Key Factors Controlling Primary Production and Cyanobacterial Harmful Algal Blooms (cHABs) in a Continuous Weir System in the Nakdong River, Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Colllection
2.3. In-situ Incubation Experiment for Primary Productivity
2.4. Meteorological and Physicochemical Characteristics
2.5. Phytoplankton Pigment Analysis Using HPLC and Application of the CHEMTAX Program
2.6. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics in the Regulated Water Environment
3.2. Seasonal and Spatial Variations in Primary Production
3.3. Seasonal and Spatial Variations in Marker Pigment Concentrations of Phytoplankton
3.4. Relative Proportion of Pigment-Based Phytoplankton Composition Calculated by the CHEMTAX Program
4. Discussion
4.1. Seasonal and Spatial Variations in Primary Production in a Continuous Weir System
4.2. Key Factors Controlling Primary Production in a Continuous Weir System
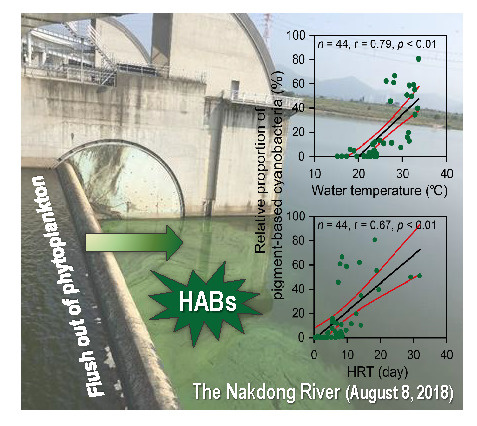
4.3. Favorable Environmental Conditions for cHABs in a Continuous Weir System
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Fuco | Neo | Viol | Diad | Anth | Myx | Allo | Lut | Zea | Chl. b | ß-car | Ech | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diatoms | 0.51 | 0 | 0 | 0.074 | 0 | 0 | 0 | 0 | 0 | 0 | 0.003 | 0 |
| Cryptophytes | 0 | 0 | 0 | 0 | 0 | 0 | 0.37 | 0 | 0 | 0 | 0.001 | 0 |
| Chlorophytes | 0 | 0.038 | 0.026 | 0 | 0.016 | 0 | 0 | 0.15 | 0 | 0.36 | 0.003 | 0 |
| Cyanobacteria | 0 | 0 | 0 | 0 | 0 | 0.14 | 0 | 0 | 0.28 | 0 | 0.097 | 0.076 |
Appendix B
| Method | Sampling Date (mm/dd) in 2018 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHEMTAX | 4/27 | 5/16 | 5/30 | 6/15 | 6/25 | 7/18 | 7/25 | 4/25 | 8/8 | 8/8 | 9/6 | 9/20 | 10/4 |
| Cell counting | 4/18 | 5/16 | 5/30 | 6/11 | 6/20 | 7/19 | 7/24 | 7/27 | 8/1 | 8/7 | 9/11 | 9/17 | 10/2 |
Appendix C
| Study Sites | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gangjeong-Goryeong Weir | Samunjin Bridge | Goryeong Bridge | Dalseong Weir | Hapcheon-Changnyeong Weir | Changnyeong-Haman Weir | |||||||||||||
| Date | LEC | Zeu | SD | LEC | Zeu | SD | LEC | Zeu | SD | LEC | Zeu | SD | LEC | Zeu | SD | LEC | Zeu | SD |
| (mm/dd) | (m−1) | (m) | (m) | (m−1) | (m) | (m) | (m−1) | (m) | (m) | (m−1) | (m) | (m) | (m−1) | (m) | (m) | (m−1) | (m) | (m) |
| 4/27 | 1.53 | 3.5 | 0.9 | 1.18 | 4.0 | 1.0 | 1.65 | 2.3 | 1.2 | 1.51 | 4.0 | 1.2 | 1.61 | 3.5 | 1.6 | 5.37 | 2.0 | 0.7 |
| 5/16 | 1.50 | 3.5 | 1.6 | 1.56 | 3.0 | 1.1 | – | 3.5 | – | 1.03 | 4.0 | 1.2 | 1.28 | 3.5 | 1.0 | 1.29 | 3.0 | 1.0 |
| 5/30 | 1.02 | 4.5 | 2.0 | 3.23 | 3.0 | 0.9 | 0.96 | 5.0 | 2.1 | 0.81 | 6.0 | 2.7 | 0.69 | 8.0 | 3.0 | 1.20 | 5.0 | 2.0 |
| 6/15 | 1.30 | 4.5 | 2.5 | 2.30 | 2.0 | 1.5 | 0.94 | 3.6 | 2.5 | 0.93 | 6.0 | 3.1 | 0.81 | 5.0 | 2.0 | 0.93 | 3.0 | 1.0 |
| 6/25 | 0.94 | 8.0 | 2.9 | 1.05 | 3.5 | 1.5 | 0.88 | 6.5 | 1.9 | 1.47 | 4.5 | 1.9 | 1.45 | 3.0 | 1.0 | 1.47 | 3.0 | 1.4 |
| 7/18 | 1.32 | 4.0 | 2.0 | 1.49 | 3.5 | 1.3 | 1.02 | 4.5 | 1.7 | 1.30 | 4.0 | 1.4 | 1.30 | 4.0 | 1.2 | 1.81 | 2.5 | 1.2 |
| 7/25 | 1.65 | 4.0 | 1.8 | 4.12 | 1.9 | 0.9 | 1.59 | 3.3 | 1.0 | 1.55 | 3.5 | 1.0 | 1.18 | 3.0 | 1.2 | 2.99 | 3.0 | 1.2 |
| 8/8 | 1.76 | 2.5 | 1.4 | 1.82 | 3.0 | 1.3 | 3.53 | 2.0 | 0.3 | 3.23 | 1.5 | 0.4 | 2.75 | 2.0 | 0.8 | 2.95 | 2.0 | 0.5 |
