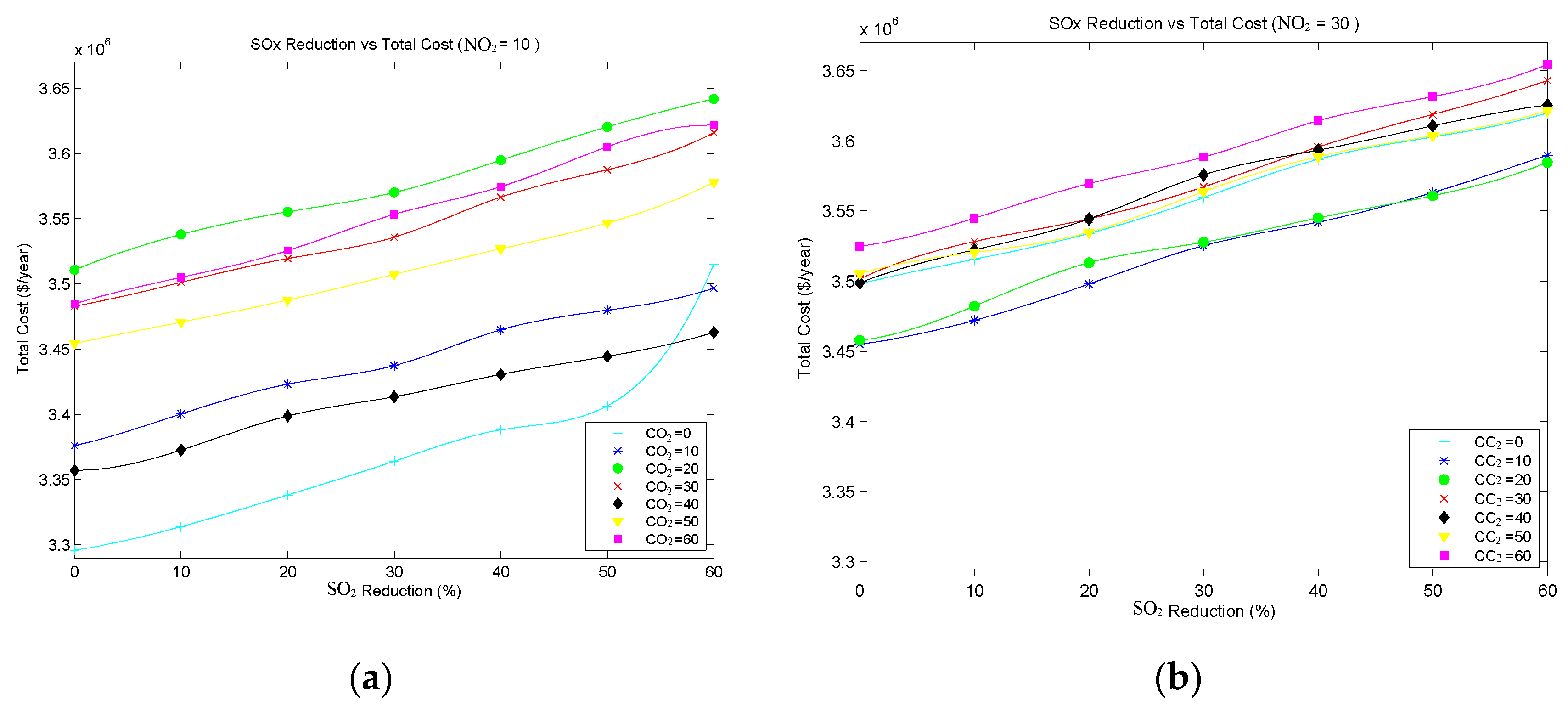
Figure 4a and
Figure 5b show the annual cost when reducing SO
2 from 0% to 60% with varying CO
2 and NO
2 reduction levels. These figures show that overall cost increases almost linearly with the emissions reduction level.
