Benthic Community Assessment of Commercial Oyster (Crassostrea virginica) Gear in Delaware Inland Bays
Abstract
:1. Introduction
2. Materials and Methods
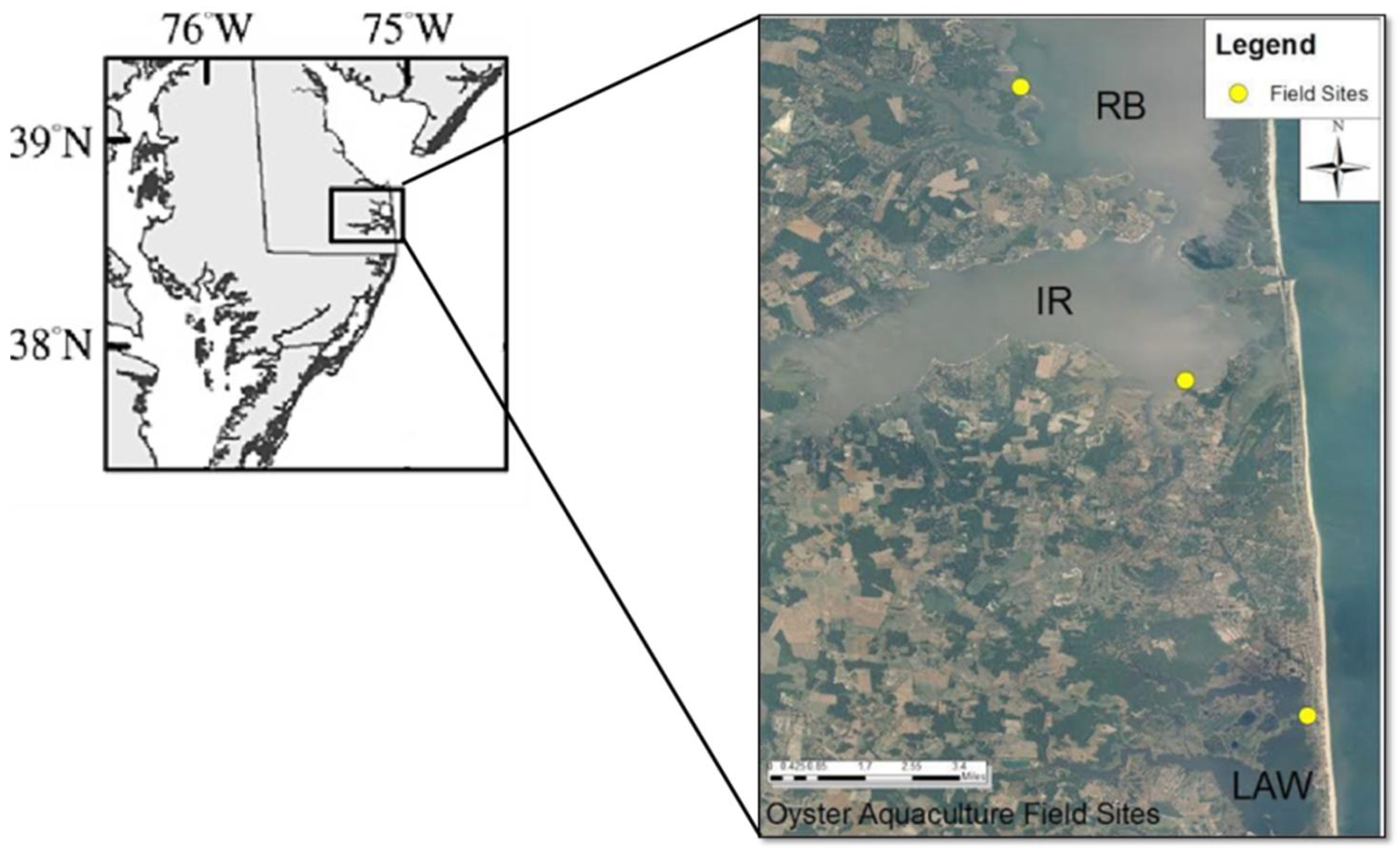
2.1. Study Area
2.2. Water Quality Monitoring
2.3. Set Up Oyster Gears and Trays
2.4. Benthic Community Assessment
2.5. Statistical Analysis
3. Results
3.1. Physicochemical Parameters in Water Samples
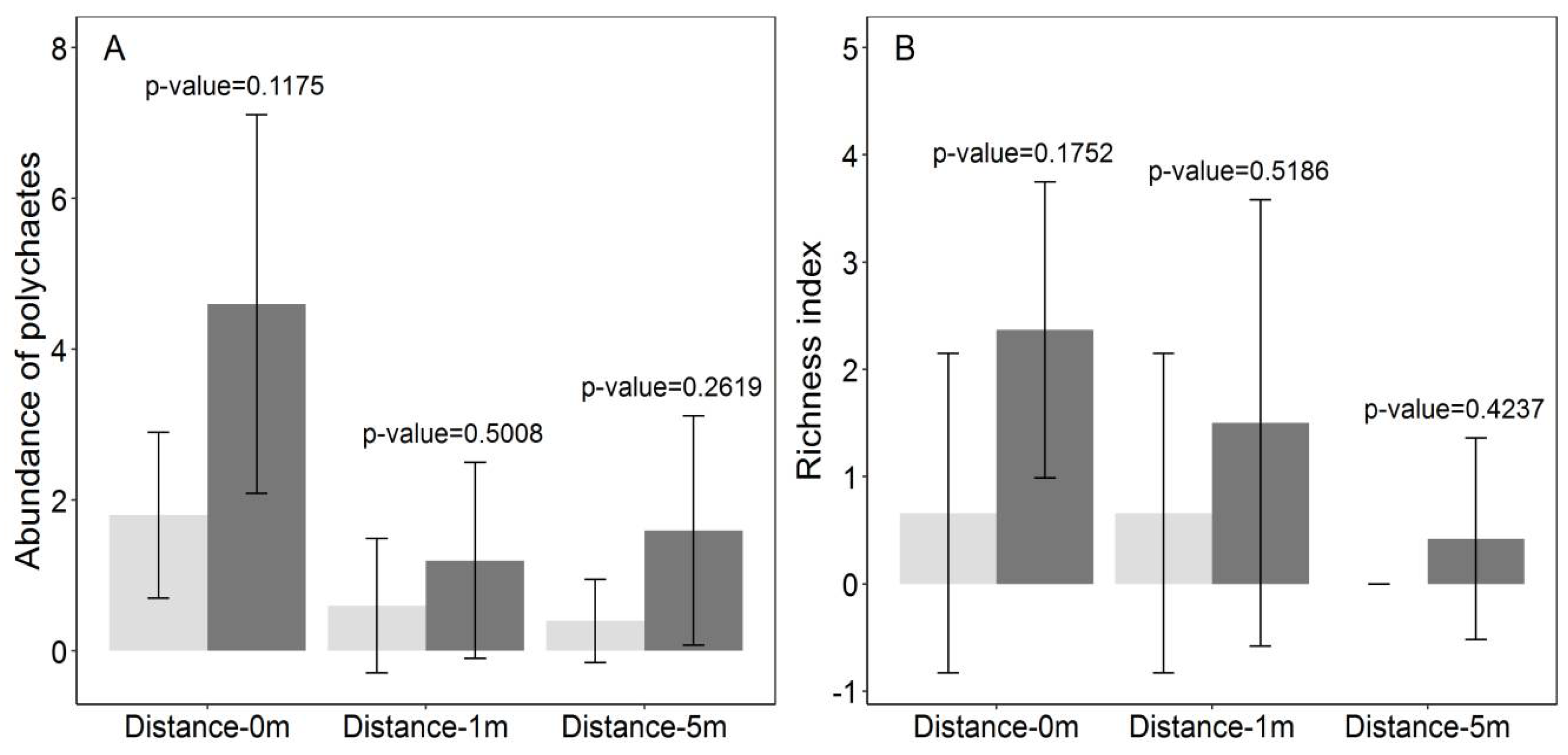
3.2. Polychaetes Abundance and Richness
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaillou, J.; Weisberg, S.; Kutz, F.; DeMoss, T.; Mangiaracina, L.; Magnien, R.; Eskin, R.; Maxted, J.; Price, K.; Summers, J. Assessment of the Ecological Condition of the Delaware and Maryland Coastal Bays; U.S. Environmental Protection Agency: Washington, DC, USA, 1994.
- Sallade, Y.; Sims, J. Phosphorus transformations in the sediments of Delaware’s agricultural drainageways: I. phosphorus forms and sorption. J. Environ. Qual. 1997, 26, 1571–1579. [Google Scholar] [CrossRef]
- Price, K. A framework for a Delaware inland bays environmental classification. Environ. Monit. Assess. 1998, 51, 285–298. [Google Scholar] [CrossRef]
- EPA. State of the Delaware Inland Bays; U.S. Environmental Protection Agency: Washington, DC, USA, 2011.
- Walch, M.; Seldomridge, E.; Mcgowan, A.; Boswell, S.; Bason, C. 2016 State of the Delaware Inland Bays; Delaware Center for the Inland Bays: Rehoboth Beach, DE, USA, 2016. [Google Scholar]
