The Effect of Biochar Used as Soil Amendment on Morphological Diversity of Collembola
Abstract
:1. Introduction
- Biochar will increase the soil biological quality mainly through improvement of the physical and chemical soil properties.
- From the analyzed life-form groups of springtails, the response of euedaphic assemblages will be most distinct after biochar amendment. On the other hand, springtails living on the soil surface and in the litter layer (epigeic and hemiedaphic) will be more sensitive to cover plants.
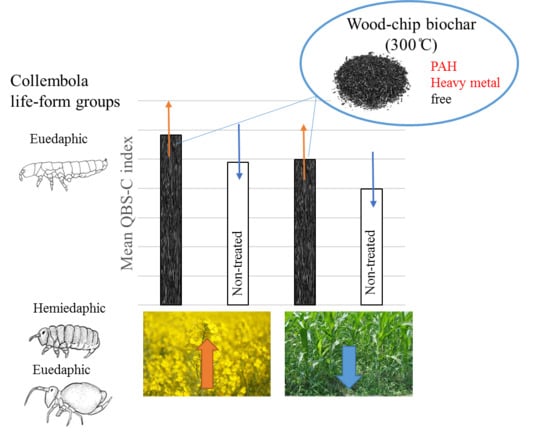
- The QBS-c index will show higher values in crops where biochar was applied.
2. Materials and Methods
2.1. Experimental Design
2.2. Biochar Characteristic and Soil Properties
2.3. Collembola Studies
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
- (1)
- Crop affected more Collembola community than the biochar application. More springtails occurred in oilseed rape.
- (2)
- In each of the life-form groups, biochar caused a significant increase in individual number of Collembola in comparison to the no-biochar treatment. The effect was significant mainly for the oilseed rape crop.
- (3)
- The QBS-C index (biological quality index based on Collembola species) was higher in treatments where biochar was applied.
- (4)
- Collembola related to biochar were characterized by reduced appendages and the absence of specific structures on the cuticle, what indicates better adaptation to live in soil.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gregory, A.S.; Ritz, K.; McGrath, S.P.; Quinton, J.N.; Goulding, K.W.; Jones, R.J.; Harris, J.A.; Bol, R.; Wallace, P.; Pilgrim, E.S.; et al. A review of the impacts of degradation threats on soil properties in the UK. Soil Use Manag. 2015, 31, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Meyer, A.; Poesen, J.; Isabirye, M.; Deckers, J.; Rates, D. Soil erosion rate in tropical villages: A case study from Lake Victoria Basin, Uganda. Catena 2011, 84, 89–98. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management Science and Technology, 1st ed.; Earthscan: London, UK, 2009; pp. 1–944. [Google Scholar]
- Latawiec, A.E.; Królczyk, J.B.; Kubon, M.; Szwedziak, K.; Drosik, A.; Polańczyk, E.; Grotkiewicz, K.; Strassburg, B. Willingness to Adopt Biochar in Agriculture: The Producer’s Perspective. Sustainability 2017, 9, 655. [Google Scholar] [CrossRef]
- Lin, Y.; Munroe, P.; Joseph, S.; Henderson, R.; Ziolkowski, A. Water extractable organic carbon in untreated and chemical treated biochars. Chemosphere 2012, 87, 151–157. [Google Scholar] [CrossRef]
- Herath, H.M.; Camps-Arbestain, M.; Hedley, M. Effect of biochar on soil physical properties in two contrasting soils: An Alfisol and an Andisol. Geoderma 2013, 209–210, 188–197. [Google Scholar] [CrossRef]
- Baiamonte, G.; De Pasquale, C.; Marsala, V.; Cimò, G.; Alonzo, G.; Crescimanno, G.; Conte, P. Structure alteration of a sandy-clay soil by biochar amendments. J. Soils Sediments 2015, 15, 816–824. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef] [Green Version]
- Latawiec, A.E.; Peake, L.; Baxter, H.; Cornelissen, G.; Grotkiewicz, K.; Hale, S.; Królczyk, J.B.; Kubon, M.; Łopatka, A.; Medynska-Juraszek, A.; et al. A reconnaissance-scale GIS-based multicriteria decision analysis to support sustainable biochar use: Poland as a case study. J. Environ. Eng. Landsc. 2017, 25, 208–222. [Google Scholar] [CrossRef]