3.2. Case Study Details and Results for Air Pollution Dispersion
In this section, CALPUFF and SCREEN3 modeling tools are used to estimate the overall concentration of SO
x, NO
x, and CO
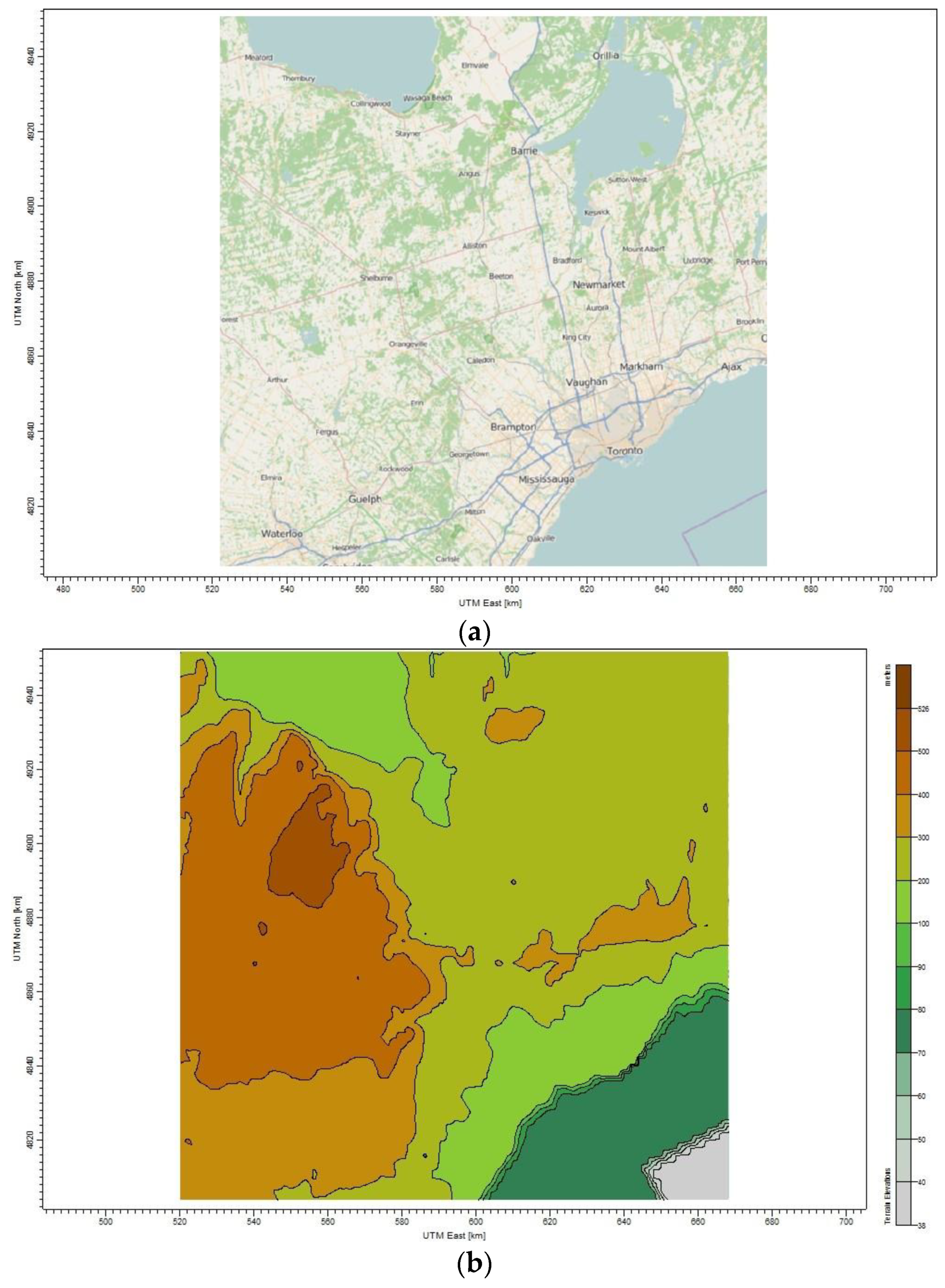
2 dispersed from an oil refinery site located in North Toronto, Ontario-Canada. This refinery has a similar process design to the one illustrated in
Figure 1. The location of this refinery is 50 km away from the urban Toronto; thus, there is a need for a screening analysis gauging to what extent the emission from the refinery under the proposed mitigation strategies would affect the surrounding area.
The first step in air pollution analysis with CALPUFF model is identifying the meteorological domain information for the case study region, which is given in
Table 7. The surface data for the Toronto area were acquired from the weather records in the Canadian government website [
37]. The surface stations were chosen based on their proximity to the source point and upper air stations. For each station, the hourly data includes the date, time, temperature, wind speed and direction, ceiling height, cloud cover, and station pressure. The hourly data for two modeling periods from (i) 1 January 2014 at 00:00 h to 31 January 2014 at 23:00 h; (ii) 1 May 2014 at 00:00 h to 31 May 2014 at 23:00 h were extracted and organized in a certain layout that is suitable for use in ‘SMERGE’ to create a formatted file ‘SURF.DAT’, which is compatible for usage with CALMET. The relevant surface station data is shown in
Table 8.
The meteorological data of the upper air for the location was obtained from the radiosonde station records in the National Oceanic and Atmospheric Administration (NOAA) Earth System Research Laboratory (NOAA/ESRL) radiosonde database [
38]. These data records contain the station ID number, date and time, and information of the sounding level followed by pressure, temperature, elevation, and wind direction and speed for each sounding level. The hourly data for the Toronto area was taken from one radiosonde station that is close to the study site for the two modeling periods mentioned above and was then prepared in a format suitable to use in “READ62” to generate the “UP.DAT” file that was used in the CALMET program. The relevant information about the radiosonde station is shown in
Table 9. The geophysical data such as land use and terrain were obtained from the website of Geographic Information Systems [
39], and used as input files in CTG-PROC and TERREL to produce “LU.DAT” and “TERREL.DAT”, respectively. All this data is compressed by a MAKEGEO program to generate the output file ‘GEO.DAT’, which was used in the CALMET program.