- Ma, S.; Whereat, E.B.; Luther, G.W. Shift of algal community structure in dead end lagoons of the Delaware Inland Bays during seasonal anoxia. Aquat. Microb. Ecol. 2006, 44, 279–290. [Google Scholar] [CrossRef] [Green Version]
- Kiddon, J.A.; Paul, J.F.; Buffum, H.W.; Strobel, C.S.; Hale, S.S.; Cobb, D.; Brown, B.S. Ecological condition of US Mid-Atlantic estuaries, 1997–1998. Mar. Pollut. Bull. 2003, 46, 1224–1244. [Google Scholar] [CrossRef]
- Bricker, S.B.; Longstaff, B.; Dennison, W.; Jones, A.; Boicourt, K.; Wicks, C.; Woerner, J. Effects of nutrient enrichment in the nation’ s estuaries: A decade of change. Harmful Algae 2008, 8, 21–32. [Google Scholar] [CrossRef]
- Handy, S.M.; Demir, E.; Hutchins, D.A.; Portune, K.J.; Whereat, E.B.; Hare, C.E.; Rose, J.M.; Warner, M.; Farestad, M.; Cary, S.C.; et al. Using quantitative real-time PCR to study competition and community dynamics among Delaware Inland Bays harmful algae in field and laboratory studies. Harmful Algae 2008, 7, 599–613. [Google Scholar] [CrossRef]
- Ozbay, G.; Smith, S.L. Are aquaculture practices sustaining our goal to restore oysters (Crassostrea virginica)? In Aquaculture—Plants and Invertebrates; Diarte-Plata, G., Escamilla-Montes, R., Eds.; IntechOpen: London, UK, 2018; p. 32. ISBN 978-1-83881-778-7. [Google Scholar]
- Marenghi, F.; Ozbay, G.; Rossi-snook, K.; Chalabala, E.J. Restoration program in Inland Bays improves ecosystems as oyster populations recover. Glob. Aquac. Advocate 2009, 16–17. Available online: https://www.aquaculturealliance.org/advocate/restoration-program-in-inland-bays-improves-ecosystems-as-oyster-populations-recover/ (accessed on 15 November 2020).
