Aspergillus foetidus Regulated the Biochemical Characteristics of Soybean and Sunflower under Heat Stress Condition: Role in Sustainability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of the Fungal Endophyte AdR-13
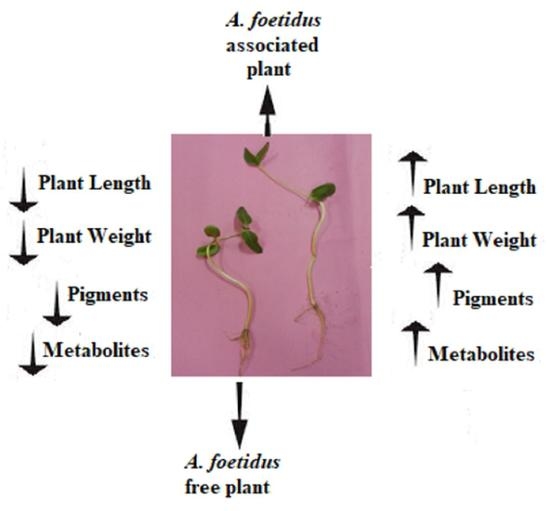
2.2. Early Assessment of the Potent Fungi Using Oryza sativa
2.3. Identification of AdR13
2.4. Inoculation of A. foetidus to G. max and H. annuus
2.5. Estimation of Antioxidants
2.6. Estimation of ABA
2.7. Estimation of Phenolics and Proline
2.8. Estimation of Total Lipids, Proteins and Sugars
2.9. Statistical Analysis
3. Results
3.1. Isolation and Plant Growth Promoting Activity of Endophytes
3.2. Molecular Identification of Isolate AdR-13
3.3. Arbitration of Enzymatic and Non-Enzymatic Antioxidants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ismail; Hamayun, M.; Hussain, A.; Afzal Khan, S.; Iqbal, A.; Lee, I.-J. Aspergillus flavus promoted the growth of soybean and sunflower seedlings at elevated temperature. Biomed Res. Int. 2019, 2019, 1295457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail; Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Lee, I.-J. Endophytic fungus Aspergillus japonicus mediates host plant growth under normal and heat stress conditions. Biomed Res. Int. 2018, 2018, 7696831. [Google Scholar] [CrossRef] [PubMed]
- Ismail; Anwar, H.; Mehmood, A.; Qadir, M.; Husna; Iqbal, A.; Hamayun, M.; Khan, N. Thermal stress alleviating potential of endophytic fungus Rhizopus oryzae inoculated to sunflower (Helianthus annuus L.) And soybean (Glycine max L.). Pak. J. Bot. 2020, 52, 1–5. [Google Scholar] [CrossRef]
- Zahid, K.R.; Ali, F.; Shah, F.; Younas, M.; Shah, T.; Shahwar, D.; Hassan, W.; Ahmad, Z.; Qi, C.; Lu, Y.; et al. Response and tolerance mechanism of cotton Gossypium hirsutum L. to elevated temperature stress: A review. Front. Plant Sci. 2016, 7, 937. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Harris, P. Potential biochemical indicators of salinity tolerance in plants. Plant. Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Abd_Hallah, E.F.; Alqarawi, A.A.; Hashem, A.; Radhakrishnan, R.; Al-Huqail, A.A.; Al-Otibi, F.O.N.; Malik, J.A.; Alharbi, R.I.; Egamberdieva, D. Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J. Plant Interact. 2018, 13, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef] [Green Version]
- Abd_Hallah, E.F.; Hashem, A.; Alqarawi, A.A.; Bahkali, A.H.; Alwhibi, M.S. Enhancing growth performance and systemic acquired resistance of medicinal plant Sesbania sesban (L.) Merr using arbuscular mycorrhizal fungi under salt stress. Saudi J. Biol. Sci. 2015, 22, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Gul Jan, F.; Hamayun, M.; Hussain, A.; Iqbal, A.; Jan, G.; Khan, S.A.; Khan, H.; Lee, I.-J. A promising growth promoting Meyerozyma caribbica from Solanum xanthocarpum alleviated stress in maize plants. Biosci. Rep. 2019, 39, 1–15. [Google Scholar]
- Raid, A.; Humaira, G.; Hamayun, M.; Mamoona, R.; Amjad, I.; Mohib, S.; Anwar, H.; Hamida, B.; In-Jung, L. Aspergillus awamori ameliorates the physicochemical characteristics and mineral profile of mung bean under salt stress. Chem. Biol. Technol. Agric. 2021, 8, 1–13. [Google Scholar]