The proposed mathematical model in
Section 2.2 was used to find the pollutant emission rates for the case study. The source parameters for the case study are shown in
Table 10. For different reduction plans, the emission rates of the three pollutants are given in
Table 11, which were used in the CALPUFF model and specified for the two modeling periods.
Initially, the CALPUFF model was used to estimate the pollutant concentrations emanated from the oil refinery over the surrounding region for a two-month modeling period of January and May of 2014. The CALPOST post-processor was used to determine their spatial distribution.
Figure 6 presents the characteristics of the study area along with the ability of the CALPUFF model to simulate the geographical condition of the area of interest.
Table 12 illustrates the maximum and minimum monthly average concentrations of CO
2 for January 2014.
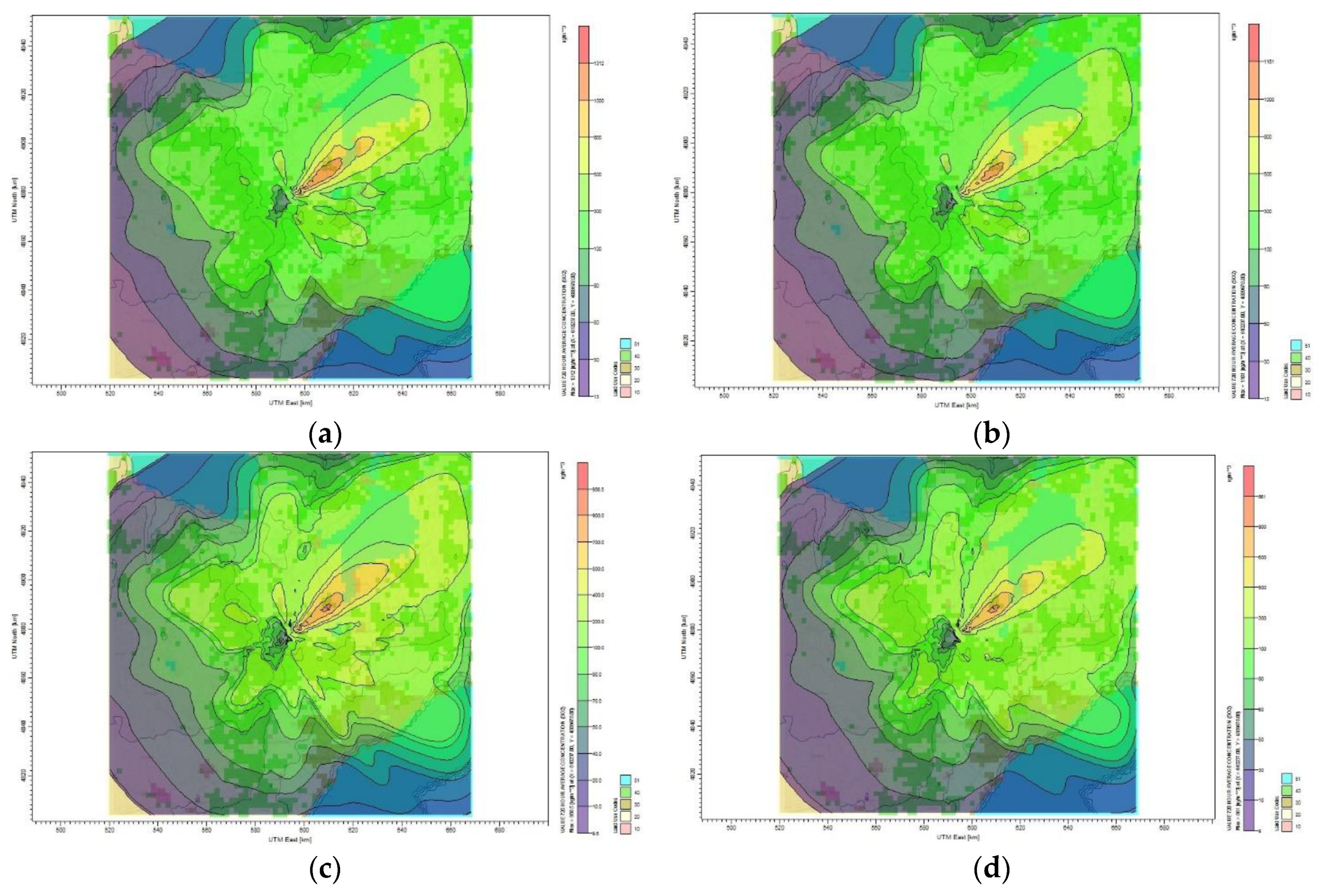
Figure 7 shows the CO
2 plume distributed significantly in the Toronto area in January 2014. The highest monthly CO
2 concentration calculated by CALPUFF view model is 216 µg/m
3 and the lowest is 2 µg/m