| 9/6 | 3.18 | 1.3 | 0.1 | 3.63 | 1.4 | 0.1 | – | 1.5 | – | 2.80 | 1.6 | 0.3 | 1.91 | 2.5 | 0.2 | 2.23 | 2.5 | 0.2 |
| 9/20 | 1.11 | 4.5 | 1.3 | 1.38 | 4.0 | 1.4 | 1.30 | 4.5 | 1.3 | 1.29 | 4.0 | 1.1 | 1.35 | 4.0 | 1.0 | 1.54 | 2.5 | 0.7 |
| 10/4 | 1.40 | 3.5 | 0.8 | 1.63 | 2.0 | 0.8 | 1.11 | 4.0 | 1.0 | 0.79 | 5.5 | 1.1 | 1.18 | 4.0 | 1.0 | 1.53 | 3.0 | 1.0 |
Appendix D
| Region | Methods | Sampling Date | Primary Production (mg C m−2 d−1) | References |
|---|---|---|---|---|
| Ganjeong weir–Haman weir | 13C | 2017.10–2017.12 | 61–933 | [55] |
| Nakdong River estuary | 14C | 1991.10–1992.09 | 543–4112 | [56] |
| Gangjeong–Hagu | 14C | 1994.07–1994.08 | 8000–23,000 | [57] |
| Seonakdong River | 14C | 1996.01–1996.10 | 2000–181,000 | [58] |
| Ganjeong weir–Haman weir | 13C | 2018.04–2018.10 | 8.9–3519 | This study |
Appendix E
| Light | LEC | Zeu | SD | HRT | WT | pH | NH4+ | NO3− + NO2− | PO43− | SiO2 | POC | PN | N/P | Chl. a | P.P. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Light | 1.000 | 0.620 ** | −0.486 ** | −0.220 | 0.303 | 0.694 ** | 0.599 ** | −0.377 * | −0.400 * | −0.141 | 0.395 * | 0.455 ** | 0.379 * | 0.027 | −0.508 ** | 0.200 |
| LEC | 1.000 | −0.784 ** | −0.566 ** | −0.134 | 0.156 | 0.106 | −0.081 | −0.106 | 0.008 | 0.256 | 0.467 ** | 0.290 | −0.089 | −0.290 | 0.092 | |
| Zeu | 1.000 | 0.771 ** | 0.142 | −0.131 | 0.033 | 0.092 | 0.162 | 0.007 | −0.150 | −0.510 ** | −0.332 * | 0.100 | 0.341 * | −0.075 | ||
| SD | 1.000 | 0.379 * | 0.185 | 0.269 | −0.026 | −0.141 | −0.250 | −0.257 | −0.302 | −0.163 | 0.323 * | 0.213 | 0.099 | |||
| HRT | 1.000 | 0.641 ** | 0.672 ** | −0.523 ** | −0.737 ** | −0.383 * | 0.157 | 0.166 | 0.237 | 0.223 | −0.155 | 0.163 | ||||
| WT | 1.000 | 0.778 ** | −0.410 ** | −0.679 ** | −0.189 | 0.249 | 0.331 * | 0.397 * | 0.026 | −0.409 ** | 0.243 | |||||
| pH | 1.000 | −0.525 ** | −0.555 ** | −0.410 ** | 0.197 | 0.361 * | 0.468 ** | 0.259 | −0.201 | 0.326 * | ||||||
| NH4+ | 1.000 | 0.481 ** | 0.311 | −0.050 | −0.159 | −0.160 | −0.200 | −0.059 | −0.051 | |||||||
| NO3− + NO2− | 1.000 | 0.242 | −0.032 | −0.152 | −0.232 | 0.015 | 0.380 * | −0.049 | ||||||||
| PO43− | 1.000 | 0.442 ** | −0.245 | −0.350 * | −0.938 ** | −0.076 | −0.408 ** | |||||||||
| SiO2 | 1.000 | 0.141 | 0.108 | −0.496 ** | −0.262 | −0.175 | ||||||||||
| POC | 1.000 | 0.922 ** | 0.223 | 0.028 | 0.627 ** | |||||||||||
| PN | 1.000 | 0.308 | 0.085 | 0.709 ** | ||||||||||||
| N/P | 1.000 | 0.199 | 0.440 ** | |||||||||||||
| Chl. a | 1.000 | 0.304 | ||||||||||||||
| P.P. | 1.000 |
References
- Kimmel, B.L.; Groeger, A.W. Factors controlling primary production in lakes and reservoirs: A perspective. Lake Reserv. Manag. 1984, 1, 277–281. [Google Scholar] [CrossRef]
- Jeong, K.S.; Kim, D.K.; Joo, G.J. Delayed influence of dam storage and discharge on the determination of seasonal proliferations of Microcystis aeruginosa and Stephanodiscus hantzschii in a regulated river system of the lower Nakdong River (South Korea). Water Res. 2007, 41, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Lürling, M.; Eshetu, F.; Faassen, E.J.; Kosten, S.; Huszar, V.L.M. Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshw. Biol. 2013, 58, 552–559. [Google Scholar] [CrossRef]