- Ismail; Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Khan, M.A.; Lee, I.-J. An Endophytic Fungus Gliocladium cibotii Regulates Metabolic and Antioxidant System of Glycine max and Helianthus annuus under Heat Stress. Pol. J. Environ. Stud. 2021, 30, 1631–1640. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Hamayun, M.; Lee, S.-K.; Lee, I.-J. Methyl jasmonate alleviated salinity stress in soybean. J. Crop. Sci. Biotechnol. 2009, 12, 63–68. [Google Scholar] [CrossRef]
- Muhammad, I.; Niaz, A.; Gul, J.; Amjad, I.; Hamayun, M.; Farzana, G.J.; Anwar, H.; In-Jung, L. Trichoderma reesei improved the nutrition status of wheat crop under salt stress. J. Plant Interact. 2019, 14, 590–602. [Google Scholar]
- Khushdil, F.; Jan, F.G.; Jan, G.; Hamayun, M.; Iqbal, A.; Hussain, A.; Bibi, N. Salt stress alleviation in Pennisetum glaucum through secondary metabolites modulation by Aspergillus terreus. Plant Physiol. Biochem. 2019, 144, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Nusrat, B.; Gul, J.; Farzana, G.J.; Hamayun, M.; Amjad, I.; Anwar, H.; Hazir, R.; Abdul, T.; Faiza, K. Cochliobolus sp. acts as a biochemical modulator to alleviate salinity stress in okra plants. Plant Physiol. Biochem. 2019, 139, 459–469. [Google Scholar]
- Ashraf, M.; Foolad, M. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Škorić, D. Sunflower breeding for resistance to abiotic stresses/mejoramiento de girasol por resistencia a estreses abióticos/sélection du tournesol pour la résistance aux stress abiotiques. Helia 2009, 32, 1–16. [Google Scholar]
- Van der Merwe, R.; Labuschagne, M.T.; Herselman, L.; Hugo, A. Effect of heat stress on seed yield components and oil composition in high-and mid-oleic sunflower hybrids. S. Afr. J. Plant. Soil 2015, 32, 121–128. [Google Scholar] [CrossRef]
- Alsajri, F.A.; Singh, B.; Wijewardana, C.; Irby, J.; Gao, W.; Reddy, K.R. Evaluating soybean cultivars for low-and high-temperature tolerance during the seedling growth stage. Agronomy 2019, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, A.; Hussain, A.; Irshad, M.; Hamayun, M.; Iqbal, A.; Khan, N. In vitro production of IAA by endophytic fungus Aspergillus awamori and its growth promoting activities in Zea mays. Symbiosis 2019, 77, 225–235. [Google Scholar] [CrossRef]
- Mehmood, A.; Hussain, A.; Irshad, M.; Hamayun, M.; Iqbal, A.; Rahman, H.; Tawab, A.; Ahmad, A.; Ayaz, S. Cinnamic acid as an inhibitor of growth, flavonoids exudation and endophytic fungus colonization in maize root. Plant Physiol. Biochem. 2019, 135, 61–68. [Google Scholar] [CrossRef]
- Kang, S.-M.; Hamayun, M.; Khan, M.A.; Iqbal, A.; Lee, I.-J. Bacillus subtilis JW1 enhances plant growth and nutrient uptake of Chinese cabbage through gibberellins secretion. J. Appl. Bot. Food Qual. 2019, 92, 172–178. [Google Scholar]
- Ismail; Hamayun, M.; Anwar, H.; Sumera Afzal, K.; Amjad, I.; In-Jung, L. An endophytic fungus Aspergillus violaceofuscus can be used as heat stress adaptive tool for Glycine max L. and Helianthus annuus L. J. Appl. Bot. Food Qual. 2020, 93, 112–120. [Google Scholar]
