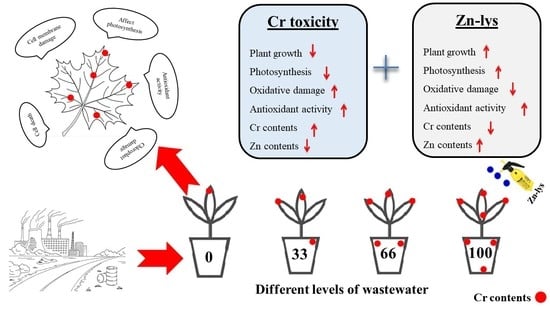
Iron–Lysine Mediated Alleviation of Chromium Toxicity in Spinach (Spinacia oleracea L.) Plants in Relation to Morpho-Physiological Traits and Iron Uptake When Irrigated with Tannery Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Analysis of Wastewater and Soil
2.2. Pot Experiment and Treatments
2.3. Plant Harvesting
2.4. Determination of Photosynthetic Pigments and Gaseous Exchange Parameters
2.5. Determination of Malondialdehyde (MDA), Hydogen Peroxide (H2O2) and Electrolyte Leakage (EL)
2.6. Determination of Superoxidase (SOD), Peroxidase (POD), Catalase (CAT) and Ascorbate Peroxidase (APX) Activity
2.7. Determination of Iron (Fe) and Cromium (Cr) Contents from the Plants
2.8. Statistical Analysis
3. Results
3.1. Effect of Foliar Application of Fe-lys on Plant Growth and Biomass under Different Levels of Tannery Wastewater
3.2. Effect of Foliar Application of Fe-lys on Chlorophyll Contents and Gaseous Exchange Attributes under Different Levels of Tannery Wastewater
3.3. Effect of Foliar Application of Fe-lys on Oxidative Stress and Antioxidant Response under Different Levels of Tannery Wastewater
3.4. Effect of Foliar Application of Fe-lys on the Uptake and Accumulation of Cr and Fe under Different Levels of Tannery Wastewater
3.5. Relationship between Morpho-Physiological Attributes and Cr Uptake in Different Parts of the Plants
3.6. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hashem, I.A.; Abbas, A.Y.; Abd El-Hamed, A.E.-N.H.; Salem, H.M.S.; El-hosseiny, O.E.M.; Abdel-Salam, M.A.; Saleem, M.H.; Zhou, W.; Hu, R. Potential of rice straw biochar, sulfur and ryegrass (Lolium perenne L.) in remediating soil contaminated with nickel through irrigation with untreated wastewater. Peer J. 2020, 8, e9267. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Liu, L.; Bashir, S.; Saleem, M.H.; Chen, C.; Peng, D.; Siddique, K.H. Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (Boehmeria nivea L.) grown as forage in aged copper-contaminated soil. Plant. Physiol. Biochem. 2019, 138, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Ali, S.; Rehman, M.; Rana, M.; Rizwan, M.; Kamran, M.; Imran, M.; Riaz, M.; Hussein, M.; Elkelish, A. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 2020, 248, 126023. [Google Scholar] [CrossRef] [PubMed]
- ŞENKAL, B.C.; USKUTOĞLU, T.; CESUR, C.; ÖZAVCI, V.; DOĞAN, H. Determination of essential oil components, mineral matter, and heavy metal content of Salvia virgata Jacq. grown in culture conditions. Turk. J. Agric. For. 2019, 43, 395–404. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Rizwan, M.; Abbas, Z.; Bukhari, S.A.H.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Zinc-lysine prevents chromium-induced morphological, photosynthetic, and oxidative alterations in spinach irrigated with tannery wastewater. Environ. Sci. Pollut. Res. 2019, 26, 28951–28961. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Malik, Z.; Parveen, A.; Huang, L.; Riaz, M.; Bashir, S.; Mustafa, A.; Abbasi, G.H.; Xue, B.; Ali, U. Ameliorative Effects of Biochar on Rapeseed (Brassica napus L.) Growth and Heavy Metal Immobilization in Soil Irrigated with Untreated Wastewater. J. Plant. Growth Regul. 2020, 39, 266–281. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, X.; Lin, Y.; Yang, C.; Tang, W.; Wu, S.; Li, D.; Lou, W. Effects of copper ions on removal of nutrients from swine wastewater and on release of dissolved organic matter in duckweed systems. Water Res. 2019, 158, 171–181. [Google Scholar]