- Li, H.M.; Tang, H.J.; Shi, X.Y.; Zhang, C.S.; Wang, X.L. Increased nutrient loads from the Changjiang (Yangtze) River have led to increased Harmful Algal Blooms. Harmful Algae 2014, 39, 92–101. [Google Scholar] [CrossRef]
- Lee, Y.; Ha, S.Y.; Park, H.K.; Han, M.S.; Shin, K.H. Identification of key factors influencing primary productivity in two river-type reservoirs by using principal component regression analysis. Environ. Monit. Assess. 2015, 187, 213. [Google Scholar] [CrossRef]
- Cao, J.; Chu, Z.; Du, Y.; Hou, Z.; Wang, S. Phytoplankton dynamics and their relationship with environmental variables of Lake Poyang. Hydrol. Res. 2016, 47, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Franchini, F.; Lepori, F.; Bruder, A. Improving estimates of primary production in lakes: A test and a case study from a peri-alpine lake (Lake Lugano). Inland Waters 2017, 7, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, T.; Tomizawa, K.; Okamoto, Y.; Watanabe, K.; Salaenoi, J.; Hayashizaki, K.; Kurokura, H.; Ishikawa, S. Nutrients, light and phytoplankton production in the shallow, tropical coastal waters of Bandon Bay, Southern Thailand. Mar. Ecol. 2017, 38, 1–12. [Google Scholar] [CrossRef]
- Jia, J.; Gao, Y.; Song, X.; Chen, S. Characteristics of phytoplankton community and water net primary productivity response to the nutrient status of the Poyang Lake and Gan River, China. Ecohydrology 2019, 12, e2136. [Google Scholar] [CrossRef]
- Elliott, J.A.; Jones, I.D.; Thackeray, S.J. Testing the sensitivity of phytoplankton communities to changes in water temperature and nutrient load, in a temperate lake. Hydrobiologia 2006, 559, 401–411. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Li, M.; Liu, B.; Liu, X.; Wu, Z.; Cai, Y.; Xu, J.; Wang, J. Water-level fluctuations are key for phytoplankton taxonomic communities and functional groups in Poyang Lake. Ecol. Indic. 2019, 104, 470–478. [Google Scholar] [CrossRef]
- Coops, H.; Beklioglu, M.; Crisman, T.L. The role of water-level fluctuations in shallow lake ecosystems—Workshop conclusions. Hydrobiologia 2003, 506, 23–27. [Google Scholar] [CrossRef]
- Cha, Y.K.; Cho, K.H.; Lee, H.; Kang, T.; Kim, J.H. The relative importance of water temperature and residence time in predicting cyanobacteria abundance in regulated rivers. Water Res. 2017, 124, 11–19. [Google Scholar] [PubMed]
- Anderson, D.M. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast. Manag. 2009, 52, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef]
- Kim, D.; Hong, S.; Choi, H.; Choi, B.; Kim, J.; Khim, J.S.; Park, H.; Shin, K.H. Multimedia distributions, bioaccumulation, and trophic transfer of microcystins in the Geum River Estuary, Korea: Application of compound-specific isotope analysis of amino acids. Environ. Int. 2019, 133, 105194. [Google Scholar] [CrossRef]
- Lee, H.-J.; Park, H.-K.; Cheon, S.-U. Effects of weir construction on phytoplankton assemblages and water quality in a large river system. Int. J. Environ. Res. Public Health 2018, 15, 2348. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.-Y.; Ahn, J.-M.; Kim, K.; Lee, I.J.; Yang, D.S. Evaluation of water quality characteristics and water quality improvement grade classification of Geumho River tributaries. J. Environ. Sci. Int. 2016, 25, 767–787. [Google Scholar] [CrossRef]
