Biomass Growth and Its Control in the Process of Biofiltration of Air Contaminated with Xylene on a Biotrickling Column Filled with Expanded Clay
Abstract
:1. Introduction
- -
- Mechanical, such as backwashing with or without fluidizing the bed, air blowing, regular stirring, or mixing of the filter bed, etc., that is, the use of shear forces;
- -
- Chemical and physical, such as the usage of pH regulators, oxidants, surfactants, bactericidal and hydrolyzing compounds, enzymes, and elevated temperature during backwashing;
- -
- Slowing of microbial growth by limiting the availability of biogenic elements: nitrogen, potassium, phosphorus, and microelements;
- -
- The use of predation, for example, by using protozoa or mites as predators of bacteria or fungi.
- Physical: the use of mechanical or hydraulic shear forces;
- Chemical: limiting the availability (control) of carbon and other nutrients, as well as washing or pouring solutions containing oxidants, surfactants, bactericidal, and hydrolyzing compounds;
- Biological: predation;
- Improving the biofilter design;
- Improvement of the biofilter mode of operation.
2. Materials and Methods
3. Results
3.1. Effect of Xylene Concentration, Gas Flow Rate, and Sprinkle Intensity on Biofiltration Efficiency
3.2. Effects of Xylene Concentration, Gas Flow Rate, and Sprinkling Intensity on Biomass Growth and Flow Resistance
3.3. Estimation of Indicators of Daily Biomass Increase and Biomass Efficiency Factors
4. Discussion
5. Conclusions
- Air contaminated with technical xylene can be effectively purified by biofiltration on a biotrickling column filled with keramzyt (fired clay) at a concentration of up to 1500 mg/m3, airflow at a linear rate of up to 0.032 m/s, and a load of the bed up to 105 g/h/m3.
- Biofiltration of air on the trickle-bed reactor filled with expanded clay is accompanied by an intensive growth in biomass, resulting in a rise of the bed’s initial mass by up to 46% and rapid increases in flow resistance, leading to a practical blockage of the gas flow through the column.
- The average values of the wet biomass yield coefficient ranged from about 0.33 to 1.82, and the daily indicators of wet biomass growth were in the range from about 0.6 to 1.8 kg/kgof the bed/day.
- Biomass growth hindering the biofilter exploitation can be effectively limited by periodically flushing the bed in the column with the medium solution fed in the opposite direction to gas flow or by the washing of the packing material after its removal from the column. The second procedure allows restoring the resistance of gas flow through the column to a value close to the initial worth.
- To ensure the availability of biogenic elements in the bed and to maintain its humidity, it is sufficient to rinse it with a standard mineral nutrient medium, at a low sprinkling intensity of up to 2.5 dm3/h/m3 of the bed, and 0.1 dm3/h/m3 of the purified air.
Author Contributions
Funding
Conflicts of Interest
References
- European Commission. Reference Document on Best Available Techniques in the Large Volume Organic Chemical Industry, Integrated Pollution Prevention and Control (IPPC). 2003. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwilsPnsopXqAhVPzqYKHQMSA-wQFjARegQIChAB&url=https%3A%2F%2Feippcb.jrc.ec.europa.eu%2Fsites%2Fdefault%2Ffiles%2F2020-03%2Fsuperseded_lvoC_bref_0203.pdf&usg=AOvVaw0n7xHhrK3QTFq4FJicdqYm (accessed on 26 June 2020).
- Nagel, H.; Frey, R.; Hartgerink, C.; Rikeit, H.E.; Greiner, R.D.; Klein, C.; Boesl, U. On-Line Analysis of Individual Aromatic Hydrocarbons in Automotive Exhaust:Dealkylation of the Aromatic Hydrocarbons in the Catalytic Converter. SAE Tech. Paper Ser. 1997, 412, 451–457. [Google Scholar] [CrossRef]
- The European Chemicals Agency Xylene. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.014.124 (accessed on 26 June 2020).
- United States Environmental Protection Agency. Available online: https://www.epa.gov/clean-air-act-overview/clean-air-act-text (accessed on 26 June 2020).