- Freddo, A.; Cai, C.; Reid, B.J. Environmental contextualization of potential toxic elements and polycyclic aromatic hydrocarbons in biochar. Environ. Pollut. 2012, 171, 18–24. [Google Scholar] [CrossRef]
- Hopkin, S.P. Biology of the Springtails (Insecta: Collembola); Oxford University Press: Oxford, UK, 1997; pp. 1–330. [Google Scholar]
- Rusek, J. Biodiversity of Collembola and their functional role in the ecosystem. Biodivers. Conserv. 1998, 7, 1207–1219. [Google Scholar] [CrossRef]
- Filser, J. The role of Collembola in carbon and nitrogen cycling in soil. Pedobiologia 2012, 46, 234–245. [Google Scholar] [CrossRef]
- Frampton, G.K. The potential of Collembola as indicators of pesticide usage: Evidence and methods from the UK arable ecosystem. Pedobiologia 1997, 41, 179–184. [Google Scholar]
- Fiera, C. Application of stable isotopes and lipid analysis to understand trophic interactions in springtails. North West. J. Zool. 2014, 10, 227–235. [Google Scholar]
- Sousa, J.P.; Bolger, T.; da Gama, M.M.; Lukkari, T.; Ponge, J.-F.; Simón, C.; Traser, G.; Vanbergen, A.J.; Brennan, A.; Dubs, F.; et al. Changes in Collembola richness and diversity along a gradient of land-use intensity: A pan European study. Pedobiologia 2006, 50, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Parisi, V. The biological soil quality, a method based on microarthropods. Acta Naturalia de L’Ateneo Parmense 2001, 37, 97–106. [Google Scholar]
- Machado, J.S.; Oliveira, F.L.; Sants, J.C.; Paulino, A.T.; Baretta, D. Morphological diversity of springtails (Hexapoda: Collembola) as soil quality bioindicators in land use systems. Biota Neotrop. 2019, 19, e20180618. [Google Scholar] [CrossRef]
- Potapov, A.; Semeninaa, E.; Korotkevich, A.; Kuznetsova, N.; Tiunova, A. Connecting taxonomy and ecology: Trophic niches of collembolans as related to taxonomic identity and life forms. Soil Biol. Biochem. 2016, 110, 20–31. [Google Scholar] [CrossRef]
- Karaban, K.; Karaban, E.; Uvarov, A. Determination of life form spectra in soil Collembola communities: A comparison of two methods. Pol. J. Ecol. 2012, 59, 381–389. [Google Scholar]
- Ponge, J.F.; Dubs, F.; Gillet, S.; Sousa, J.P.; Lavelle, P. Decreased biodiversity in soil springtail communities: The importance of dispersal and land use history in heterogeneous landscapes. Soil Biol. Biochem. 2006, 38, 1158–1161. [Google Scholar] [CrossRef]
- Ellers, J.; Berg, M.P.; Dias, A.T.; Fontana, S.; Ooms, A.; Moretti, M. Diversity in form and function: Vertical distribution of soil fauna mediates multidimensional trait variation. J. Anim. Ecol. 2018, 87, 933–944. [Google Scholar] [CrossRef] [Green Version]
- Greenslade, P.; Vaughan, G.T. A comparison of Collembola species for toxicity testing of Australian soils. Pedobiologia 2003, 47, 171–179. [Google Scholar] [CrossRef]
- Marks, E.A.N.; Mattana, S.; Alcañiz, J.M.; Domene, X. Biochars provoke diverse soil mesofauna reproductive responses in laboratory bioassays. Eur. J. Soil Biol. 2014, 60, 104–111. [Google Scholar] [CrossRef]