3 when considering the base case scenario as shown in
Figure 7a. The plume affecting Toronto residential area concentration is 20 µg/m
3. For the 10% reduction target, the maximum monthly average concentration of CO
2 was 194 µg/m
3, and the minimum average concentration was 2 µg/m
3, as seen in
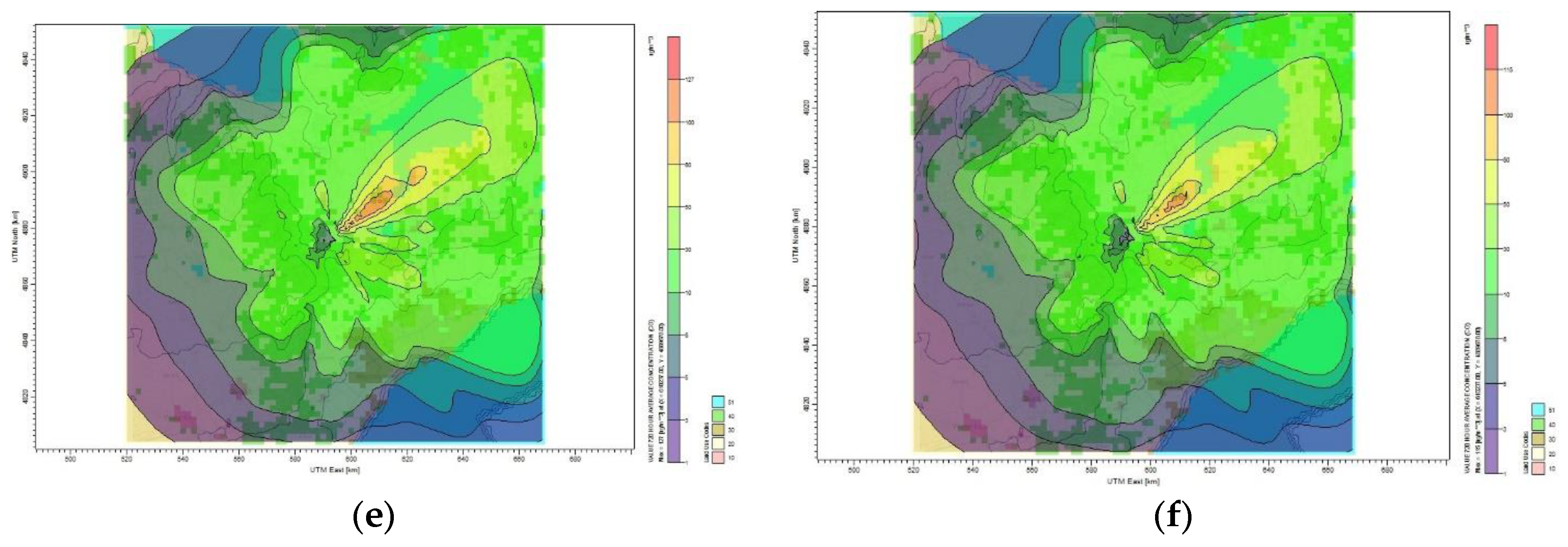
Figure 7b. The pollutant is dispersed in all the directions, especially in the northwest direction as seen in
Figure 7c. For the 50% reduction target of CO
2, the maximum monthly concentration was 127 µg/m
3 at a distance of 2 km from the source, as shown in
Figure 7e. The dispersion of CO
2 was heading significantly in the south and northeast direction as presented in
Figure 7d, thus affecting people in that area. As displayed in
Figure 7f, the plume dispersed significantly towards the northwest and it shows that the maximum monthly average concentration of CO
2 obtained for 60% reduction target reached 115 µg/m
3 within 2 km northeast of the source. It is clear how the plumes are covering the Toronto residential area with the plumes concentration ranging from 216 to 1 µg/m
3 represented by the color code.