- Xi, J.; Kang, I.; Hu, H.; Zhang, X. A biofilter model for simultaneous simulation of toluene removal and bed pressure drop under varied inlet loadings. Front. Environ. Sci. Eng. 2014, 9, 554–562. [Google Scholar] [CrossRef]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm Formation as Microbial Development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Liu, J. Temporal variation of microbial population in a thermophilic biofilter for SO2 removal. J. Environ. Sci. 2016, 39, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lu, L.; Kennes, C.; Yu, J.; Chen, J. Treatment of gaseous toluene in three biofilters inoculated with fungi/bacteria: Microbial analysis, performance and starvation response. J. Hazard. Mater. 2016, 303, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, H.; Zeng, G.; Yu, G.; Luo, S. Biomass accumulation and control strategies in gas biofiltration. Biotechnol. Adv. 2010, 28, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, G.; Mohseni, M. Using UV pretreatment to enhance biofiltration of mixtures of aromatic VOCs. J. Hazard. Mater. 2007, 144, 59–66. [Google Scholar] [CrossRef]
- Wang, C.; Kong, X.; Zhang, X.Y. Mesophilic and thermophilic biofiltration of gaseous toluene in a long-term operation: Performance evaluation, biomass accumulation, mass balance analysis and isolation identification. J. Hazard. Mater. 2012, 229, 94–99. [Google Scholar] [CrossRef]
- Saingam, P.; Xi, J.; Xu, Y.; Hu, H.Y. Investigation of the characteristics of biofilms grown in gas-phase biofilters with and without ozone injection by CLSM technique. Appl. Microbiol. Biotechnol. 2015, 100, 2023–2031. [Google Scholar] [CrossRef]
- Kim, D.; Sorial, G.A. Role of biological activity and biomass distribution in air biofilter performance. Chemosphere 2007, 66, 1758–1764. [Google Scholar] [CrossRef]
- Kennes, C.; Veiga, M.C. Inert filter media for the biofiltration of waste gases—Characteristics and biomass control. Rev. Environ. Sci. Bio/Technol. 2002, 1, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.; Hernández, S.; Cornabé, T.; Revah, S.; Auria, R. Effect of Drying on Biofilter Performance: Modeling and Experimental Approach. Environ. Sci. Technol. 2003, 37, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Rene, E.R.; Montes, M.; Veiga, M.C.; Kennes, C. Styrene removal from polluted air in one and two-liquid phase biotrickling filter: Steady and transient-state performance and pressure drop control. Bioresour. Technol. 2011, 102, 6791–6800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Liang, Z.; Ao, T.; Li, G. Comparative elimination of dimethyl disulfide by maifanite and ceramic-packed biotrickling filters and their response to microbial community. Bioresour. Technol. 2016, 202, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.M.; Quijano, G.; Lebrero, R.; Munoz, R. Step-feed biofiltration: A low cost alternative configuration for off-gas treatment. Water Res. 2013, 47, 4312–4321. [Google Scholar] [CrossRef]
- Flores-Valle, S.O.; Rios-Bernÿ, O.; Chanona-Perez, J.; Fregoso-Aguilar, T.; Morales-Gonzalez, J.A.; Prado-Rubianes, O.J.; Herrera-Bucio, R.; Lopez-Albarran, P.; Morales-Gonzalez, A.; Garibay-Febles, V.; et al. A Comparative Study of Physical and Chemical Processes for Removal of Biomass in Biofilters. Molecules 2011, 16, 6927–6949. [Google Scholar] [CrossRef] [Green Version]
- Prado, Ó.J.; Mendoza, J.A.; Veiga, M.C.; Kennes, C.O. Optimization of nutrient supply in a downflow gas-phase biofilter packed with an inert carrier. Appl. Microbiol. Biotechnol. 2002, 59, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Trejo-Aguilar, G.; Revah, S.; Lobo-Oehmichen, R. Hydrodynamic characterization of a trickle bed air biofilter. Chem. Eng. J. 2005, 113, 145–152. [Google Scholar] [CrossRef]