- Zimmerman, R.; Minello, T.; Baumer, T.; Castiglione, M. Oyster Reef as Habitat for Estuarine Macrofauna; NOAA Technical Memorandum NMFS-SEFC-249; National Oceanic and Atmospheric Administration: Washington, DC, USA, 1989; p. 19.
- Grabowski, J.H.; Brumbaugh, R.D.; Conrad, R.F.; Keeler, A.G.; James, J.; Peterson, C.H.; Piehler, M.F.; Sean, P.; Smyth, A.R. Economic valuation of ecosystem services provided by oyster reefs. Bioscience 2012, 62, 900–909. [Google Scholar] [CrossRef] [Green Version]
- Erbland, P.J.; Ozbay, G. A comparison of the macrofaunal communities inhabiting a Crassostrea virginica oyster reef and oyster aquaculture gear in Indian River Bay, Delaware. BioOne 2008, 27, 757–768. [Google Scholar] [CrossRef]
- Marenghi, F.; Ozbay, G. Preliminary habitat assessment of floating oyster (Crassostrea virginica) gardens (Delaware). Ecol. Restor. 2010, 28, 254–257. [Google Scholar] [CrossRef]
- Marenghi, F.; Ozbay, G. Floating oyster, Crassostrea virginica Gmelin 1791, aquaculture as habitat for fishes and macroinvertebrates in Delaware Inland Bays: The comparative value of oyster clusters and loose shell. BioOne 2010, 29, 889–904. [Google Scholar] [CrossRef]
- Rossi-snook, K.; Ozbay, G.; Marenghi, F. Oyster (Crassostrea virginica) gardening program for restoration in Delaware’s Inland Bays, USA. Aquac. Int. 2010, 18, 61–67. [Google Scholar] [CrossRef]
- DNREC. DNREC’s Division of Fish & Wildlife Announces Shellfish Aquaculture Leasing Lottery for Inland Bays; Department of Natural Resources and Environmental Conservation: Dover, DE, USA, 2017.
- Gutierrez, J.L.; Jones, C.G.; Strayer, D.L.; Iribarne, O.O. Mollusks as ecosystem engineers: The role of shell production in aquatic habitats. OIKOS 2003, 101, 79–90. [Google Scholar] [CrossRef]
- O’Beirn, F.; Ross, P.; Luckenbach, M. Organisms associated with oysters cultured in floating systems in Virginia, USA. J. Shellfish Res. 2004, 23, 825–829. [Google Scholar] [CrossRef]
- Ruesink, J.L.; Lenihan, H.S.; Trimble, A.C.; Heiman, K.W.; Micheli, F.; Byers, J.E.; Kay, M.C. Introduction of non-native oysters: Ecosystem effects and restoration implications. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 643–689. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, J.; Fernandes, T.F.; Read, P.; Nickell, T.D.; Davies, I.M. Impacts of biodeposits from suspended mussel (Mytilus edulis L.) culture on the surrounding surficial sediments. ICES J. Mar. Sci. 2001, 58, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Christensen, P.B.; Glud, R.N.; Dalsgaard, T.; Gillespie, P. Impacts of longline mussel farming on oxygen and nitrogen dynamics and biological communities of coastal sediments. Aquaculture 2003, 218, 567–588. [Google Scholar] [CrossRef]
- Hartstein, N.D.; Rowden, A.A. Effect of biodeposits from mussel culture on macroinvertebrate assemblages at sites of different hydrodynamic regime. Mar. Environ. Res. 2004, 57, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Callier, M.D.; McKindsey, C.W.; Desrosiers, G. Multi-scale spatial variations in benthic sediment geochemistry and macrofaunal communities under a suspended mussel culture. Mar. Ecol. Prog. Ser. 2007, 348, 103–115. [Google Scholar] [CrossRef]
- Forrest, B.M.; Keeley, N.B.; Hopkins, G.A.; Webb, S.C.; Clement, D.M. Bivalve aquaculture in estuaries: Review and synthesis of oyster cultivation effects. Aquaculture 2009, 298, 1–15. [Google Scholar] [CrossRef]
- Coe, H.C. Effects of Longline Oyster Aquaculture on Benthic Invertebrate Communities in Humboldt Bay, California; Humboldt State University: Arcata, CA, USA, 2019. [Google Scholar]
- Callier, M.D.; McKindsey, C.W.; Desrosiers, G. Evaluation of indicators used to detect mussel farm influence on the benthos: Two case studies in the Magdalen Islands, Eastern Canada. Aquaculture 2008, 278, 77–88. [Google Scholar] [CrossRef]
- Crawford, C.M.; Macleod, C.K.A.; Mitchell, I.M. Effects of shellfish farming on the benthic environment. Aquaculture 2003, 224, 117–140. [Google Scholar] [CrossRef] [Green Version]
- Gallardi, D. Effects of bivalve aquaculture on the environment and their possible mitigation: A review. Fish. Aquac. J. 2014, 5, 1000105. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Shou, L.; Jiang, Z.; Tang, Y.; Du, P.; Zeng, J.; Chen, Q.; Yan, X.; Chen, J. Effects of fish cage culture and suspended oyster culture on macrobenthic communities in Xiangshan Bay, a semi-enclosed subtropical bay in eastern China. Mar. Pollut. Bull. 2019, 142, 475–483. [Google Scholar] [CrossRef]