- Tekile, A.; Kim, I.; Kim, J. Mini-review on river eutrophication and bottom improvement techniques, with special emphasis on the Nakdong River. J. Environ. Sci. 2015, 30, 113–121. [Google Scholar] [CrossRef]
- Jun, K.S.; Kim, J.S. The four major rivers restoration project: Impacts on river flows. KSCE J. Civ. Eng. 2011, 15, 217–224. [Google Scholar] [CrossRef]
- Park, J.; Wang, D.; Lee, W.H. Evaluation of weir construction on water quality related to algal blooms in the Nakdong River. Environ. Earth Sci. 2018, 77, 1–11. [Google Scholar] [CrossRef]
- Ha, K.; Cho, E.-A.; Kim, H.-W.; Joo, G.-J. Microcystis bloom formation in the lower Nakdong River, South Korea: Importance of hydrodynamics and nutrient loading. Mar. Freshw. Res. 1999, 50, 89–94. [Google Scholar] [CrossRef]
- Marinas, M.; Sa, E.; Rojas, M.M.; Moalem, M.; Urbano, F.J.; Guillou, C.; Rallo, L. A nuclear magnetic resonance (1H and 13C) and isotope ratio mass spectrometry (δ13C, δ2H and δ18O) study of Andalusian olive oils. Rapid Commun. Mass Spectrom. 2010, 24, 1457–1466. [Google Scholar]
- Hama, T.; Miyazaki, T.; Ogawa, Y.; Iwakuma, T.; Takahashi, M.; Otsuki, A.; Ichimura, S. Measurement of photosynthetic productions of a marine phytoplankton population using stable 13C isotope. Mar. Biol. 1983, 73, 31–36. [Google Scholar] [CrossRef]
- Garneau, M.È.; Gosselin, M.; Klein, B.; Tremblay, J.É.; Fouilland, E. New and regenerated production during a late summer bloom in an Arctic polynya. Mar. Ecol. Prog. Ser. 2007, 345, 13–26. [Google Scholar] [CrossRef]
- Gordon, L.I.; Jennings, J.C.; Ross, A.A.; Krest, J.M. A Suggested protocol for continuous flow automated analysis of seawater nutrients (phosphate, nitrate, nitrite and silicic acid) in the WOCE Hydrographic Program and the Joint Global Ocean Fluxes Study. WOCE Hydrogr. Progr. Off. Methods Man. WHPO 1993, 91-1, 1–52. [Google Scholar]
- Zapata, M.; Rodríguez, F.; Garrido, J.L. Separation of chlorophylls and carotenoids from marine phytoplankton: A new HPLC method using a reserved phase C8 column and pyridine containing mobile phases. Mar. Ecol. Prog. Ser. 2000, 195, 29–45. [Google Scholar] [CrossRef] [Green Version]
- Park, M.O. Composition and distribution of phytoplankton with size fraction results at southwestern East/Japan Sea. Ocean Sci. J. 2006, 41, 301–313. [Google Scholar] [CrossRef]
- Roy, R.; Pratihary, A.; Mangesh, G.; Naqvi, S.W.A. Spatial variation of phytoplankton pigments along the southwest coast of India. Estuar. Coast. Shelf Sci. 2006, 69, 189–195. [Google Scholar] [CrossRef]
- Wong, C.K.; Wong, C.K. HPLC pigment analysis of marine phytoplankton during a red tide occurrence in Tolo Harbour, Hong Kong. Chemosphere 2003, 52, 1633–1640. [Google Scholar] [CrossRef]
- Tamm, M.; Freiberg, R.; Tõnno, I.; Nõges, P.; Nõges, T. Pigment-based Chemotaxonomy—A quick alternative to determine algal assemblages in large shallow eutrophic lake? PLoS ONE 2015, 10, e0122526. [Google Scholar] [CrossRef] [PubMed]
- Mackey, M.D.; Mackey, D.J.; Higgins, H.; Wright, S.W. CHEMTAX—A program for estimating class abundances from chemical markers: Application to HPLC measurements of phytoplankton. Mar. Ecol. Prog. Ser. 1996, 144, 265–283. [Google Scholar] [CrossRef] [Green Version]