- Rana, M.S.; Hu, C.X.; Shaaban, M.; Imran, M.; Afzal, J.; Moussa, M.G.; Elyamine, A.M.; Bhantana, P.; Saleem, M.H.; Syaifudin, M. Soil phosphorus transformation characteristics in response to molybdenum supply in leguminous crops. J. Environ. Manag. 2020, 268, 110610. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Hussain, S.; Kamran, M.; Chattha, M.S.; Ahmad, S.; Aqeel, M.; Rizwan, M.; Aljarba, N.H.; Alkahtani, S. Flax (Linum usitatissimum L.): A Potential Candidate for Phytoremediation? Biological and Economical Points of View. Plants 2020, 9, 496. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.H.; Ali, S.; Rehman, M.; Hasanuzzaman, M.; Rizwan, M.; Irshad, S.; Shafiq, F.; Iqbal, M.; Alharbi, B.M.; Alnusaire, T.S. Jute: A Potential Candidate for Phytoremediation of Metals—A Review. Plants 2020, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Maqbool, A.; Ali, S.; Rizwan, M.; Ishaque, W.; Rasool, N.; ur Rehman, M.Z.; Bashir, A.; Abid, M.; Wu, L. Management of tannery wastewater for improving growth attributes and reducing chromium uptake in spinach through citric acid application. Environ. Sci. Pollut. Res. 2018, 25, 10848–10856. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Yang, Y.; Li, Z.; Yang, Y.; Wang, J.; Zhuang, P.; Zou, B. Phytoextraction of 55-year-old wastewater-irrigated soil in a Zn–Pb mine district: Effect of plant species and chelators. Environ. Technol. 2018, 39, 2138–2150. [Google Scholar] [CrossRef] [PubMed]
- Zia, M.H.; Watts, M.J.; Niaz, A.; Middleton, D.R.; Kim, A.W. Health risk assessment of potentially harmful elements and dietary minerals from vegetables irrigated with untreated wastewater, Pakistan. Environ. Geochem. Health 2017, 39, 707–728. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Zia-ur-Rehman, M.; Farid, M.; Asma, M. Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, F.M.; Jebur, A.B.; Nasr, H.M.; Hamid, H.M. Modulatory effect of Turnera diffusa against testicular toxicity induced by fenitrothion and/or hexavalent chromium in rats. Environ. Toxicol. 2019, 34, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Tang, R.; Li, X.; Mo, Y.; Ma, Y.; Ding, C.; Wang, J.; Zhang, T.; Wang, X. Toxic responses of metabolites, organelles and gut microorganisms of Eisenia fetida in a soil with chromium contamination. Environ. Pollut. 2019, 251, 910–920. [Google Scholar] [CrossRef]
- Gill, R.A.; Ali, B.; Cui, P.; Shen, E.; Farooq, M.A.; Islam, F.; Ali, S.; Mao, B.; Zhou, W. Comparative transcriptome profiling of two Brassica napus cultivars under chromium toxicity and its alleviation by reduced glutathione. BMC Genom. 2016, 17, 885. [Google Scholar] [CrossRef] [Green Version]
- Riaz, M.; Yasmeen, T.; Arif, M.S.; Ashraf, M.A.; Hussain, Q.; Shahzad, S.M.; Rizwan, M.; Mehmood, M.W.; Zia, A.; Mian, I.A. Variations in morphological and physiological traits of wheat regulated by chromium species in long-term tannery effluent irrigated soils. Chemosphere 2019, 222, 891–903. [Google Scholar] [CrossRef]