- Benizri, E.; Courtade, A.; Picard, C.; Guckert, A. Role of maize root exudates in the production of auxins by Pseudomonas fluorescens M. 3.1. Soil Biol. Biochem. 1998, 30, 1481–1484. [Google Scholar] [CrossRef]
- Warrier, R.; Paul, M.; Vineetha, M. Estimation of salicylic acid in Eucalyptus leaves using spectrophotometric methods. Genet. Plant. Physiol. 2013, 3, 90–97. [Google Scholar]
- Khan, S.A.; Hamayun, M.; Yoon, H.; Kim, H.-Y.; Suh, S.-J.; Hwang, S.-K.; Kim, J.-M.; Lee, I.-J.; Choo, Y.-S.; Yoon, U.-H. Plant growth promotion and Penicillium citrinum. BMC Microbiol. 2008, 8, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.-p.; Kuo, T.-T. A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res. 1993, 21, 2260. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.; Dwivedi, U. Genotypic difference in salinity tolerance of green gram cultivars. Plant. Sci. 2004, 166, 1135–1142. [Google Scholar] [CrossRef]
- Ismail; Hamayun, M.; Hussain, A.; Afzal Khan, S.; Iqbal, A.; Lee, I.-J. Aspergillus niger boosted heat stress tolerance in sunflower and soybean via regulating their metabolic and antioxidant system. J. Plant Interact. 2020, 15, 223–232. [Google Scholar] [CrossRef]
- Luck, H. Methods in Enzymatic Analysis, 2nd ed.; Academic Press: New York, NY, USA, 1974. [Google Scholar]
- Kar, M.; Mishra, D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Oberbacher, M.; Vines, H. Spectrophotometric assay of ascorbic acid oxidase. Nature 1963, 197, 1203–1204. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Glutathione reductase. In Methods Enzymol; Elsevier: Amsterdam, The Netherlands, 1985; Volume 113, pp. 484–490. [Google Scholar]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Van Handel, E. Rapid determination of total lipids in mosquitoes. J. Am. Mosq. Control Assoc. 1985, 1, 302–304. [Google Scholar] [PubMed]
- Mohammadkhani, N.; Heidari, R. Drought-induced accumulation of soluble sugars and proline in two maize varieties. World Appl. Sci. J. 2008, 3, 448–453. [Google Scholar]
- Rodriguez, R.J.; Redman, R.S.; Henson, J.M. The role of fungal symbioses in the adaptation of plants to high stress environments. Mitig. Adapt. Strateg. Glob. Chang. 2004, 9, 261–272. [Google Scholar] [CrossRef]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Kim, H.-Y.; Khan, A.L.; Waqas, M.; Irshad, M.; Iqbal, A.; Rehman, G.; Jan, S. Gibberellins producing endophytic fungus Porostereum spadiceum AGH786 rescues growth of salt affected soybean. Front. Microbiol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilal, L.; Asaf, S.; Hamayun, M.; Gul, H.; Iqbal, A.; Ullah, I.; Lee, I.-J.; Hussain, A. Plant growth promoting endophytic fungi Asprgillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis 2018, 76, 117–127. [Google Scholar] [CrossRef]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Irshad, M.; Khan, A.L.; Waqas, M.; Shahzad, R.; Iqbal, A.; Ullah, N.; Rehman, G. Kinetin modulates physio-hormonal attributes and isoflavone contents of Soybean grown under salinity stress. Front. Plant Sci. 2015, 6, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, A.; Hamayun, M.; Rahman, H.; Iqbal, A.; Shah, M.; Irshad, M.; Qasim, M.; Islam, B. Bioremediation of hexavalent chromium by endophytic fungi; safe and improved production of Lactuca sativa L. Chemosphere 2018, 211, 653–663. [Google Scholar]