- Mirto, S.; Rosa, T.; Danovaro, R.; Mazzola, A. Microbial and meiofaunal response to intensive mussel-farm biodeposition in coastal sediments of the Western Mediterranean. Mar. Pollut. Bull. 2000, 40, 244–252. [Google Scholar] [CrossRef]
- Han, Q.; Wang, Y.; Zhang, Y.; Keesing, J.; Dongyan, L. Effects of intensive scallop mariculture on macrobenthic assemblages in Sishili Bay, the northern Yellow Sea of China. Hydrobiologia 2013, 718, 1–15. [Google Scholar] [CrossRef]
- Pezy, J.; Delecrin, C.; Ba, A.; Basuyaux, O.; Dauvin, J. Anthropogenic impact of oyster farming on macrofauna biodiversity in an eelgrass (Zostera marina) ecosystem of the English Channel. Ecol. Indic. 2019, 106, 1–10. [Google Scholar] [CrossRef]
- Mallet, A.L.; Carver, C.E.; Landry, T. Impact of suspended and off-bottom Eastern oyster culture on the benthic environment in eastern Canada. Aquaculture 2006, 255, 362–373. [Google Scholar] [CrossRef]
- van der Zee, E.M.; Tielens, E.; Holthuijsen, S.; Donadi, S.; Erikson, B.K.; van der Veer, H.W.; Piersma, T.; Olff, H.; van der Heide, T. Habitat modification drives benthic trophic diversity in an intertidal soft-bottom ecosystem. J. Exp. Mar. Biol. Ecol. 2015, 465, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Mendez, N.; Linke-Gamenick, I.; Forbes, V.E.; Balrd, D.J. Sediment processing in Capitella spp. (Polychaeta: Capitellidae): Strain-specic differences and effects of the organic toxicant fluoranthene. Mar. Biol. 2001, 138, 311–319. [Google Scholar] [CrossRef]
- Silva, C.F.; Shimabukuro, M.; Alfaro-Lucas, J.M.; Fujiwara, Y.; Sumida, P.YG; Amaral, A.C.Z. A new Capitella polychaete worms (Annelida: Capitellidae) living inside whale bones in the abyssal South Atlantic. Deep Sea Res. Part I Oceanogr. Res. Pap. 2015, 108, 23–31. [Google Scholar] [CrossRef]
- Forrest, B.M.; Creese, R.G. Benthic impacts of intertidal oyster culture, with consideration of taxonomic sufficiency. Environ. Monit. Assess. 2006, 112, 159–176. [Google Scholar] [CrossRef]
- Sarà, G.; Scilipoti, D.; Milazzo, M.; Modica, A. Use of stable isotopes to investigate dispersal of waste from fish farms as a function of hydrodynamics. Mar. Ecol. Prog. Ser. 2006, 313, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Miron, G.; Landry, T.; Archambault, P.; Frenette, B. Effects of mussel culture husbandry practices on various benthic characteristics. Aquaculture 2005, 250, 138–154. [Google Scholar] [CrossRef]
- Heffernan, M. A Review of the Ecological Implications of Mariculture and Intertidal Harvesting in Ireland; Irish Wildlife Manuals: Dublin, Ireland, 1999. [Google Scholar]
- Solomon, O.O.; Ahmed, O.O. Ecological consequences of oysters culture: A review. Int. J. Fish. Aquat. Stud. 2016, 4, 1–6. [Google Scholar]
- Cranford, P.J.; Strain, P.M.; Dowd, M.; Hargrave, B.T.; Grant, J.; Archambault, M. Influence of mussel aquaculture on nitrogen dynamics in a nutrient enriched coastal embayment. Mar. Ecol. Prog. Ser. 2007, 347, 61–78. [Google Scholar] [CrossRef]
- Ferreira, J.G.; Corner, R.A.; Moore, H.; Service, M.; Bricker, S.B.; Rheault, R.; Nova, U.; Quinta, D.L.; Street, W.; Ecv, L.; et al. Ecological carrying capacity for shellfish aquaculture-sustainability of naturally occurring filter-feeders and cultivated bivalves. J. Shellfish Res. 2018, 37, 1–18. [Google Scholar] [CrossRef]
- Beadman, H.; Kaiser, M.; Galandi, M.; Shucksmith, R.; Willows, R. Changes in species richness with stocking density of marine bivalves. J. Appl. Ecol. 2004, 41, 464–475. [Google Scholar] [CrossRef]
- Kaiser, M.; Laing, I.; Utting, S.; GM, B. Environmental impacts of bivalve mariculture. J. Shellfish Res. 1998, 17, 59–66. [Google Scholar]
- Giangrande, A.; Cavallo, A.; Licciano, M.; Mola, E.; Pierri, C.; Trianni, L. Utilization of the filter feeder polychaete Sabella spallanzanii Gmelin (Sabellidae) as bioremediator in aquaculture. Aquac. Int. 2005, 13, 129–136. [Google Scholar] [CrossRef]
- Tomassetti, P.; Porrello, S. Polychaetes as indicators of marine fish farm organic enrichment. Aquac. Int. 2005, 13, 109–128. [Google Scholar] [CrossRef]