- Ali, S.; Chaudhary, A.; Rizwan, M.; Anwar, H.T.; Adrees, M.; Farid, M.; Irshad, M.K.; Hayat, T.; Anjum, S.A. Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 2015, 22, 10669–10678. [Google Scholar] [CrossRef]
- Li, L.; Zhang, K.; Gill, R.A.; Islam, F.; Farooq, M.A.; Wang, J.; Zhou, W. Ecotoxicological and Interactive Effects of Copper and Chromium on Physiochemical, Ultrastructural, and Molecular Profiling in Brassica napus L. BioMed Res. Int. 2018, 2018, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallah-Ud-Din, R.; Farid, M.; Saeed, R.; Ali, S.; Rizwan, M.; Tauqeer, H.M.; Bukhari, S.A.H. Citric acid enhanced the antioxidant defense system and chromium uptake by Lemna minor L. grown in hydroponics under Cr stress. Environ. Sci. Pollut. Res. 2017, 24, 17669–17678. [Google Scholar] [CrossRef] [PubMed]
- Medda, S.; Mondal, N.K. Chromium toxicity and ultrastructural deformation of Cicer arietinum with special reference of root elongation and coleoptile growth. Ann. Agrar. Sci. 2017, 15, 396–401. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Rizwan, M.; Ali, Q.; Saeed, R.; Nasir, T.; Abbasi, G.H.; Rehmani, M.I.A.; Ata-Ul-Karim, S.T.; Bukhari, S.A.H. Phyto-management of chromium contaminated soils through sunflower under exogenously applied 5-aminolevulinic acid. Ecotoxicol. Environ. Saf. 2018, 151, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Ali, S.; Rizwan, M.; Ali, Q.; Abbas, F.; Bukhari, S.A.H.; Saeed, R.; Wu, L. Citric acid assisted phytoextraction of chromium by sunflower; morpho-physiological and biochemical alterations in plants. Ecotoxicol. Environ. Saf. 2017, 145, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Ali, S.; Rizwan, M.; ur Rehman, M.Z.; Hameed, A.; Hafeez, F.; Alamri, S.A.; Alyemeni, M.N.; Wijaya, L. Role of zinc–lysine on growth and chromium uptake in rice plants under Cr stress. J. Plant Growth Regul. 2018, 37, 1413–1422. [Google Scholar] [CrossRef]
- Yu, X.-Z.; Lu, C.-J.; Li, Y.-H. Role of cytochrome c in modulating chromium-induced oxidative stress in Oryza sativa. Environ. Sci. Pollut. Res. 2018, 25, 27639–27649. [Google Scholar] [CrossRef]
- Yu, X.-Z.; Gu, J.-D. The role of EDTA in phytoextraction of hexavalent and trivalent chromium by two willow trees. Ecotoxicology 2008, 17, 143–152. [Google Scholar] [CrossRef]
- Danish, S.; Kiran, S.; Fahad, S.; Ahmad, N.; Ali, M.A.; Tahir, F.A.; Rasheed, M.K.; Shahzad, K.; Li, X.; Wang, D. Alleviation of chromium toxicity in maize by Fe fortification and chromium tolerant ACC deaminase producing plant growth promoting rhizobacteria. Ecotoxicol. Environ. Saf. 2019, 185, 109706. [Google Scholar] [CrossRef]
- Danish, S.; Tahir, F.; Rasheed, M.; Ahmad, N.; Ali, M.; Kiran, S.; Younis, U.; Irshad, I.; Butt, B. Comparative effect of foliar application of Fe and banana peel biochar addition in spinach for alleviation of chromium (IV) toxicity. Open Agric 2019, 4, 381–390. [Google Scholar] [CrossRef]
- Jiraungkoorskul, W. Review of neuro-nutrition used as anti-alzheimer plant, spinach, Spinacia oleracea. Pharmacogn. Rev. 2016, 10, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Gupta, S.D.; Barman, M.; Mitra, A. Photosynthetic apparatus plays a central role in photosensitive physiological acclimations affecting spinach (Spinacia oleracea L.) growth in response to blue and red photon flux ratios. Environ. Exp. Bot. 2018, 156, 170–182. [Google Scholar] [CrossRef]