- Domene, X.; Hanley, K.; Enders, A.; Lehmann, J. Short-term mesofauna responses to soil additions of corn stover biochar and the role of microbial biomass. Appl. Soil Ecol. 2015, 89, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, Q.; Liang, W.; Zhang, M.; Bao, X.; Xie, Z. Soil nematode response to biochar addition in a Chinese wheat field. Pedosphere 2013, 23, 98–103. [Google Scholar] [CrossRef]
- Tammeorg, P.; Parviainen, T.; Nuutinen, V.; Simojoki, A.; Vaara, E.; Helenius, J. Effects of biochar on earthworms in arable soil: Avoidance test and field trial in boreal loamy sand. Agric. Ecosyst. Environ. 2014, 191, 150–157. [Google Scholar] [CrossRef]
- Kolton, M.; Harel, Y.M.; Pasternak, Z.; Graber, E.R.; Elad, Y.; Cytryn, E. Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl. Environ. Microbiol. 2011, 77, 4924–4930. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Cole, E.; Zandvakili, O.R.; Blanchard, J.; Xing, B.; Hashemi, M.; Etemadi, F. Investigating responses of soil bacterial community composition to hardwood biochar amendment using high-throughput PCR sequencing. Appl. Soil Ecol. 2019, 136, 80–85. [Google Scholar] [CrossRef]
- Van Straalen, N.M.; Verhoef, H.A. The development of a bioindicator system for soil acidity based on arthropod pH preferences. J. Appl. Ecol. 1997, 34, 217–232. [Google Scholar] [CrossRef]
- Bardgett, R. The Biology of Soil: A Community and Ecosystem Approach; Oxford University Press: Oxford, UK, 2005; pp. 1–254. [Google Scholar]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char Sequestration in Terrestrial Ecosystems—A Review. MITIG ADAPT STRAT GL 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Berg, M.P.; Stoffer, M.; van den Heuvel, H.H. Feeding guilds in Collembola based on digestive enzymes. Pedobiologia 2004, 48, 589–601. [Google Scholar] [CrossRef]
- Chahartaghi, M.; Scheu, S.; Ruess, L. Sex Ratio and Mode of Reproduction in Collembola in an Oak-Beech Forest. Pedobiologia 2006, 50, 331–340. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic Matter and Water-Stable Aggregates in Soils. Eur. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- FAO-WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports 106; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- Standardized Product Definition and Product Testing Guidelines for Biochar that is Used in Soil; IBI-STD-2.1; IBI (International Biochar Initiative): Canandaigua, NY, USA, 2014.
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizao, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Sohi, S.P.; Thies, J.; O’Neill, B.; Truillo, L.; Gaunt, J.; Solomon, D.; Grossman, J.; Neves, E.G.; et al. Black carbon affects the cycling of non-black carbon in soil. Org. Geochem. 2010, 41, 206–213. [Google Scholar] [CrossRef]
- Gruss, I.; Twardowski, J.P.; Latawiec, A.; Medyńska-Juraszek, A.; Królczyk, J. Risk assessment of low temperature biochar used as soil amendment on soil mesofauna. Environ. Sci. Pollut. Res. 2019, 26, 18230–18239. [Google Scholar] [CrossRef] [PubMed]
- Meier, U. Growth Stages of Mono-and Dicotyledonous Plants; Federal Biological Research Centre for Agriculture and Forestry: Berlin, Germany, 2001; pp. 1–204. [Google Scholar]
- Murphy, P.W. Extraction methods for soil animals. I. Dynamic methods with particular reference to funnel processes. In Progress in Soil Zoology; Butterworths: London, UK, 1962; pp. 75–114. [Google Scholar]