- Martinez-garcia, E.; Sanchez-jerez, P.; Aguado-giménez, F.; Ávila, P.; Guerrero, A.; Sánchez-lizaso, J.L.; Fernandez-gonzalez, V.; González, N.; Ignasi, J.; Carballeira, C.; et al. A meta-analysis approach to the effects of fish farming on soft bottom polychaeta assemblages in temperate regions. Mar. Pollut. Bull. 2013, 69, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, J.; Vibin, A.; Deivakumari, M.; Muruganantham, M.; Ramasubburayam, R.; Palavesam, A.; Immanuel, G. Comparison of polychaete diversity and distribution along the south Tamilnadu coast (Lat. 8.08° to 10.79° N), India Jesudoss. Reg. Stud. Mar. Sci. 2019, 28, 100654. [Google Scholar] [CrossRef]
- Dean, H.K. The use of polychaetes (Annelida) as indicator species of marine pollution: A review. Int. J. Trop. Biol. 2008, 56, 11–38. [Google Scholar] [CrossRef]
- Diaz-Castaneda, V.; Reish, D. Part IV: Environmental and ecological studies. In Annelids in Modern Biology; Shain, D.H., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 203–227. ISBN 9780470455203. [Google Scholar]
- Olsgard, F.; Brattegard, T.; Holthe, T. Polychaetes as surrogates for marine biodiversity: Lower taxonomic resolution and indicator groups. Biodivers. Conserv. 2003, 12, 1033–1049. [Google Scholar] [CrossRef]
- Wlodarska-Kowalczuk, M.; Kedra, M. Surrogacy in natural patterns of benthic distribution and diversity: Selected taxa versus lower taxonomic resolution. Mar. Ecol. Prog. Ser. 2007, 351, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Errera, R.M.; Yvon-lewis, S.; Kessler, J.D.; Campbell, L. Reponses of the dinoflagellate Karenia brevis to climate change: pCO2 and sea surface temperatures. Harmful Algae 2014, 37, 110–116. [Google Scholar] [CrossRef]
- Aguado-Giméneza, F.; Gairínb, J.; Martinez-Garciac, E.; Fernandez-Gonzalez, V.; Ballester Moltó, M.; Cerezo-Valverde, J.; Sanchez-Jerez, P. Application of “taxocene surrogation” and “taxonomic sufficiency” concepts to fish farming environmental monitoring. Comparison of BOPA index versus polychaete assemblage structure. Mar. Environ. Res. 2014, 103, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Mangion, M.; Borg, J.A.; Schembri, P.J.; Scanchez-Jerez, P. Assessment of benthic biological indicators for evaluating the environmental impact of tuna farming. Aquac. Res. 2017, 48, 5797–5811. [Google Scholar] [CrossRef]
- DNREC. Inland Bays/Alantic Ocean Basin Assessment Report; Department of Natural Resources and Environmental Conservation: Dover, DE, USA, 2001.
- DNREC. Sediment Management Plan, Rehoboth Bay, Sussex County, Delaware; Department of Natural Resources and Environmental Conservation: Dover, DE, USA, 2007.
- Eichler, P.P.; Billups, K.; Velez, C.C.; Cardona, V. Investigating faunal and geochemical methods for tracing salinity in an Atlantic coastal Lagoon, Delaware, USA. J. Foraminifer. Res. 2010, 40, 16–35. [Google Scholar] [CrossRef]
- Wang, L.T.; Mckenna, T.E.; Deliberty, T.L. Locating Ground-Water Discharge Areas in Rehoboth, Indian River Bays, and Indian River, Delaware Using Landsat 7 Imagery; University of Delaware: Newark, DE, USA, 2008. [Google Scholar]
- Karpas, R. The Hydrography of Indian River and Rehoboth-Delaware’s Small Bays; University of Delaware: Newark, DE, USA, 1978. [Google Scholar]
- Lee, K. Molecular Assessment of Benthic Diatom Assemblages in Delaware’s Inland Bays; University of Delaware: Newark, DE, USA, 2012. [Google Scholar]
- Fuoco, M. Nitrogen Source Tracking and Benthic Community Assessment in Relation to Oyster Aquaculture in the Delaware Inland Bays; Delaware State University: Dover, DE, USA, 2018. [Google Scholar]
- Cerco, C.F.; Seitzinger, S.P. Measured and modeled effects of benthic algae on eutrophication in Indian River-Rehoboth Bay, Delaware. Estuaries 1997, 20, 231–248. [Google Scholar] [CrossRef]
- Wong, K. On the wind-induced exchange between Indian River Bay, Delaware and the adjacent continental shelf. Cont. Shelf Res. 2002, 22, 1651–1668. [Google Scholar] [CrossRef]
- Wong, K.-C. The effect of coastal sea level forcing on Indian River Bay and Rehoboth Bay, Delaware. Estuar. Coast. Shelf Sci. 1991, 32, 213–229. [Google Scholar] [CrossRef]
- Smullen, J. Water Quality of Inland Bays; Roy F. Weston Inc.: West Chester, PA, USA, 1992. [Google Scholar]
- Weston, R.F. Inc. Report to the Delaware Inland Bays National Estuary Program; Delaware Department of Natural Resources and Environmental Control: Dover, DE, USA, 1993.