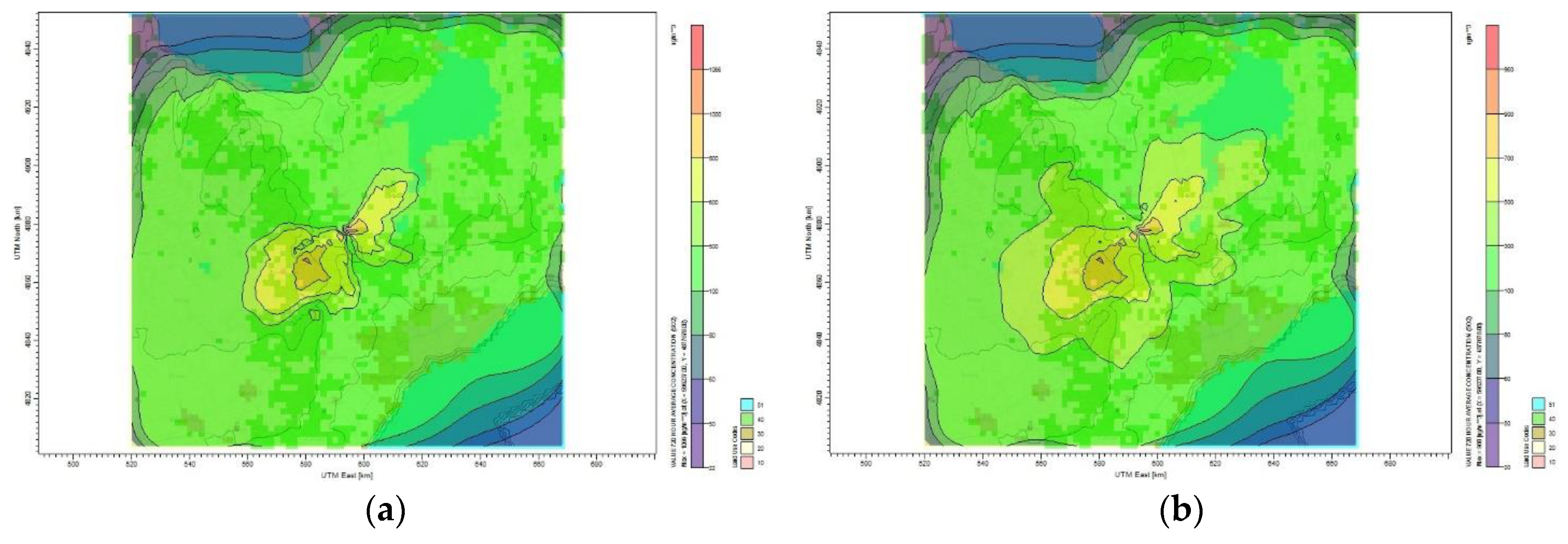
For the second period of May 2014,
Table 13 shows the maximum and minimum monthly average concentrations of CO
2 for the six reduction plans.
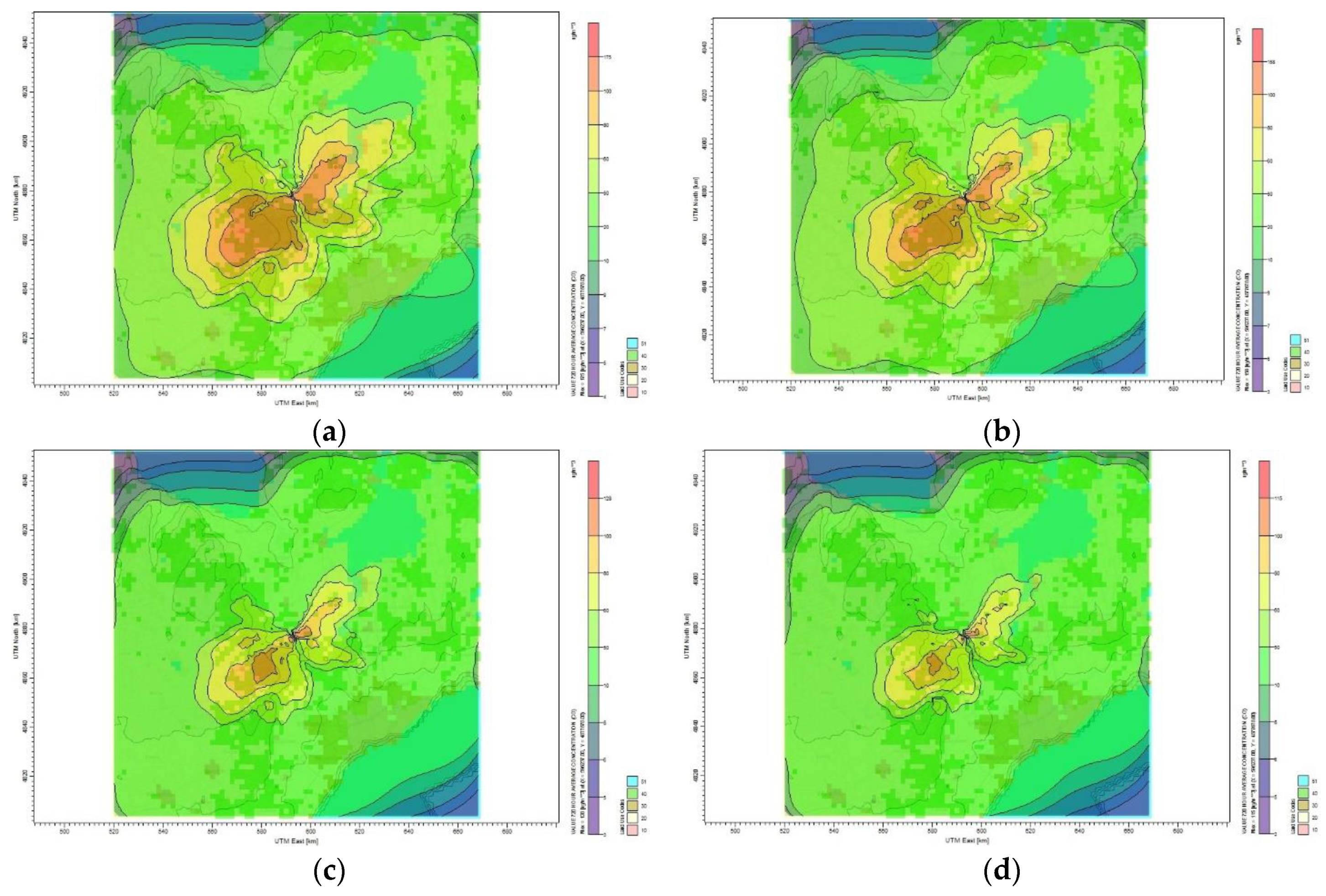
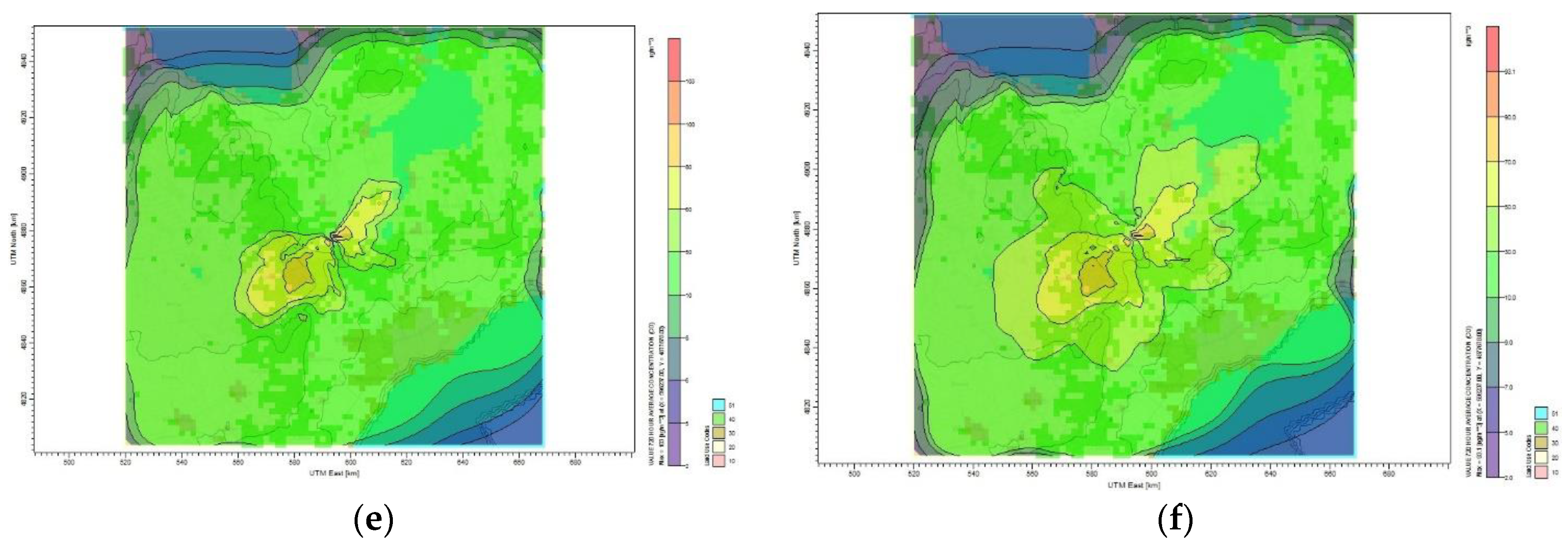
Figure 8 shows the dispersion of CO
2 emission to the surrounding area of Toronto for different mitigation plans. The maximum monthly CO
2 concentration calculated by the CALPUFF model for the base case scenario is 175 µg/m
3 and the lowest is 4 µg/m
3. The plume affecting the Toronto residential area has a concentration of 20 µg/m
3, as shown in
Figure 8a. The maximum CO
2 monthly average concentration became 158 µg/m
3 once the oil refinery site reduces its emission by 10%, as shown in
Figure 8b. For the case of 30% CO
2 reduction,
Figure 8c shows that the maximum average concentration reduced to 128 µg/m
3 and the minimum concentration became 3 µg/m
3. Furthermore,
Figure 8f shows that reducing the CO
2 emission by 60% results in reducing the monthly average concentration to 93.1 µg/m
3. The maximum monthly average concentration of CO
2 for all reduction plans that affect the Toronto area is between 2 and 30 µg/m
3. No health symptoms are associated with this range of concentration values according to air quality standard and guideline [
40].