- Ismail; Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Gul, S.; Khan, H.; Rehman, K.U.; Bibi, H.; Lee, I.-J. Penicillium Glabrum acted as a heat stress relieving endophyte in soybean and sunflower. Pol. J. Environ. Stud. 2021, 30, 3099–3110. [Google Scholar] [CrossRef]
- Sun, C.; Johnson, J.M.; Cai, D.; Sherameti, I.; Oelmüller, R.; Lou, B. Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized CAS protein. J. Plant Physiol. 2010, 167, 1009–1017. [Google Scholar] [CrossRef]
- Ilyas, N.; Mumtaz, K.; Akhtar, N.; Yasmin, H.; Sayyed, R.; Khan, W.; Enshasy, H.A.E.; Dailin, D.J.; Elsayed, E.A.; Ali, Z. Exopolysaccharides producing bacteria for the amelioration of drought stress in wheat. Sustainability 2020, 12, 8876. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.; Ikram, R.; Rizwan, M.; Akhtar, N.; Yasmin, H.; Sayyed, R.; Shafaqat, A.; Ilyas, N. Effect of 24-Epibrassinolide regulated antioxidants and osmolyte defense and endogenous hormones in two wheat varieties under drought stress. Physiol. Planta 2020, 172, 696–706. [Google Scholar] [CrossRef]
- Hare, P.; Cress, W. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; Van Staden, J. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998, 21, 535–553. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Y.; Shao, L.; Laberge, R.-M.; Demaria, M.; Campisi, J.; Janakiraman, K.; Sharpless, N.E.; Ding, S.; Feng, W. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016, 22, 78. [Google Scholar] [CrossRef] [Green Version]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Mittal, S.; Kumari, N.; Sharma, V. Differential response of salt stress on Brassica juncea: Photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiol. Biochem. 2012, 54, 17–26. [Google Scholar] [CrossRef]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Simultaneous measurement of foliar glutathione, γ-glutamylcysteine, and amino acids by high-performance liquid chromatography: Comparison with two other assay methods for glutathione. Anal. Biochem. 1998, 264, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA perception and signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Toh, S.; Imamura, A.; Watanabe, A.; Nakabayashi, K.; Okamoto, M.; Jikumaru, Y.; Hanada, A.; Aso, Y.; Ishiyama, K.; Tamura, N. High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol. 2008, 146, 1368–1385. [Google Scholar] [CrossRef] [Green Version]




| Growth Attributes | Ctrl (DW) | Ctrl (Czk) | A. foetidus |
|---|---|---|---|
| SL (cm) | 10.4 ± 0.8 a | 10.6 ± 1.5 a | 15 ± 0.6 b |
| RL (cm) | 4.9 ± 0.8 a | 6.3 ± 0.3 a | 8 ± 0.6 b |
| SFW (g) | 0.03 ± 0.0002 a | 0.0317 ± 0.0003 a | 0.0409 ± 0.0002 b |
| RFW (g) | 0.08 ± 0.007 a | 0.082 ± 0.006 a | 0.1031 ± 0.005 b |
| SDW (g) | 0.0047 ± 0.0003 a | 0.0043 ± 0.0003 a | 0.0063 ± 0.0009 b |
| RDW (g) | 0.0137 ± 0.0009 a | 0.015 ± 0.0001 a | 0.020 ± 0.0006 b |
| Chlorophyll content (SPAD) | 18.9 ± 1.3 a | 21.4 ± 0.3 a | 23.4 ± 0.7 b |
| Growth Attributes | 25 °C | 40 °C | ||
|---|---|---|---|---|
| Ctrl | A. foetidus | Control | A. foetidus | |
| SL (cm) | 39 ± 1.7 b | 40 ± 0.6 b | 26 ± 0.9 a | 27 ± 2.0 a |
| RL (cm) | 13 ± 1.4 a,b | 14 ± 1.4 b | 10 ± 0.6 a | 11 ± 0.3 a,b |