- Xu, L.; Ditroro, D.; Kirby, J. Numerical Study in Delaware Inland Bays; University of Delaware: Newark, DE, USA, 2006. [Google Scholar]
- Chrzastowski, M.J. Stratigraphy and Geologic History of a Holocene Lagoon: Rehoboth Bay and Indian River Bay, Delaware; University of Delaware: Newark, DE, USA, 1986. [Google Scholar]
- DNREC. Total Maximum Daily Loads for the Little Assawomn Bay and Tributaries and Ponds of the Indian River, Indian River Bay, and Reboth Bay; Department of Natural Resources and Environmental Conservation: Dover, DE, USA, 2004.
- Allison, J. Maryland Coastal Basin Existing Water Quality Conditions: Water Resources Administration; Maryland Department of Natural Resources: Annapolis, MD, USA, 1975.
- Wells, D.; Ortt, R. Bathymetric Survey of Assawoman Bay, St.Martin River, Sinepuxent Bay and Newport Bay; Maryland Department of Natural Resources: Annapolis, MD, USA, 2001.
- MDE. Priority Areas for Wetland Restoration, Preservation, and Mitigation in Maryland’s Coastal Bays; Maryland Department of the Environment: Baltimore, MD, USA, 2004.
- DIBEP. Delaware Inland Bays Estuary: Technical Appendix; Delaware Center for the Inland Bays: Rehoboth Beach, DE, USA, 1993. [Google Scholar]
- DIBEP. A day in the Life of Delaware’s Forgotten Bay: A Scientific Survey of Little Assawoman Bay; Delaware Center for the Inland Bays: Rehoboth Beach, DE, USA, 1991. [Google Scholar]
- VIMS. Polychaete Key for Chesapeake Bay and Coastal Virginia; College of William & Mary: Gloucester Point, VA, USA, 2001. [Google Scholar]
- Margalef, R. Information Theory in Ecology. Gen. Syst. 1957, 3, 36–71. [Google Scholar]
- He, F.; Gaston, K.J. Occupancy-abundance relationships and sampling scales. Ecography 2000, 23, 503–511. [Google Scholar] [CrossRef]
- R Development Core Team: A Language and Environment for Statistical Computing. Available online: https://cran.r-project.org/bin/windows/base/old/3.6.2/ (accessed on 12 December 2019).
- Nazeer, S.; Khan, M.U.; Malik, R.N. Phytoplankton spatio-temporal dynamics and its relation to nutrients and water retention time in multi-trophic system of Soan River, Pakistan. Environ. Technol. Innov. 2018, 9, 38–50. [Google Scholar] [CrossRef]
- Dobler, D.; Friedrich, S.; Pauly, M. Nonparametric MANOVA in meaningful effects. Ann. Inst. Stat. Math. 2019, 72, 997–1022. [Google Scholar] [CrossRef]
- Herna, I.; Pe, A.; Mateo, J.J.; Megina, C.; Vergara, J.J.; Steentoft, G.; Farnham, L.M.I. Growth dynamic of Ulva rotundata (Chlorophyta) in a fish farm: Implications for biomitigation at a large scale. J. Phycol. 2008, 1089, 1080–1089. [Google Scholar] [CrossRef]
- Beer, S.; Israel, A. Photosynthesis of Ulva fasciata. IV. pH, carbonic anhydrase and inorganic carbon conversions in the unstirred layer. Plant Cell Environ. 1990, 13, 555–560. [Google Scholar] [CrossRef]
- Björk, M.; Axelsson, L.; Beer, S. Why is Ulva intestinalis the only macroalga inhabiting isolated rockpools along the Swedish Atlantic coast ? Mrine Ecol. Prog. Ser. 2004, 284, 109–116. [Google Scholar] [CrossRef]
- Rybak, A.S.; Gabka, M. The influence of abiotic factors on the bloom-forming alga Ulva flexuosa (Ulvaceae, Chlorophyta): Possibilities for the control of the green tides in freshwater ecosystems. J. Appl. Phycol. 2018, 30, 1405–1416. [Google Scholar] [CrossRef] [Green Version]
- Castaldelli, G.; Welsh, D.; Flachi, G.; Zucchini, G.; Colombo, G.; Rossi, R.; Fano, E. Decomposition dynamics of the bloom forming macroalga Ulva rigida determined using a 14C-carbon radio-tracer technique. Aquat. Bot. 2003, 75, 111–122. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Arvanitidis, C.; Eleftheriou, A. Multicausal environmental severity: A flexible framework for microtidal sandy beaches and the role of polychaetes as an indicator taxon. Estuar. Coast. Mar. Sci. 2006, 70, 643–653. [Google Scholar] [CrossRef]
- CIB. About the Bays: Issues Affecting the Bays; Delaware Center for the Inland Bays: Rehoboth Beach, DE, USA, 2014. [Google Scholar]
- MacKenzie, J.; Martin, J.; Pintea, L.; Boonmee, B.; Gedamu, N.; Thomas, T. Delaware Inland Bays Watershed Nutrient Management Project; University of Delaware: Newark, DE, USA, 2016. [Google Scholar]