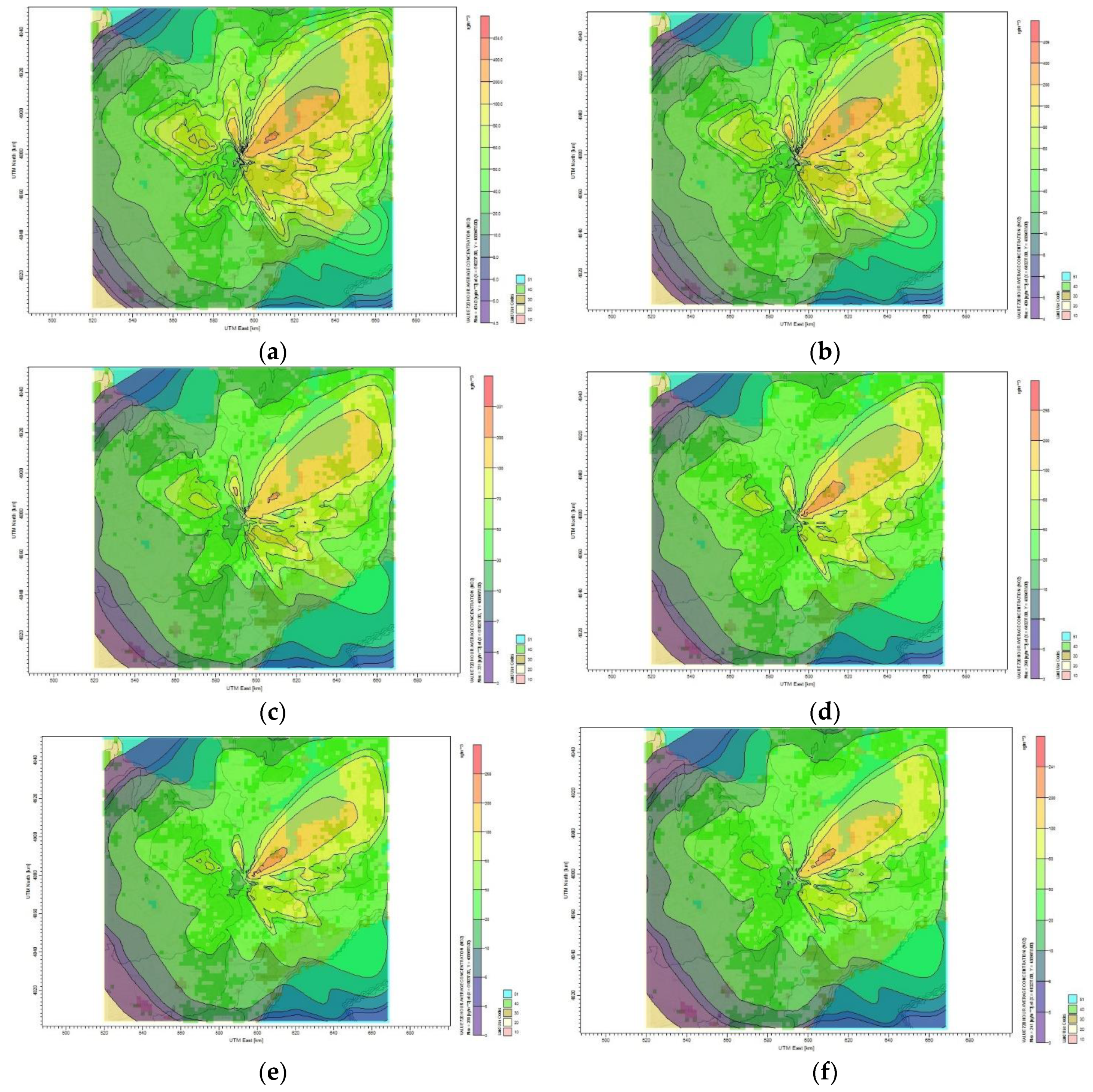
Similarly, the following set of results are related to the dispersion of SO
x and NO
x for the periods of January and May 2014, the tables and figures for which are excluded here for brevity and can be found in
Appendix B. For January 2014, the maximum monthly average concentration of SO
2 when no reduction plan is applied to the refinery was 1312 µg/m
3 and the plumes dispersed 5 km northeast of the source. The maximum and minimum average monthly concentrations of SO
2 were 1181 and 12 µg/m
3 for 10% reduction target. It was observed that when the pollutants from the source were reduced by certain percentages, the concentration of the pollutants in the receptor area reduced accordingly. Therefore, reducing the SO
2 by 30%, 50%, and 60% results in reducing the maximum monthly average concentration to 956.5, 775, and 697 µg/m
3, respectively. For May 2014, the maximum and minimum monthly average concentrations of SO
2 are 1066 and 22 µg/m
3 for the base case scenario, whereas the plume affecting the Toronto residential area has a concentration of 100 µg/m
3. Reducing 10% of SO
2 in the oil refinery decreases the maximum and minimum monthly average concentration of SO
2 to 960 and 20 µg/m
3, respectively. The highest monthly SO
2 concentration computed by CALPUFF when reducing the SO
2 emission by 30% is 777 µg/m
3 and the lowest is 16 µg/m
3. The monthly concentration of plume covering the Toronto residential area is 80 µg/m
3.