| SFW (g) | 1.12 ± 0.02 a | 1.47 ± 0.20 a | 0.82 ± 0.04 a | 1.19 ± 0.06 a |
| RFW (g) | 0.18 ± 0.02 a | 0.25 ± 0.09 a | 0.13 ± 0.02 a | 0.16 ± 0.02 a |
| SDW(g) | 0.14 ± 0.012 b | 0.14 ± 0.0051 b | 0.08 ± 0.001 a | 0.11 ± 0.009 a |
| RDW (g) | 0.075 ± 0.002 b | 0.078 ± 0.003 b | 0.01 ± 0.001 a | 0.012 ± 0.001 a |
| Chlorophyll (SPAD) | 31 ± 0.2 a | 33 ± 1.6 a | 29 ± 2.3 a | 32 ± 3.1 a |
| Growth Attributes/Temperature Stress | 25 °C | 40 °C | ||
|---|---|---|---|---|
| Ctrl | A. foetidus | Ctrl | A. foetidus | |
| SL (cm) | 23.7 ± 0.9 bc | 25.9 ± 0.9 c | 20.5 ± 0.4 a | 22.5 ± 0.5 ab |
| RL (cm) | 9.3 ± 0.6 b | 10.9 ± 0.5 b | 6.2 ± 0.6 a | 6.7 ± 0.9 a |
| SFW (g) | 1.22 ± 0.09 a | 1.32 ± 0.14 a | 0.82 ± 0.31 a | 0.93 ± 0.22 a |
| RFW (g) | 0.13± 0.021 b,c | 0.14 ± 0.084 c | 0.08 ± 0.074 a | 0.09 ± 0.006 a,b |
| SDW (g) | 0.08 ± 0.024 a,b | 0.09 ± 0.001 b | 0.04 ± 0.0001 a | 0.06 ± 0.009 a,b |
| RDW (g) | 0.024 ± 0.001 b | 0.032 ± 0.001 c | 0.014 ± 0.001 a | 0.016 ± 0.002 a |
| Chlorophyll (SPAD) | 40 ± 4.7 a | 45.6 ± 2.6 a | 38.4 ± 1.9 a | 39 ± 1.3 a |
| Growth Attributes | 25 °C | 40 °C | ||
|---|---|---|---|---|
| Ctrl | A. foetidus | Ctrl | A. foetidus | |
| AAO (EU/mg ptn) | 1.51 ± 0.09 a | 1.61 ± 0.07 b | 1.85 ± 0.12 c | 2.04 + 0.14 d |
| CAT (EU/mg ptn) | 0.46 ± 0.02 a | 0.58 ± 0.04 b | 0.95 ± 0.41 c | 1.04 ± 0.17 d |
| POD (EU/mg ptn) | 1.42 ± 0.03 a | 1.64 + 0.11 a | 3.90 ± 0.22 b | 5.01 ± 0.64 c |
| SOD (EU/mg ptn) | 18 ± 0.64 a | 19 ± 0.55 a | 37 ± 0.73 b | 43 ± 1.34 c |
| GR (EU/mg ptn) | 1.54 ± 0.61 a | 0.16 ± 0.13 a | 1.86 ± 0.05 b | 2.09 ± 0.11 c |
| Growth Attributes | 25 °C | 40 °C | ||
|---|---|---|---|---|
| Ctrl | A. foetidus | Ctrl | A. foetidus | |
| AAO (EU/mg ptn) | 0.65 ± 0.06 a | 0.77 ± 0.05 b | 2.28 ± 0.36 c | 2.55 ± 0.28 c |
| CAT (EU/mg ptn) | 0.26 ± 0.03 a | 0.34 ± 0.03 b | 0.60 ± 0.43 c | 0.72 ± 0.04 d |
| POD (EU/mg ptn) | 1.11 ± 0.08 a | 1.37 ± 0.26 a | 2.73 ± 0.16 b | 3.78 ± 0.18 c |
| SOD (EU/mg ptn) | 12 ± 0.47 a | 13 ± 0.64 a | 22 ± 1.05 b | 26 ± 0.78 c |
| GR (EU/mg ptn) | 0.89 ± 0.05 a | 0.94 ± 0.04 a | 2.11 ± 0.19 b | 2.63 ± 0.13 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail; Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Ahmad, A.; Gul, S.; Kim, H.-Y.; Lee, I.-J. Aspergillus foetidus Regulated the Biochemical Characteristics of Soybean and Sunflower under Heat Stress Condition: Role in Sustainability. Sustainability 2021, 13, 7159. https://0-doi-org.brum.beds.ac.uk/10.3390/su13137159
Ismail, Hamayun M, Hussain A, Iqbal A, Khan SA, Ahmad A, Gul S, Kim H-Y, Lee I-J. Aspergillus foetidus Regulated the Biochemical Characteristics of Soybean and Sunflower under Heat Stress Condition: Role in Sustainability. Sustainability. 2021; 13(13):7159. https://0-doi-org.brum.beds.ac.uk/10.3390/su13137159
Chicago/Turabian StyleIsmail, Muhammad Hamayun, Anwar Hussain, Amjad Iqbal, Sumera Afzal Khan, Ayaz Ahmad, Sarah Gul, Ho-Youn Kim, and In-Jung Lee. 2021. "Aspergillus foetidus Regulated the Biochemical Characteristics of Soybean and Sunflower under Heat Stress Condition: Role in Sustainability" Sustainability 13, no. 13: 7159. https://0-doi-org.brum.beds.ac.uk/10.3390/su13137159