- Vallarino, E.A.; Rivero, M.S.; Gravina, M.C.; Elías, R. The community-level response to sewage impact in intertidal mytilid beds of the Southwestern Atlantic, and the use of the Shannon index to assess pollution. Rev. Biol. Mar. Oceanogr. 2002, 37, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Belan, T.A. Benthos abundance pattern and species composition in conditions of pollution in Amursky Bay (the Peter the Great Bay, the Sea of Japan). Mar. Pollut. Bull. 2003, 46, 1111–1119. [Google Scholar] [CrossRef]
- Rivero, M.S.; Elias, R.; Vallarino, E.A. First survey of macroinfauna in the Mar del Plata Harbor (Argentina), and the use of polychaetes as pollution indicators. Rev. Biol. Mar. Oceanogr. 2005, 40, 101–108. [Google Scholar] [CrossRef]
- Dix, T.; Karlen, D.; Grabe, S.; Goetting, B.; Holden, S.; Markahm, C. Spionid polychaetes as environmental indicators: An example from Tampa Bay, Florida. In Estuarine Indicators; Bortone, S.A., Ed.; CRC Press: Boca Raton, FL, USA, 2004; p. 20. ISBN 9780429122231. [Google Scholar]
- Levin, L.A.; Gage, J.D.; Martin, C.; Lamont, P.A. Macrobenthic community structure within and beneath the oxygen minimum zone, NW Arabian Sea. Deep Sea Res. II 2000, 47, 189–226. [Google Scholar] [CrossRef]
- Jose, J.J.; Udayakumar, P.; Deepak, M.P.; Rajesh, B.R.; Babu, K.N.; Chandran, A. Assemblages of marine polychaete genus Glycera (Phyllodocida: Glyceridae) along the Kerala Coast as an indicator of organic enrichment. Nat. Environ. Pollut. Technol. 2011, 10, 395–398. [Google Scholar]
- Bailey-brock, J.H.; Paavo, B.; Barrett, B.M.; Dreyer, J. Polychaetes associated with a tropical ocean outfall: Synthesis of a biomonitoring program off O’ahu, Hawaii. Pac. Sci. 2002, 56, 459–479. [Google Scholar] [CrossRef] [Green Version]
- Kruse, I.; Strasser, M.; Thiermann, F. The role of ecological divergence in speciation between intertidal and subtidal Scoloplos armiger (Polychaeta, Orbiniidae). J. Sea Res. 2004, 51, 53–62. [Google Scholar] [CrossRef]
- Bellan, G.; Desrosierst, G.; Willsie, A. Use of an annelid pollution index for monitoring a moderately polluted littoral zone. Mar. Pollut. Bull. 1988, 19, 662–665. [Google Scholar] [CrossRef]
- Gao, Q.F.; Cheung, K.L.; Cheung, S.G.; Shin, P.K.S. Effects of nutrient enrichment derived from fish farming activities on macroinvertebrate assemblages in a subtropical region of Hong Kong. Mar. Pollut. Bull. 2005, 51, 994–1002. [Google Scholar] [CrossRef]
- Hernández-Alcántara, P.; Solís-Weiss, V. Distribution of the polychaete assemblages on the continental shelf of the Northern Gulf of California, Eastern Pacific. Ital. J. Zool. 2011, 78, 280–289. [Google Scholar] [CrossRef]
- Newell, R. Ecosystem influences of natural and cultivated populations of suspension-feeding bivalve molluscs: A review. J. Shellfish Res. 2004, 23, 51–61. [Google Scholar]
- Fabi, G.; Manoukian, S.; Spagnolo, A. Impact of an open-sea suspended mussel culture on macrobenthic community (Western Adriatic Sea). Aquaculture 2009, 289, 54–63. [Google Scholar] [CrossRef]
- Wu, R.; Lam, K.; MacKay, D.; Lau, T.; Yam, V. Impact of marine fish farming on water quality and bottom sediment: A case study in the sub-tropical environment. Mar. Environ. Res. 1994, 38, 115–145. [Google Scholar] [CrossRef]
- Duball, C.E. Environmental Impacts of Oyster Aquaculture on the Coastal Lagoons of Southern Rhode Island; University of Rhode Island: Kingstown, RI, USA, 2017. [Google Scholar]
- Dubois, S.; Marin-Léal, J.C.; Ropert, M.; Lefebvre, S. Effects of oyster farming on macrofaunal assemblages associated with Lanice conchilega tubeworm populations: A trophic analysis using natural stable isotopes. Aquaculture 2007, 271, 336–349. [Google Scholar] [CrossRef]
- Prins, T.C.; Smaal, A.C.; Dame, R.F. A review of the feedbacks between bivalve grazing and ecosystem processes. Aquat. Ecol. 1997, 31, 349–359. [Google Scholar] [CrossRef]