- Schlüter, L.; Lauridsen, T.L.; Krogh, G.; Jørgensen, T. Identification and quantification of phytoplankton groups in lakes using new pigment ratios—A comparison between pigment analysis by HPLC and microscopy. Freshw. Biol. 2006, 51, 1474–1485. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; Hall, N.S.; Rossignol, K.L.; Joyner, A.R.; Zhu, G.; Qin, B. Nutrient limitation dynamics examined on a multi-annual scale in Lake Taihu, China: Implications for controlling eutrophication and harmful algal blooms. J. Freshw. Ecol. 2015, 30, 5–24. [Google Scholar] [CrossRef]
- Schlüter, L.; Behl, S.; Striebel, M.; Stibor, H. Comparing microscopic counts and pigment analyses in 46 phytoplankton communities from lakes of different trophic state. Freshw. Biol. 2016, 61, 1627–1639. [Google Scholar] [CrossRef]
- Korea Water Resources Corporation. A Study on Causes and Prediction of Cyanobacterial Blooms in Korea’s 3 Major Rivers; Korea Water Resources Corporation: Daejeon, Korea, 2018; pp. 68–84. [Google Scholar]
- Kim, S.; Chung, S.; Park, H.; Cho, Y.; Lee, H. Analysis of environmental factors associated with cyanobacterial dominance after river weir installation. Water 2019, 11, 1163. [Google Scholar] [CrossRef] [Green Version]
- Wetzel, R.G. Limnology; Saunders College Publishing: Philadelphia, PA, USA, 1983. [Google Scholar]
- Devercelli, M.; O’Farrell, I. Factors affecting the structure and maintenance of phytoplankton functional groups in a nutrient rich lowland river. Limnologica 2013, 43, 67–78. [Google Scholar] [CrossRef]
- Park, D.-Y.; Park, H.-S.; Kim, S.-J.; Chung, S.-W. Analysis of water quality variation by lowering of water level in Gangjeong-Goryong Weir in Nakdong River. J. Environ. Impact Assess. 2019, 28, 245–262. [Google Scholar]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Tadonléké, R.D. Evidence of warming effects on phytoplankton productivity rates and their dependence on eutrophication status. Limnol. Oceanogr. 2010, 55, 973–982. [Google Scholar] [CrossRef]
- Jung, S.-Y.; Kim, I.-K. Analysis of Water quality factor and correlation between water quality and Chl-a in middle and downstream weir section of Nakdong River. J. Korean Soc. Environ. Eng. 2017, 39, 89–96. [Google Scholar] [CrossRef]
- Justić, D.; Rabalais, N.N.; Turner, R.E.; Dortch, Q. Changes in nutrient structure of river-dominated coastal waters: Stoichiometric nutrient balance and its consequences. Estuar. Coast. Shelf Sci. 1995, 40, 339–356. [Google Scholar] [CrossRef]
- Cloern, J.E.; Foster, S.Q.; Kleckner, A.E. Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences 2014, 11, 2477–2501. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.T.; Chou, T.L. Silicate as the limiting nutrient for phytoplankton in a subtropical eutrophic estuary of Taiwan. Estuar. Coast. Shelf Sci. 2003, 58, 155–162. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Freshwater Phytoplankton; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Barone, R.; Naselli-Flores, L. Distribution and seasonal dynamics of cryptomonads in sicilian water bodies. Hydrobiologia 2003, 502, 325–329. [Google Scholar] [CrossRef]
- Water Environment Information System. Available online: http://water.nier.go.kr/publicMain/mainContent.do (accessed on 11 March 2019).