- Zimdars, B.; Dunger, W. Synopses on Palaearctic Collembola Part I. Tullbergiinae Bagnall, 1935; Abhandlungen und Berichte des Naturkundemuseums: Görlitz, Germany, 1994; pp. 1–71. [Google Scholar]
- Fjellberg, A. The Collembola of Fennoscandia and Denmark. Part II: Entomobryomorpha and Symphypleona; Fauna Entomologica Scandinavica; Brill Publishers: Leiden, The Netherlands, 2007; pp. 1–264. [Google Scholar]
- Hopkin, S.P. A Key to the Springtails (Collembola) of Britain and Ireland; Field Studies Council (AIDGAP Project): London, UK, 2007; pp. 1–245. [Google Scholar]
- Conti, F.; Visioli, G.; Malcevschi, A.; Menta, C. Safety assessment of gasification biochars using Folsomia candida (Collembola) ecotoxicological bioassays. ESPR 2017, 25, 6668–6679. [Google Scholar] [CrossRef] [PubMed]
- Liesch, A.M.; Weyers, S.L.; Gaskin, J.W.; Das, K.C. Impact of two different biochars on earthworm growth and survival. Ann. Environ. Sci. 2010, 4, 1–9. [Google Scholar]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A Review of Biochar and Its Use and Function in Soil. Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2010; pp. 47–82. [Google Scholar]
- Xie, T.; Reddy, K.R.; Wang, C.; Yargicoglu, E.; Spokas, K. Characteristics and applications of biochar for environmental remediation: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 939–969. [Google Scholar] [CrossRef]
- Vaccari, F.P.; Maienza, A.; Miglietta, F.; Baronti, S.; Di Lonardo, S.; Giagnoni, L.; Lagomarsino, L.; Pozzi, A.; Pusceddu, E.; Ranieri, R.; et al. Biochar stimulates plant growth but not fruit yield of processing tomato in a fertile soil. Agric. Ecosyst. Environ. 2015, 207, 163–170. [Google Scholar] [CrossRef]
- Głąb, T.; Palmowska, J.; Zaleski, T.; Gondek, K. Effect of biochar application on soil hydrological properties and physical quality of sandy soil. Geoderma 2016, 28, 11–20. [Google Scholar] [CrossRef]
- Yang, D.; Yunguo, L.; Liu, S.; Huang, X.; Li, Z.; Tan, X.; Zeng, G.; Zhou, L. Potential benefits of biochar in agricultural soils: A review. Pedosphere 2017, 27, 645–661. [Google Scholar]
- Baronti, S.; Vaccari, F.P.; Miglietta, F.; Calzolari, C.; Lugato, E.; Orlandini, S.; Pini, R.; Zulian Genesio, C.L. Impact of biochar application on plant water relations in Vitis vinifera (L.). Eur. J. Agron. 2014, 53, 38–44. [Google Scholar] [CrossRef]
- Wang, L.; Butterly, C.R.; Wang, Y.; Herath, H.M.S.K.; Xi, Y.G.; Xiao, X.J. Effect of crop residue biochar on soil acidity amelioration in strongly acidic tea garden soils. Soil Use Manag. 2013, 30, 119–138. [Google Scholar] [CrossRef]
- Obia, A.; Cornelissen, G.; Mulder, J.; Dörsch, P. Effect of Soil pH Increase by Biochar on NO, N2O and N2 Production during Denitrification in Acid Soils. PLoS ONE 2015, 10, e0138781. [Google Scholar] [CrossRef] [PubMed]
- Hågvar, S. Reactions to soil acidification in microarthropods: Is competition a key factor? Biol. Fertil. Soils 1990, 9, 178–181. [Google Scholar] [CrossRef]
- Domene, X.; Mattana, S.; Hanley, K.; Enders, A. Medium-term effects of corn biochar addition on soil biota activities and functions in a temperate soil cropped to corn. Soil Biol. Biochem. 2014, 72, 152–162. [Google Scholar] [CrossRef] [Green Version]