- Weber, F.J.; Hartmans, S. Prevention of clogging in a biological trickle-bed reactor removing toluene from contaminated air. Biotechnol. Bioeng. 1996, 50, 91–97. [Google Scholar] [CrossRef]
- Yang, H.; Minuth, B.; Allen, D.G. Effects of nitrogen and oxygen on biofilter performance. J. Air Waste Manag. Assoc. 2002, 52, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.; Revah, S.; Auria, R. Start-up and the effect of gaseous ammonia additions on a biofilter for the elimination of toluene vapors. Biotechnol. Bioeng. 1998, 60, 483–491. [Google Scholar] [CrossRef]
- Delhoménie, M.-C.; Bibeau, L.; Roy, S.; Brzezinski, R.; Heitz, M. Influence of nitrogen on the degradation of toluene in a compost-based biofilter. J. Chem. Technol. Biotechnol. 2001, 76, 997–1006. [Google Scholar] [CrossRef]
- Acuña, M.E.; Villanueva, C.; Cárdenas, B.; Christen, P.; Revah, S. The effect of nutrient concentration on biofilm formation on peat and gas phase toluene biodegradation under biofiltration conditions. Process. Biochem. 2002, 38, 7–13. [Google Scholar] [CrossRef]
- Song, J.H.; Kinney, K.A. Effect of vapor-phase bioreactor operation on biomass accumulation, distribution, and activity: Linking biofilm properties to bioreactor performance. Biotechnol. Bioeng. 2000, 68, 508–516. [Google Scholar] [CrossRef]
- Wright, W.F.; Schroeder, E.D.; Chang, D.P.Y. Transient Response of Flow-Direction-Switching Vapor-Phase Biofilters. J. Environ. Eng. 2005, 131, 999–1009. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, J.; Veiga, M.C.; Kennes, C. Biofiltration of waste gases in a reactor with a split-feed. J. Chem. Technol. Biotechnol. 2003, 78, 703–708. [Google Scholar] [CrossRef]
- Sempere, F.; Gabaldón, C.; Martínez-Soria, V.; Marzal, P.; Penya-Roja, J.M.; Álvarez-Hornos, F.J. Performance evaluation of a biotrickling filter treating a mixture of oxygenated VOCs during intermittent loading. Chemosphere 2008, 73, 1533–1539. [Google Scholar] [CrossRef]
- Atoche, J.C.; Moe, W.M. Treatment of MEK and toluene mixtures in biofilters: Effect of operating strategy an performance during transient loading. Biotechnol. Bioeng. 2004, 86, 468–481. [Google Scholar] [CrossRef]
- Xi, J.; Saingam, P.; Gu, F.; Hu, H.Y.; Zhao, X. Effect of continuous ozone injection on performance and biomass accumulation of biofilters treating gaseous toluene. Appl. Microbiol. Biotechnol. 2014, 99, 33–42. [Google Scholar] [CrossRef]
- Wang, L.; Yang, C.; Cheng, Y.; Huang, J.; Yang, H.; Zeng, G.; Lu, L.; He, S. Enhanced removal of ethylbenzene from gas streams in biotrickling filters by Tween-20 and Zn(II). J. Environ. Sci. 2014, 26, 2500–2507. [Google Scholar] [CrossRef]
- Kasperczyk, D.; Bartelmus, G.; Gąszczak, A. Removal of styrene from dilute gaseous waste streams using a trickle-bed bioreactor: Kinetics, mass transfer and modeling of biodegradation process. J. Chem. Technol. Biotechnol. 2012, 87, 758–763. [Google Scholar] [CrossRef]
- Hwang, J.W.; Choi, C.Y.; Park, S.; Lee, E.Y. Biodegradation of gaseous styrene by Brevibacillus sp. using a novel agitating biotrickling filter. Biotechnol. Lett. 2008, 30, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.; Prado, Ó.J.; Veiga, M.C.; Kennes, C. Hydrodynamic behaviour and comparison of technologies for the removal of excess biomass in gas-phase biofilters. Water Res. 2004, 38, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Roshani, B.; Torkian, A.; Aslani, H.; Dehghanzadeh, R.; Aslani, H. Bed mixing and leachate recycling strategies to overcome pressure drop buildup in the biofiltration of hydrogen sulfide. Bioresour. Technol. 2012, 109, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Zápotocký, L.; Halecký, M.; Vaněk, T.; Páca, J.; Kozliak, E.; Jones, K.D.; Soccol, C.R. Biofiltration of a styrene/acetone vapor mixture in two reactor types under conditions of styrene overloading. Braz. Arch. Boil. Technol. 2014, 57, 782–788. [Google Scholar] [CrossRef] [Green Version]