- Black, K. Environmental Impacts of Aquaculture; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Magill, S.H.; Thetmeyer, H.; Cromey, C.J. Settling velocity of faecal pellets of gilthead sea bream (Sparus aurata L.) and sea bass (Dicentrarchus labrax L.) and sensitivity analysis using measured data in a deposition model. Aquaculture 2006, 251, 295–305. [Google Scholar] [CrossRef]
- Rice, M. Environmental impacts of shellfish aquaculture: Filter feeding to control eutrophication. In Proceedings of the Marine Aquaculture and the Marine Environment: A Meeting for the Stakeholders in the Northeast, Boston, MA, USA, 11–13 January 2001; Tlusty, M., Bengtson, D., Halvorson, H., Oktay, S., Pearce, J., Rheualt, R., Eds.; Cape Cod Press: Falmouth, MA, USA, 2001; pp. 77–86. [Google Scholar]
- Sturdivant, S.K.; Díaz, R.J.; Llansó, R.; Dauer, D.M. Relationship between Hypoxia and Macrobenthic Production in Chesapeake Bay. Estuaries Coasts 2014, 37, 1219–1232. [Google Scholar] [CrossRef]


| Parameter | Location Effect | Year Effect | Month Effect | |||
|---|---|---|---|---|---|---|
| F2, 109 | p-Value | F1, 109 | p-Value | F4, 109 | p-Value | |
| Surface temperature | 1.641 | 0.1990 n.s | 0.692 | 0.4070 n.s | 33.575 | <2.2−16 *** |
| Surface salinity | 59.981 | <2.2−16 *** | 3.018 | 0.0852 n.s | 5.381 | 0.0006 *** |
| pH | 0.605 | 0.5481 n.s | 4.581 | 0.0345 * | 8.681 | 4.03 × 10−6 *** |
| Turbidity | 3.784 | 0.0258 * | 6.676 | 0.0111 * | 8.971 | 2.66 × 10−6 *** |
| Dissolved oxygen | 2.358 | 0.0994 n.s | 14.667 | 0.0002 *** | 7.303 | 3−05 *** |
| Chlorophyll a | 8.959 | 0.0003 *** | 47.411 | 3.85−10 *** | 2.875 | 0.0262 * |
| Family | Number of Specimens | Month | Distance | Bay | Year 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| June | July | August | September | October | Under gear | One Meter | Five Meters | RB | IR | LAW | 2016 | 2017 | ||
| Arenicolidae | 3 | + | + | + | + | + | + | |||||||
| Capitellidae | 299 | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Chaetopteridae | 4 | + | + | + | + | + | ||||||||
| Eunicidae | 1 | + | + | + | + | |||||||||
| Glyceridae | 19 | + | + | + | + | + | + | + | + | + | + | + | ||
| 2 | + | + | + | + | + | + | ||||||||
| 2 | + | + | + | + | ||||||||||
| 199 | + | + | + | + | + | + | + | + | + | + | + | + | ||
| Orbiniidae | 60 | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Oweniidae | 29 | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Phyllodocidae | 3 | + | + | + | + | + | + | |||||||
| Spionidae | 46 | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Spirobidae | 3 | + | + | + | + | + | + | |||||||
| Not identified | 39 | + | + | + | + | + | + | + | + | + | ||||
| Polycladida | 1 | + | + | + | + | |||||||||
| Source | df | Multivariate | Abundance | Richness | |||
|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | ||
| Bay | 2.72 | 12.069 | 0.009 ** | 6.732 | 0.020 * | 5.336 | 0.042 * |
| Density | 1.72 | 6.239 | 0.034 * | 0.854 | 0.053 n.s | 5.385 | 0.021 * |
| Distance | 2.72 | 60.855 | <0.001 *** | 48.709 | <0.001 *** | 12.146 | <0.001 *** |
| Bay × Density | 2.72 | 2.400 | 0.494 n.s | 0.171 | 0.642 n.s | 2.229 | 0.304 n.s |
| Bay × Distance | 4.72 | 11.404 | 0.090 n.s | 3.684 | 0.160 n.s | 7.719 | 0.082 n.s |
| Density × Distance | 2.72 | 23.512 | <0.001 *** | 14.915 | <0.001*** | 8.597 | 0.010*** |
| Bay × Density × Distance | 4.72 | 2.792 | 0.824 n.s | 1.942 | 0.931 n.s | 0.850 | 0.914 n.s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuoco, M.; Borsum, S.; Kouhanestani, Z.M.; Ozbay, G. Benthic Community Assessment of Commercial Oyster (Crassostrea virginica) Gear in Delaware Inland Bays. Sustainability 2021, 13, 6480. https://0-doi-org.brum.beds.ac.uk/10.3390/su13116480
Fuoco M, Borsum S, Kouhanestani ZM, Ozbay G. Benthic Community Assessment of Commercial Oyster (Crassostrea virginica) Gear in Delaware Inland Bays. Sustainability. 2021; 13(11):6480. https://0-doi-org.brum.beds.ac.uk/10.3390/su13116480
Chicago/Turabian StyleFuoco, Melanie, Scott Borsum, Zohreh Mazaheri Kouhanestani, and Gulnihal Ozbay. 2021. "Benthic Community Assessment of Commercial Oyster (Crassostrea virginica) Gear in Delaware Inland Bays" Sustainability 13, no. 11: 6480. https://0-doi-org.brum.beds.ac.uk/10.3390/su13116480