- Jabeen, M.; Akram, N.A.; Aziz, M.A.; Aniqa, A. Assessment of Biochemical Changes in Spinach (Spinacea oleracea L.) Subjected to Varying Water Regimes. Sains Malays. 2019, 48, 533–541. [Google Scholar] [CrossRef]
- Ahmed, D.A.; Slima, D.F. Heavy metal accumulation by Corchorus olitorius L. irrigated with wastewater. Environ. Sci. Pollut. Res. 2018, 25, 14996–15005. [Google Scholar] [CrossRef]
- Sangeetha, V.; Sharavanan, P. Use of tannery effluent for irrigation: An evaluative study on the response of Sorghum plants its growth and biochemical characteristics. J. Appl. Adv. Res. 2018, 3, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Zeng, F.; Qiu, L.; Zhang, G. The effect of chromium and aluminum on growth, root morphology, photosynthetic parameters and transpiration of the two barley cultivars. Biol. Plant. 2011, 55, 291–296. [Google Scholar] [CrossRef]
- Saleem, M.H.; Kamran, M.; Zhou, Y.; Parveen, A.; Rehman, M.; Ahmar, S.; Malik, Z.; Mustafa, A.; Anjum, R.M.A.; Wang, B. Appraising growth, oxidative stress and copper phytoextraction potential of flax (Linum usitatissimum L.) grown in soil differentially spiked with copper. J. Environ. Manag. 2020, 257, 109994. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Rehman, M.; Saud, S.; Jamal, Y.; Khan, S.; Liu, L. Morpho-physiological traits, biochemical response and phytoextraction potential of short-term copper stress on kenaf (Hibiscus cannabinus L.) seedlings. Peer J. 2020, 8, e8321. [Google Scholar] [CrossRef] [Green Version]
- Parveen, A.; Saleem, M.H.; Kamran, M.; Haider, M.Z.; Chen, J.-T.; Malik, Z.; Rana, M.S.; Hassan, A.; Hur, G.; Javed, M.T. Effect of Citric Acid on Growth, Ecophysiology, Chloroplast Ultrastructure, and Phytoremediation Potential of Jute (Corchorus capsularis L.) Seedlings Exposed to Copper Stress. Biomolecules 2020, 10, 592. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Rehman, M.; Rizwan, M.; Kamran, M.; Mohamed, I.A.; Bamagoos, A.A.; Alharby, H.F.; Hakeem, K.R.; Liu, L. Individual and combined application of EDTA and citric acid assisted phytoextraction of copper using jute (Corchorus capsularis L.) seedlings. Environ. Technol. Innov. 2020, 19, 100895. [Google Scholar] [CrossRef]
- Gill, R.A.; Ali, B.; Islam, F.; Farooq, M.A.; Gill, M.B.; Mwamba, T.M.; Zhou, W. Physiological and molecular analyses of black and yellow seeded Brassica napus regulated by 5-aminolivulinic acid under chromium stress. Plant Physiol. Biochem. 2015, 94, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Souri, M.K. Aminochelate fertilizers: The new approach to the old problem; a review. Open Agric. 2016, 1, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhang, C.; Wang, C.; Huang, Y.; Liu, Z. Increasing phosphate inhibits cadmium uptake in plants and promotes synthesis of amino acids in grains of rice. Environ. Pollut. 2019, 257, 113496. [Google Scholar] [CrossRef]
- Bashir, A.; Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Ishaque, W.; Riaz, M.A.; Maqbool, A. Effect of foliar-applied iron complexed with lysine on growth and cadmium (Cd) uptake in rice under Cd stress. Environ. Sci. Pollut. Res. 2018, 25, 20691–20699. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils 1. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Page, A. Methods of Soil Analysis. Part. 2. Chemical and Microbiological Properties; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1965. [Google Scholar]