- Alonso, C.; Suidan, M.T.; Sorial, G.A.; Smith, F.L.; Biswas, P.; Smith, P.J.; Brenner, R.C. Gas treatment in trickle-bed biofilters: Biomass, how much is enough? Biotechnol. Bioeng. 1997, 54, 583–594. [Google Scholar] [CrossRef]
- Kim, D.; Cai, Z.; Sorial, G.A. Behavior of trickle-bed air biofilter for toluene removal: Effect of non-use periods. Environ. Prog. 2005, 24, 155–161. [Google Scholar] [CrossRef]
- Han, M.-F.; Wang, C.; Liu, H. Comparison of physical technologies for biomass control in biofilters treating gaseous toluene. J. Air Waste Manag. Assoc. 2018, 68, 1–8. [Google Scholar] [CrossRef]
- Smith, F.L.; Sorial, G.A.; Suidan, M.T.; Breen, A.W.; Biswas, P.; Brenner, R.C. Development of Two Biomass Control Strategies for Extended, Stable Operation of Highly Efficient Biofilters with High Toluene Loadings. Environ. Sci. Technol. 1996, 30, 1744–1751. [Google Scholar] [CrossRef]
- Wieczorek, A. Pilot-scale biofiltration of waste gases containing aliphatic and aromatic hydrocarbons, phenol, cresols, and other volatile organic compounds. Environ. Prog. 2005, 24, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Kojima, Y.; Itada, N.; Hayaishi, O. Metapyrocatechase: A New Catechol-cleaving Enzyme. J. Biol.Chem. 1961, 236, 2223–2228. [Google Scholar] [PubMed]
- Chan, W.C.; Lai, T.Y. Interaction of compounds on biodegradation of ketone mixtures in a biofilter. J. Chem. Technol. Biotechnol. 2010, 85, 416–422. [Google Scholar] [CrossRef]
- Amin, M.M.; Rahimi, A.; Bina, B.; Heidari, M.; Moghadam, F.M. Performance evaluation of a scoria-compost biofilter treating xylene vapors. J. Environ. Heal. Sci. Eng. 2014, 12, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorio, H.; Bibeau, L.; Viel, G.; Heitz, M. Effects of gas flow rate and inlet concentration on xylene vapors biofiltration performance. Chem. Eng. J. 2000, 76, 209–221. [Google Scholar] [CrossRef]
- Mirpuri, R.; Jones, W.; Bryers, J.D. Toluene degradation kinetics for planktonic and biofilm-grown cells of Pseudomonas putida 54G. Biotechnol. Bioeng. 1997, 53, 535–546. [Google Scholar] [CrossRef]
- Znad, H.; Katoh, K.; Kawase, Y. High loading toluene treatment in a compost based biofilter using up-flow and down-flow swing operation. J. Hazard. Mater. 2007, 141, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Spigno, G.; Pagella, C.; Fumi, M.D.; Molteni, R.; De Faveri, D.M. VOCs removal from waste gases: Gas-phase bioreactor for the abatement of hexane by Aspergillus niger. Chem. Eng. Sci. 2003, 58, 739–746. [Google Scholar] [CrossRef]
- Kennes, C.; Veiga, M.C. Fungal biocatalysts in the biofiltration of VOC-polluted air. J. Biotechnol. 2004, 113, 305–319. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.R.; Tudryn, G.; Bucinell, R.; Schadler, L.S.; Picu, C. Morphology and mechanics of fungal mycelium. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tarao, M.; Seto, M. Estimation of the Yield Coefficient ofPseudomonas sp. Strain DP-4 with a Low Substrate (2,4-Dichlorophenol [DCP]) Concentration in a Mineral Medium from Which Uncharacterized Organic Compounds Were Eliminated by a Non-DCP-Degrading Organism. Appl. Environ. Microbiol. 2000, 66, 566–570. [Google Scholar] [CrossRef] [Green Version]
- Sarma, A.K.; Singh, V.P.; Bhattacharjya, R.K.; Kartha, S.A. Urban Ecology, Water Quality and Climate Change, 1st ed.; Springer International Publishing: New York, NY, USA, 2018. [Google Scholar]
- Wang, J.H.; Liao, J.C. Kinetics and Dynamics of o-Xylene Biodegradation with Pseudomonus putida (ATCC 11172). In Proceedings of the 16th Conference of Biochemical Engineering in Taiwan, Taipei, Taiwan, June 2011; Available online: https://www.researchgate.net/publication/328302631_Kinetics_and_Dynamics_of_o-Xylene_Biodegradation_with_Pseudomonus_putida_ATCC_11172 (accessed on 26 June 2020).