- Castracani, C.; Maienza, A.; Grasso, D.A.; Genesio, L.; Malcevschi, A.; Miglietta, F.; Vaccari, F.P.; Mori, A. Biochar–macrofauna interplay: Searching for new bioindicators. Sci. Total Environ. 2015, 536, 449–456. [Google Scholar] [CrossRef]
- Larsen, T.; Schjønning, P.; Axelsen, J. The impact of soil compaction on euedaphic Collembola. Appl. Soil Ecol. 2004, 26, 273–281. [Google Scholar] [CrossRef]
- Twardowski, J.P.; Hurej, M.; Gruss, I. Diversity and abundance of springtails (Hexapoda: Collembola) in soil under 90-year potato monoculture in relation to crop rotation. Arch. Agron. Soil Sci. 2016, 62, 1158–1168. [Google Scholar] [CrossRef]
- Jacomini, C.; Nappi, P.; Sbrilli, G.; Mancini, L. Indicatori ed Indici Ecotossicologicie Biologici Applicati al Suolo: Stato Dell’arte; Agenzia Nazionale per la Protezione dell’Ambiente (ANPA): Roma, Italy, 2000. [Google Scholar]
- Op Akkerhuis, G.J.; de Ley, F.; Zwetsloot, H.; Ponge, J.-F.; Brussaard, L. Soil microarthropods (Acari and Collembola) in two crop rotations on a heavy marine clay soil. Rev. Ecol. Biol. Sol. 1988, 25, 175–202. [Google Scholar]




| Parameter | Value |
|---|---|
| pH H2O | 8.2 |
| CEC (cmol/kg) | 16.8 |
| Carbon content (% of DM) | 52.3 |
| H/Corg ratio | 0.026 |
| Pb (g/t DM) | 1.57 |
| Mn (g/t DM) | 29.7 |
| Cu (g/t DM) | 0.50 |
| Hg (g/t DM) | 0.32 |
| Zn (g/t DM) | 13.04 |
| Parameter | Oilseed Rape | Maize | ||
|---|---|---|---|---|
| Biochar | Control | Biochar | Control | |
| Corg (%) | 0.94 | 0.92 | 0.94 | 0.78 |
| pH H2O | 6.88 | 7.26 | 6.49 | 7.22 |
| Na+ (cmol/kg) | 0.20 * | 0.12 | 0.12 | 0.18 |
| Mg2+ (cmol/kg) | 3.14 | 1.13 | 2.76 | 0.86 |
| K+ (cmol/kg) | 0.30 | 0.19 | 0.34 | 0.31 |
| Ca2+ (cmol/kg) | 5.12 | 2.29 | 5.72 | 2.28 |
| CEC (cmol/kg) | 8.76 | 3.74 | 8.98 | 4.73 |
| Species | Abbr. on the CCA Biplot | Life-form Group | Size * | Pigmentation | Structures on Cuticle | Ocelli | Antennae | Legs | Furcula | EMI Scores (QBS-c) |
|---|---|---|---|---|---|---|---|---|---|---|
| Bourietiella hortensis (Fitch) | Bou_hor | Epedaphic | 4 | 0 | 1 | 0 | 0 | 0 | 0 | 5 |
| Brachystomella parvula (Schaffer) | Bra_par | Hemiedaphic | 4 | 0 | 1 | 0 | 3 | 0 | 3 | 14 |
| Caprainea marginata (Schoett) | Cap_mar | Epedaphic | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Desoria multisetis (Carpenter & Phillips) | Des_mul | Epedaphic | 4 | 3 | 0 | 0 | 0 | 0 | 0 | 7 |
| Desoria tigrina (Nicolet) | Des_tig | Hemiedaphic | 4 | 6 | 0 | 0 | 0 | 0 | 0 | 10 |
| Folsomia sexuolata (Tullberg) | Fol_sex | Hemiedaphic | 4 | 6 | 3 | 6 | 2 | 2 | 2 | 25 |
| Folsomides angularis (Axelson) | Fol_ang | Hemiedaphic | 4 | 6 | 3 | 6 | 3 | 2 | 3 | 27 |
| Folsomides parvulus (Stach) | Fol_par | Hemiedaphic | 4 | 6 | 3 | 3 | 3 | 3 | 3 | 25 |
| Friesea mirabilis (Tullberg) | Fri_mir | Hemiedaphic | 4 | 3 | 1 | 0 | 2 | 2 | 2 | 14 |
| Hypogastrura spp. | Hopogast | Hemiedaphic | 4 | 0 | 3 | 0 | 2 | 2 | 2 | 13 |
| Isotoma anglicana (Lubbock) | Iso_ang | Epedaphic | 4 | 3 | 1 | 0 | 0 | 0 | 0 | 8 |
| Isotoma antennalis (Bagnall) | Iso_ant | Epedaphic | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 5 |
| Isotoma viridis (Bourlet) | Iso_vir | Epedaphic | 4 | 3 | 0 | 0 | 0 | 0 | 0 | 7 |
| Isotomiella minor (Schaeffer) | Iso_min | Hemiedaphic | 4 | 6 | 3 | 3 | 3 | 3 | 3 | 25 |
| Isotomodes productus (Axelson) | Iso_pro | Hemiedaphic | 4 | 6 | 3 | 6 | 2 | 3 | 2 | 26 |