- Amacher, M.C. Nickel, cadmium, and lead. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 739–768. [Google Scholar]
- Soltanpour, P. Use of ammonium bicarbonate DTPA soil test to evaluate elemental availability and toxicity. Commun. Soil Sci. Plant. Anal. 1985, 16, 323–338. [Google Scholar] [CrossRef]
- Apha, A. WPCF, Standard Methods for the Examination of Water and Wastewater; American Public Health Association/American Water Works Association/Water Environment Federation: Washington DC, USA, 1995. [Google Scholar]
- Ayers, R.; Westcot, D. FAO Irrigation and Drainage Paper 29 Rev. 1. In Water Quality for Agriculture; Food and Agriculture Organization of the United Nations: Roma, Italy, 1985; Volume 15, p. 2016. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Chen, C.-N.; Pan, S.-M. Assay of superoxide dismutase activity by combining electrophoresis and densitometry. Bot. Bull. Acad. Sin. 1996, 37, 107–111. [Google Scholar]
- Sakharov, I.Y.; Ardila, G.B. Variations of peroxidase activity in cocoa (Theobroma cacao L.) beans during their ripening, fermentation and drying. Food Chem. 1999, 65, 51–54. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Rehman, M.Z.-u.; Rizwan, M.; Ghafoor, A.; Naeem, A.; Ali, S.; Sabir, M.; Qayyum, M.F. Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phyto-availability to wheat and rice under rotation. Environ. Sci. Pollut. Res. 2015, 22, 16897–16906. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Seleiman, M.F.; Rizwan, M.; Rehman, M.; Akram, N.A.; Liu, L.; Alotaibi, M.; Al-Ashkar, I.; Mubushar, M. Assessing the Correlations between Different Traits in Copper-Sensitive and Copper-Resistant Varieties of Jute (Corchorus capsularis L.). Plants 2019, 8, 545. [Google Scholar] [CrossRef] [Green Version]
- El-Esawi, M.A.; Elkelish, A.; Elansary, H.O.; Ali, H.M.; Elshikh, M.; Witczak, J.; Ahmad, M. Genetic transformation and hairy root induction enhance the antioxidant potential of Lactuca serriola L. Oxid. Med. Cell. Longev. 2017, 2017, 5604746. [Google Scholar] [CrossRef]
- El-Esawi, M.A. Genetic diversity and evolution of Brassica genetic resources: From morphology to novel genomic technologies—A review. Plant. Genet. Resour. 2017, 15, 388–399. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Germaine, K.; Bourke, P.; Malone, R. AFLP analysis of genetic diversity and phylogenetic relationships of Brassica oleracea in Ireland. C. R. Biol. 2016, 339, 163–170. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Din, M.; Ullah, A.; Sabagh, A.E.L.; Hossain, A.; Llanes, A.; Liu, L. Copper-induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China. Environ. Sci. Pollut. Res. 2020, 27, 5211–5221. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Ali, S.; Kamran, M.; Iqbal, N.; Azeem, M.; Tariq Javed, M.; Ali, Q.; Zulqurnain Haider, M.; Irshad, S.; Rizwan, M. Ethylenediaminetetraacetic Acid (EDTA) Mitigates the Toxic Effect of Excessive Copper Concentrations on Growth, Gaseous Exchange and Chloroplast Ultrastructure of Corchorus capsularis L. and Improves Copper Accumulation Capabilities. Plants 2020, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Fahad, S.; Adnan, M.; Ali, M.; Rana, M.S.; Kamran, M.; Ali, Q.; Hashem, I.A.; Bhantana, P.; Ali, M.; et al. Foliar application of gibberellic acid endorsed phytoextraction of copper and alleviates oxidative stress in jute (Corchorus capsularis L.) plant grown in highly copper-contaminated soil of China. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Adhikari, S.; Ghosh, S.; Azahar, I.; Shaw, A.K.; Roy, D.; Roy, S.; Saha, S.; Hossain, Z. Imbalance of redox homeostasis and antioxidant defense status in maize under chromium (VI) stress. Environ. Exp. Bot. 2020, 169, 103873. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Ali, H.M.; Alayafi, A.A.; Witczak, J.; Ahmad, M. Genetic variation and alleviation of salinity stress in barley (Hordeum vulgare L.). Molecules 2018, 23, 2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A.; Witczak, J.; Ahmad, M. Analysis of Genetic Variation and Enhancement of Salt Tolerance in French Pea (Pisum Sativum L.). Int. J. Mol. Sci. 2018, 19, 2433. [Google Scholar] [CrossRef] [Green Version]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of rice Rab7 gene improves drought and heat tolerance and increases grain yield in rice (Oryza sativa L.). Genes 2019, 10, 56. [Google Scholar] [CrossRef] [Green Version]
- Sehrish, A.K.; Aziz, R.; Hussain, M.M.; Rafiq, M.T.; Rizwan, M.; Muhammad, N.; Rafiq, M.K.; Sehar, A.; ud Din, J.; Al-Wabel, M.I. Effect of poultry litter biochar on chromium (Cr) bioavailability and accumulation in spinach (Spinacia oleracea) grown in Cr-polluted soil. Arab. J. Geosci. 2019, 12, 57. [Google Scholar] [CrossRef]
- Sadak, M.S.; Abdelhamid, M.T. Influence of amino acids mixture application on some biochemical aspects, antioxidant enzymes and endogenous polyamines of Vicia faba plant grown under seawater salinity stress. Gesunde Pflanz. 2015, 67, 119–129. [Google Scholar] [CrossRef]
- Ghasemi, S.; Khoshgoftarmanesh, A.H.; Hadadzadeh, H.; Jafari, M. Synthesis of iron-amino acid chelates and evaluation of their efficacy as iron source and growth stimulator for tomato in nutrient solution culture. J. Plant Growth Regul. 2012, 31, 498–508. [Google Scholar] [CrossRef]
- Ghasemi, S.; Khoshgoftarmanesh, A.H.; Afyuni, M.; Hadadzadeh, H. Iron (II)–amino acid chelates alleviate salt-stress induced oxidative damages on tomato grown in nutrient solution culture. Sci. Hortic. 2014, 165, 91–98. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alzahrani, S.M.; Ali, H.M.; Alayafi, A.A.; Ahmad, M. Serratia liquefaciens KM4 Improves Salt Stress Tolerance in Maize by Regulating Redox Potential, Ion Homeostasis, Leaf Gas Exchange and Stress-Related Gene Expression. Int. J. Mol. Sci. 2018, 19, 3310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Ahmad, M. Overexpression of AtWRKY30 Transcription Factor Enhances Heat and Drought Stress Tolerance in Wheat (Triticum aestivum L.). Genes 2019, 10, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nafees, M.; Ali, S.; Naveed, M.; Rizwan, M. Efficiency of biogas slurry and Burkholderia phytofirmans PsJN to improve growth, physiology, and antioxidant activity of Brassica napus L. in chromium-contaminated soil. Environ. Sci. Pollut. Res. 2018, 25, 6387–6397. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Saeed, R.; Rizwan, M.; Bukhari, S.A.H.; Abbasi, G.H.; Hussain, A.; Ali, B.; Zamir, M.S.I.; Ahmad, I. Combined application of citric acid and 5-aminolevulinic acid improved biomass, photosynthesis and gas exchange attributes of sunflower (Helianthus annuus L.) grown on chromium contaminated soil. Int. J. Phytoremediation 2019, 21, 1–8. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Soliman, M.H.; Alhaithloul, H.A.; El-Esawi, M.A. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant. Physiol. Biochem. 2019, 137, 144–153. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A. Azospirillum lipoferum FK1 confers improved salt tolerance in chickpea (Cicer arietinum L.) by modulating osmolytes, antioxidant machinery and stress-related genes expression. Environ. Exp. Bot. 2019, 159, 55–65. [Google Scholar] [CrossRef]
- Vwioko, E.; Adinkwu, O.; El-Esawi, M.A. Comparative Physiological, Biochemical and Genetic Responses to Prolonged Waterlogging Stress in Okra and Maize Given Exogenous Ethylene Priming. Front. Physiol. 2017, 8, 632. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]











| Texture | Clay Loam |
|---|---|
| Silt | 11.9 |
| Clay | 23.4 |
| pH | 7.1 |
| ECE (dS m−1) | 3.86 |
| Cation exchange capacity (CEC) (cmol kg−1) | 4.78 |
| Soluble CO3−2 (mmol L−1) | 0.85 |
| Soluble HCO3 (mmol L−1) | 3.45 |
| Soluble Cl− (mmol L−1) | 5.91 |
| Soluble Ca2+ + Mg2+ (mmol L−1) | 14.93 |
| Organic matter (%) | 0.52 |
| Ni (mg kg−1) | 0.21 |
| Cu (mg kg−1) | 0.39 |
| Zn (mg kg−1) | 0.64 |
| Cr (mg kg−1) | 0.10 |
| Parameters | Values | Permissible Limits ** |
|---|---|---|
| EC (dS m−1) | 1.41 | <1.5 |
| SAR (mmol L−1)1/2 | 4.02 | <7.5 |
| RSC (mmol c L−1) | 2.24 | <2.0 |
| Ni (mg L−1) | 0.09 | 0.20 |
| Cd (mg L−1) | 0.04 | 0.01 |
| Pb (mg L−1) | 1.24 | 5.0 |
| Co (mg L−1) | 0.02 | 0.05 |
| Cr (mg L−1) | 4.03 | 0.10 |
| Zn (mg L−1) | 1.95 | 2.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaheer, I.E.; Ali, S.; Saleem, M.H.; Noor, I.; El-Esawi, M.A.; Hayat, K.; Rizwan, M.; Abbas, Z.; El-Sheikh, M.A.; Alyemeni, M.N.; et al. Iron–Lysine Mediated Alleviation of Chromium Toxicity in Spinach (Spinacia oleracea L.) Plants in Relation to Morpho-Physiological Traits and Iron Uptake When Irrigated with Tannery Wastewater. Sustainability 2020, 12, 6690. https://0-doi-org.brum.beds.ac.uk/10.3390/su12166690
Zaheer IE, Ali S, Saleem MH, Noor I, El-Esawi MA, Hayat K, Rizwan M, Abbas Z, El-Sheikh MA, Alyemeni MN, et al. Iron–Lysine Mediated Alleviation of Chromium Toxicity in Spinach (Spinacia oleracea L.) Plants in Relation to Morpho-Physiological Traits and Iron Uptake When Irrigated with Tannery Wastewater. Sustainability. 2020; 12(16):6690. https://0-doi-org.brum.beds.ac.uk/10.3390/su12166690
Chicago/Turabian StyleZaheer, Ihsan Elahi, Shafaqat Ali, Muhammad Hamzah Saleem, Iqra Noor, Mohamed A. El-Esawi, Kashif Hayat, Muhammad Rizwan, Zohaib Abbas, Mohamed A. El-Sheikh, Mohammed Nasser Alyemeni, and et al. 2020. "Iron–Lysine Mediated Alleviation of Chromium Toxicity in Spinach (Spinacia oleracea L.) Plants in Relation to Morpho-Physiological Traits and Iron Uptake When Irrigated with Tannery Wastewater" Sustainability 12, no. 16: 6690. https://0-doi-org.brum.beds.ac.uk/10.3390/su12166690