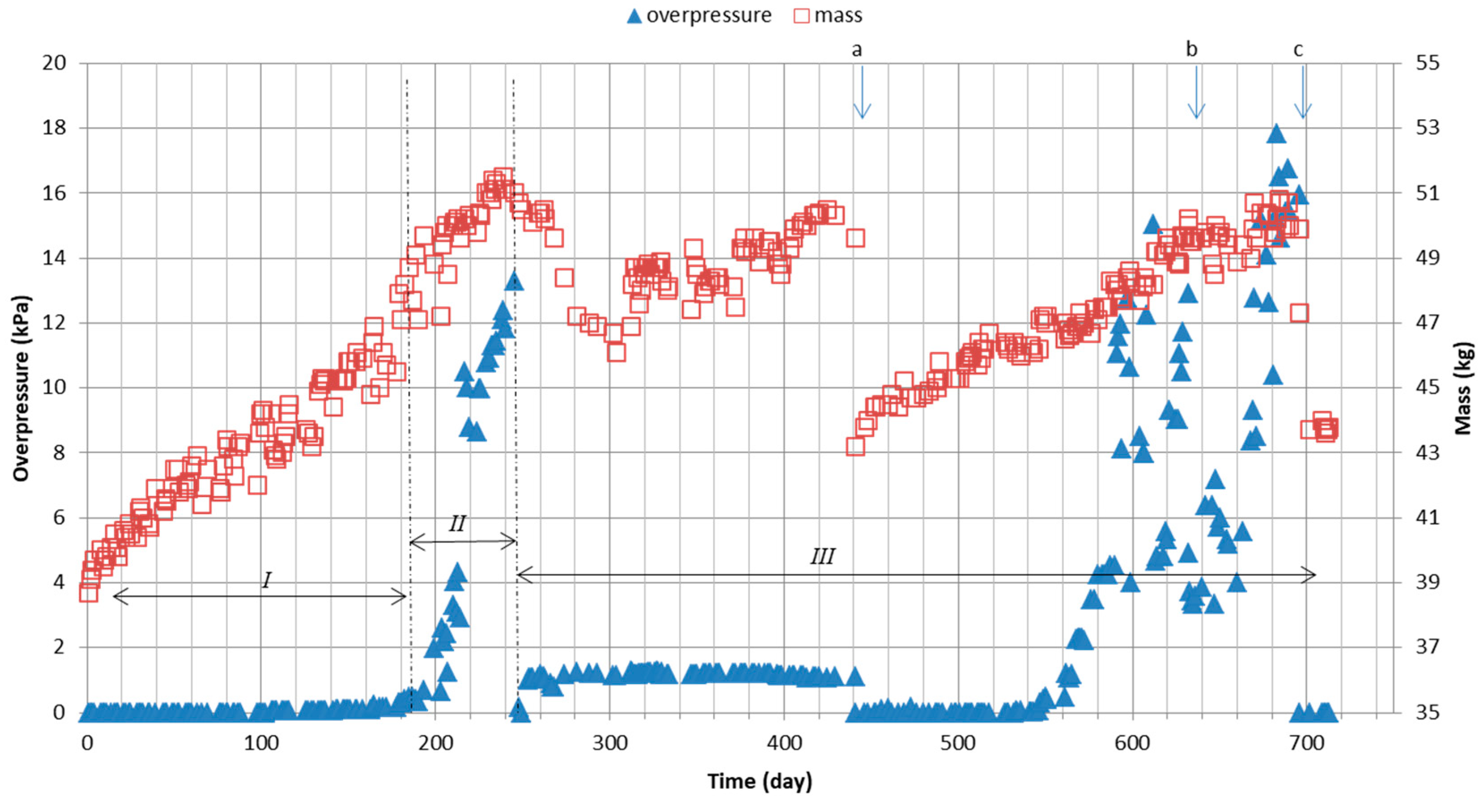
| Measurement Day | Gas Flow Rate, dm3/min | Xylene Inlet Concentration Set, mg/m3 | Average Xylene Inlet Concentration | Column Mass, kg (on the First Day of the Period) | Sprinkling Intensity per Cycle, cm3 | Sprinkling Intensity per Day, cm3 | Description of Activities on the First Day of the Period/during the Period |
|---|---|---|---|---|---|---|---|
| 1–29 | 28 | 700 | 575 | 38.7 | 120 | 960 | Start of column work |
| 30–84 | 500 | 487 | 41.2 | Change in inlet concentration | |||
| 85–112 | 1000 | 1021 | 42.3 | Change in inlet concentration | |||
| 113–146 | 300 | 275 | 43.3 | Change in inlet concentration | |||
| 147–181 | 1500 | 1407 | 45.2 | Change in inlet concentration | |||
| 182–213 | 48.2 | 240 | 1920 | Change of nutrient dosing time | |||
| 214–239 | 1000 | 1133 | 49.6 | Change in inlet concentration | |||
| 240–244 | 51.1 | 120 * | 960 * | Change of nutrient dosing time | |||
| 245–313 | 2.8 | 0 | 0 | 51 | Holiday break: no nutrient, only water | ||
| 314–347 | 28 | 1500 | 1588 | 48.7 | Normal operation after the holiday period | ||
| 348–495 | 650 | 672 | 48.7 | Change in inlet concentration | |||
| 441 | 28 | 650 | 672 | 49.6 | 120 | 960 | Flushing and mixing of the bed |
| 43.2 | After flushing and mixing of the bed | ||||||