| Isotomurus palustris (Mueller) | Iso_pal | Epedaphic | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 5 |
| Isotomutus gallicus (Carapelli et al.) | Iso_gal | Epedaphic | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 5 |
| Lepidocyrtus violaceus (Fourcroy) | Lep_vio | Epedaphic | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Mesaphorura spp. | Mesaphor | Euedaphic | 4 | 6 | 3 | 6 | 3 | 3 | 6 | 31 |
| Parisotoma notabilis (Schaeffer) | Par_not | Hemiedaphic | 4 | 6 | 1 | 3 | 0 | 2 | 0 | 16 |
| Proisotoma minima (Absolon) | Pro_mini | Hemiedaphic | 4 | 3 | 1 | 3 | 2 | 2 | 2 | 17 |
| Proisotoma minuta (Tullberg) | Pro_minu | Hemiedaphic | 4 | 3 | 1 | 0 | 2 | 2 | 2 | 14 |
| Proisotoma tenella (Reuter) | Pro_ten | Hemiedaphic | 4 | 3 | 1 | 0 | 2 | 2 | 2 | 14 |
| Protaphorura spp. | Protapho | Euedaphic | 4 | 6 | 3 | 6 | 3 | 3 | 6 | 31 |
| Pseudosinnela sexoculata (Schott) | Pse_sex | Hemiedaphic | 4 | 6 | 1 | 3 | 0 | 0 | 0 | 14 |
| Sminthurides parvulus (Krausbauer) | Smi_par | Epedaphic | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Sminthurinus alpinus (Gisin) | Smi_alp | Epedaphic | 4 | 0 | 1 | 0 | 2 | 0 | 0 | 7 |
| Sphaeridia pumilis (Krausbauer) | Sph_pum | Epedaphic | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Stenacidia violacea (Reuter) | Ste_vio | Epedaphic | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Stenaphorura spp. | Stenapho | Euedaphic | 4 | 6 | 3 | 6 | 3 | 3 | 6 | 31 |
| Dependent Variable | Treatment | Plant | Treatment × Plant | |||
|---|---|---|---|---|---|---|
| F * | p | F * | p | F | p | |
| Epedaphic | 11.10 | 0.0009 | 20.53 | <0.0001 | 12.43 | 0.0004 |
| Hemiedaphic | 7.65 | 0.0058 | 22.04 | <0.0001 | 11.53 | 0.0007 |
| Euedaphic | 13.09 | 0.0003 | 0.04 | 0.8501 | 2.15 | 0.1425 |
| QBS-c | 6.16 | 0.01132 | 4.77 | 0.0292 | 0.02 | 0.8943 |
| CCA Axes | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Eigenvalues | 0.013 | 0.002 | 0.000 | 0.103 |
| Morphometric traits-environment correlations | 0.013 | 0.002 | 0.000 | 0.103 |
| Significance of the first canonical axis | F = 26.362, p = 0.002 | |||
| Significance of all canonical axes | F = 10.296, p = 0.002 | |||
| CCA Axes | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Eigenvalues | 0.102 | 0.041 | 0.013 | 0.476 |
| Species-environment correlations | 0.563 | 0.388 | 0.270 | 0.000 |
| Significance of the first canonical axis | F = 7.754, p = 0.002 | |||
| Significance of all canonical axes | F = 4.016, p = 0.002 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruss, I.; Twardowski, J.P.; Latawiec, A.; Królczyk, J.; Medyńska-Juraszek, A. The Effect of Biochar Used as Soil Amendment on Morphological Diversity of Collembola. Sustainability 2019, 11, 5126. https://0-doi-org.brum.beds.ac.uk/10.3390/su11185126
Gruss I, Twardowski JP, Latawiec A, Królczyk J, Medyńska-Juraszek A. The Effect of Biochar Used as Soil Amendment on Morphological Diversity of Collembola. Sustainability. 2019; 11(18):5126. https://0-doi-org.brum.beds.ac.uk/10.3390/su11185126
Chicago/Turabian StyleGruss, Iwona, Jacek P. Twardowski, Agnieszka Latawiec, Jolanta Królczyk, and Agnieszka Medyńska-Juraszek. 2019. "The Effect of Biochar Used as Soil Amendment on Morphological Diversity of Collembola" Sustainability 11, no. 18: 5126. https://0-doi-org.brum.beds.ac.uk/10.3390/su11185126