- Chorus, I.; Falconer, I.R.; Salas, H.J.; Bartram, J. Health risks caused by freshwater cyanobacteria in recreational waters. J. Toxicol. Environ. Heal. Part. B Crit. Rev. 2000, 3, 323–347. [Google Scholar]
- Soares, M.C.S.; Maria, M.I.; Marinho, M.M.; Azevedo, S.M.F.O.; Branco, C.W.C.; Huszar, V.L.M. Changes in species composition during annual cyanobacterial dominance in a tropical reservoir: Physical factors, nutrients and grazing effects. Aquat. Microb. Ecol. 2009, 57, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Xie, P.; Guo, L.; Li, L.; Miyabara, Y.; Park, H.D. Organ distribution and bioaccumulation of microcystins in freshwater fish at different trophic levels from the eutrophic Lake Chaohu, China. Environ. Toxicol. 2005, 20, 293–300. [Google Scholar] [CrossRef]
- Umehara, A.; Komorita, T.; Tai, A.; Takahashi, T.; Orita, R.; Tsutsumi, H. Short-term dynamics of cyanobacterial toxins (microcystins) following a discharge from a coastal reservoir in Isahaya Bay, Japan. Mar. Pollut. Bull. 2015, 92, 73–79. [Google Scholar] [CrossRef]
- Van Donk, E.; Kilham, S.S. Temperature Effects on Silicon- and Phosphorus-Limited Growth and Competitive Interactions Among Three Diatoms. J. Phycol. 1990, 26, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Min, J.O.; Choi, B.; Kang, J.J.; Choi, K.; Lee, S.H.; Shin, K.H. Variation of primary productivity and phytoplankton community in the weirs of mid and downstream of the Nakdong River during fall and early winter. Korean J. Ecol. Environ 2019, 52, 81–93. [Google Scholar] [CrossRef]
- Lee, J.A.; Cho, K.J.; Kwon, O.S.; Chung, I.K. Primary production of phytoplankton in Natong Estuarine Ecosystem. Korean J. Limnol. 1994, 27, 69–78. [Google Scholar]
- Kim, B.; Kim, D.-S.; Hwang, G.; Choi, K.; Heo, W.-M.; Park, W.-K. Contribution of primary production of phytoplankton to organic pollution in a eutrophic river, the Naktong River. Korean J. Limnol. 1996, 11, 231–237. [Google Scholar]
- Jeon, S.-I.; Cho, K.-J. Primary productivity of phytoplankton in the Shallow and hypertrophic river (Seonakdong River). Korean. J. Limnol. 2004, 37, 57–63. [Google Scholar]











| Site No. | Study Sites | Distance between Study Sites | Water Level | Water Storage | Inflow | Discharge | Hydraulic Retention Time (HRT) | Water Temperature (WT) | pH |
|---|---|---|---|---|---|---|---|---|---|
| (km) | (EL.m) | (× 106 m3) | (m3 s−1) | (m3 s−1) | (days) | (°C) | |||
| ND-1 | Gangjeong-Goryeong weir | – | 18.3 | 73.5 | 28.6 | 11.6 | 1.3 | 15.3 | 7.3 |
| 18.7 | 80.1 | 702 | 686 | 32 | 32.1 | 9.7 | |||
| 18.5 ± 0.19 | 77.0 ± 2.7 | 153 ± 196 | 147 ± 194 | 13 ± 9.8 | 25.0 ± 5.4 | 8.5 ± 0.7 | |||