| 496–586 | 14 | 700 | 748 | 45.3 | 120 | 960 | Change of airflow |
| 587–688 | 42 | 47.1 | Change of airflow | ||||
| 632 | 28 | 700 | 748 | 50.2 | 120 | 960 | Backwashing without fluidization |
| 49.5 | After backwashing | ||||||
| 689–712 | 28 | 700 | 748 | 50.7 | 120 | 960 | Change of airflow |
| 696 | 28 | 700 | 748 | 47.3 | 120 | 960 | Flushing and mixing of the bed |
| 43.7 | After flushing and mixing of the bed | ||||||
| 713 | 28 | 700 | 748 | 43.8 | 120 | 960 | End of column work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turała, A.; Wieczorek, A. Biomass Growth and Its Control in the Process of Biofiltration of Air Contaminated with Xylene on a Biotrickling Column Filled with Expanded Clay. Sustainability 2020, 12, 5412. https://0-doi-org.brum.beds.ac.uk/10.3390/su12135412
Turała A, Wieczorek A. Biomass Growth and Its Control in the Process of Biofiltration of Air Contaminated with Xylene on a Biotrickling Column Filled with Expanded Clay. Sustainability. 2020; 12(13):5412. https://0-doi-org.brum.beds.ac.uk/10.3390/su12135412
Chicago/Turabian StyleTurała, Anita, and Andrzej Wieczorek. 2020. "Biomass Growth and Its Control in the Process of Biofiltration of Air Contaminated with Xylene on a Biotrickling Column Filled with Expanded Clay" Sustainability 12, no. 13: 5412. https://0-doi-org.brum.beds.ac.uk/10.3390/su12135412