For January 2014, the maximum NO2 concentration for the oil refinery was about 454 µg/m3 when no reduction plan was applied. For the 10% reduction plan, it was observed that NO2 was dispersed from the northwest to the northeast of the Toronto area with maximum and minimum monthly average concentrations of 409 and 4 µg/m3, respectively. The maximum monthly average concentration of NO2 for the plant was about 331 µg/m3 when reducing the emission by 30%. The monthly average of SO2 concentration for 40%, 50%, and 60% reduction plans was 298, 268, and 241 µg/m3, respectively. For May 2014, the maximum monthly average concentration of NO2 was 332 µg/m3 for the 30% reduction plan.
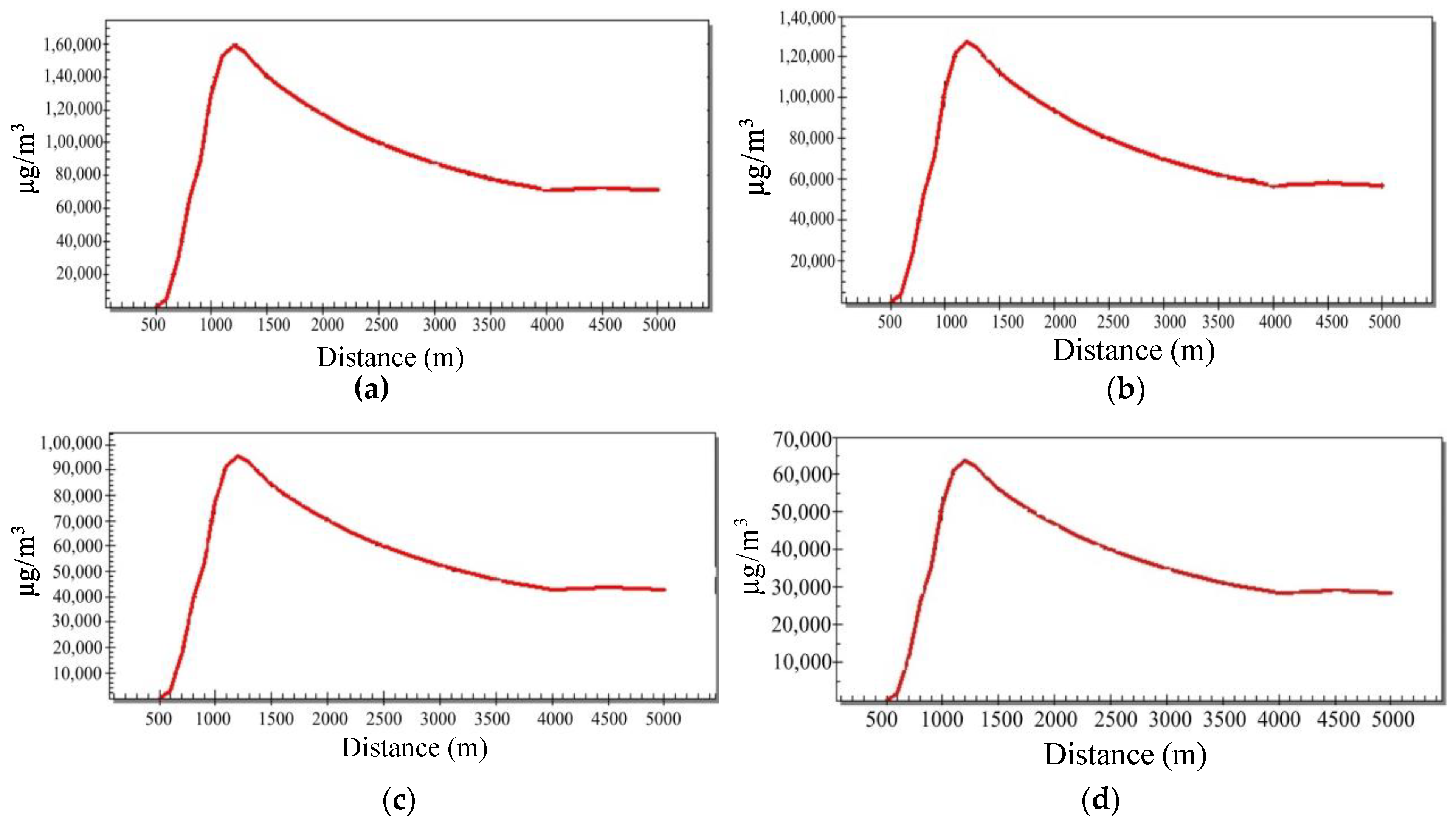
For SCREEN3, the input data used is for the atmospheric emission rates of SOx, NOx, and CO2 from the oil refinery. The results of SCREEN3 are expressed in concentration units (µg/m3) and distance (m). The simulations were performed considering the option of ‘full meteorology’; that is, defining the type of atmospheric stability class where the program assumes the ‘C’ class was omitted. The maximum concentration was calculated for SOx for the base case and various mitigation plans. In all scenarios, the maximum concentration was found at 1200 m away from the source and the emission concentrations were 1.6 × 105, 1.3 × 105, 9.5 × 104, and 6.4 × 104 µg/m3 for the base case, 20%, 40%, and 60% reduction plans, respectively.