| ND-2 | Samunjin bridge | ND-1–ND-2 (4) | – | – | – | – | – | 15.1 | 7.4 |
| – | – | – | – | – | 32.4 | 9.4 | |||
| – | – | – | – | – | 24.6 ± 5.0 | 8.3 ± 0.6 | |||
| ND-3 | Goryeong bridge | ND-2–ND-3 (12) | – | – | – | – | – | 16.3 | 7.5 |
| – | – | – | – | – | 32.0 | 9.7 | |||
| – | – | – | – | – | 24.9 ± 5.1 | 8.5 ± 0.7 | |||
| ND-4 | Dalseong weir | ND-3–ND-4 (3) | 13.6 | 53.9 | 29.2 | 29.2 | 0.43 | 16.3 | 7.0 |
| 13.9 | 57.4 | 580 | 577 | 19 | 32.8 | 10.0 | |||
| 13.7 ± 0.12 | 55.5 ± 1.3 | 151 ± 155 | 146 ± 154 | 6.9 ± 4.8 | 25.2 ± 5.4 | 8.6 ± 1 | |||
| ND-5 | Hapcheon-Changnyeong weir | ND-4–ND-5 (29) | 9.17 | 54.5 | 32.4 | 41.5 | 1.0 | 19.7 | 7.2 |
| 9.34 | 56.0 | 646 | 649 | 20 | 33.7 | 10.1 | |||
| 9.27 ± 0.059 | 55.4 ± 0.51 | 165 ± 179 | 170 ± 178 | 7.7 ± 6.1 | 26.2 ± 4.9 | 8.5 ± 0.8 | |||
| ND-6 | Changnyeong-Haman weir | ND-5–ND-6 (44) | 4.82 | 97.3 | 78.9 | 83.6 | 0.96 | 17.4 | 7.1 |
| 4.99 | 101 | 1209 | 1214 | 14 | 32.9 | 9.4 | |||
| 4.90 ± 0.059 | 98.8 ± 1.2 | 274 ± 320 | 276 ± 320 | 6.9 ± 3.7 | 25.4 ± 4.8 | 8.1 ± 0.9 |
| Light | SD | HRT | WT | pH | NH4+ | NO3− + NO2− | PO43− | SiO2 | Chl. a | |
|---|---|---|---|---|---|---|---|---|---|---|
| Diatoms | −0.223 | −0.231 | −0.732 ** | −0.779 ** | −0.698 ** | 0.357 * | 0.617 ** | 0.146 | −0.322 * | 0.338 * |
| Cryptophytes | −0.349 * | 0.157 | 0.162 | 0.003 | −0.053 | −0.134 | −0.071 | 0.028 | 0.141 | −0.382 * |
| Chlorophytes | 0.253 | 0.196 | 0.509 ** | 0.653 ** | 0.466 ** | 0.000 | −0.446 ** | 0.135 | 0.379 * | −0.019 |
| Cyanobacteria | 0.275 | 0.201 | 0.762 ** | 0.794 ** | 0.713 ** | −0.494 ** | −0.752 ** | −0.134 | 0.247 | −0.246 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Min, J.O.; Choi, B.; Kim, D.; Kang, J.J.; Lee, S.H.; Choi, K.; Lee, H.; Jung, J.; Shin, K.-H. Key Factors Controlling Primary Production and Cyanobacterial Harmful Algal Blooms (cHABs) in a Continuous Weir System in the Nakdong River, Korea. Sustainability 2020, 12, 6224. https://0-doi-org.brum.beds.ac.uk/10.3390/su12156224
Choi J, Min JO, Choi B, Kim D, Kang JJ, Lee SH, Choi K, Lee H, Jung J, Shin K-H. Key Factors Controlling Primary Production and Cyanobacterial Harmful Algal Blooms (cHABs) in a Continuous Weir System in the Nakdong River, Korea. Sustainability. 2020; 12(15):6224. https://0-doi-org.brum.beds.ac.uk/10.3390/su12156224
Chicago/Turabian StyleChoi, Jisoo, Jun Oh Min, Bohyung Choi, Dokyun Kim, Jae Joong Kang, Sang Heon Lee, Kwangsoon Choi, Heesuk Lee, Jinyoung Jung, and Kyung-Hoon Shin. 2020. "Key Factors Controlling Primary Production and Cyanobacterial Harmful Algal Blooms (cHABs) in a Continuous Weir System in the Nakdong River, Korea" Sustainability 12, no. 15: 6224. https://0-doi-org.brum.beds.ac.uk/10.3390/su12156224